Abstract
Background
Glaucoma and dry eye disorders (DEDs) are frequent comorbidities. The antioxidant and anti-inflammatory properties of essential polyunsaturated fatty acids have been extensively studied in relation to eye diseases.
Objective
Our objective was to determine the effects of oral supplementation with a combined formulation of antioxidants and essential polyunsaturated fatty acids on expression of cytokines and chemokines in tears from patients with DEDs or primary open-angle glaucoma (POAG).
Methods
Participants (n = 97) were distributed into three groups: (1) individuals with nonsevere DEDs (DEDG), (2) individuals with nonadvanced POAG (POAGG), and (3) healthy controls. These groups were randomized into two subgroups: one received a daily antioxidant and essential polyunsaturated fatty acid supplement (two pills) for 3 months (+S), and the other did not (−NS). Participants were interviewed and ophthalmologically examined. Concentrations of specific cytokines and chemokines in reflex tears were determined by multiplexed particle-based flow cytometry. The data were analyzed statistically (SPSS version 15.0).
Results
Comparison of the results from the DEDG and POAGG patients showed significant differences in tear expression of granulocyte-macrophage colony-stimulating factor (P = 0.008), tumor necrosis factor α (P = 0.005), vascular endothelial growth factor (P = 0.038), interleukin-4 (P = 0.030), and interleukin-6 (P = 0.044). The main signs and symptoms of dry eyes such as dryness, burning, photophobia, eye heaviness, and blurred vision, as well as positive changes in eyelashes, hair, nails and skin, were significantly improved in DEDG +S and POAGG +S patients relative to unsupplemented patients.
Conclusion
Inflammation biomarkers were differentially expressed in glaucomatous tears, but the differences changed upon antioxidant/essential polyunsaturated fatty acid supplementation. Chronic instillation of antihypertensive eye drops must be considered for integrating protocols to glaucoma standards of care.
Introduction
Owing to advances in disease prevention, detection, and treatment, life expectancyCitation1 has increased in most countries. From 2005 to 2010, the worldwide average life expectancy at birth was 67.88 years (70.14 years for females and 65.71 years for males).Citation2 As a result, biomedical research on the diseases of aging is especially relevant. Age is an important risk factor for ocular diseases such as glaucoma, dry eye disorders (DEDs), cataracts, and age-related macular degeneration, and in elderly people, such diseases can occur together, significantly impairing visual acuity and thus reducing quality of life.
DEDs are complex pathological conditions involving the eyelids, lacrimal glands, tear film, and ocular surface tissues.Citation3 There are two major forms of DEDs – the deficient aqueous tear production form (due to lacrimal gland dysfunction) and the increased evaporative loss form (due to meibomian gland disorder) – but combinations of the two forms are usually seen in clinical practice.Citation4,Citation5 DEDs are also classified according to severity, ranging from normal to mild-to-moderate to severe. Dry eyes usually affect people aged above 60 years old. Significant reduction in body water associated with aging may play a role in the onset or progression of DEDs. Meibomian gland dropout, reduced goblet cells, and laxity of the eyelids may also be contributing factors. In addition, diverse pathologic manifestations on the ocular surface may be triggered by external or internal injury, as well as by menopause, topical or systemic medications, light, computer use, environmental pollutants, and air conditioning.Citation3–Citation5
Glaucoma is a group of diseases characterized by increased intraocular pressure as the main risk factor, leading to progressive visual field defects and visual impairment secondary to loss of retinal ganglion cells and optic nerve fibers.Citation6 People with a glaucomatous family history, diabetes, hypertension, or myopia; African-Americans, Hispanics, and Asians; and people over forty years are at increased risk of glaucoma. Among the various types of glaucoma, the most prevalent is primary open-angle glaucoma (POAG), which accounts for almost 80% of all glaucomas.Citation7,Citation8 The prevalence of POAG has been estimated as 1.1%–3.0% of Western populations aged 40 years or more. Glaucoma remains the second leading cause of blindness worldwide.Citation8
Therefore, both glaucoma and DEDs are highly correlated with age. It is not atypical to see patients chronically treated with antihypertensive eye drops who also exhibit the clinical DED manifestations, from a lesser to a greater degree of severity categories. Recent studies have indicated that 66% of patients with severe DEDs also have glaucoma,Citation9 and approximately 60% of glaucomatous individuals undergoing topical antiglaucoma therapy report DED symptoms.Citation10 However, most cases of glaucoma and DED comorbidity remain undiagnosed, or misclassified as chronic eye irritation, and this is the most important point to be addressed in the present work.
Oxidative stress (OS) plays a significant role in a wide spectrum of ocular conditions.Citation11 OS, an imbalance between pro-oxidant and antioxidant species, can result from the accumulation or uncontrolled generation of reactive oxygen species, partially reduced byproducts of molecular oxygen,Citation11 which may trigger damage to lipids, proteins, and nucleic acids, resulting in cell lesions and death. A significant increase in oxidative activity and a decrease in antioxidant defenses in ocular fluids and tissues as well as in plasma samples have been associated with DEDs,Citation5 POAG,Citation12,Citation13 cataracts,Citation14 diabetic retinopathy,Citation15,Citation16 and age-related macular degeneration.Citation17 However, the pathogenesis of OS and the contribution of this condition to the initial stages or progression of DEDs in patients with POAG remain unclear.
The eyes are continuously exposed to environmental irritants, which stimulate inflammation and immune response (IIR) processes intended to prevent and heal external and internal injuries. The main mediators of IIR processes include leukocytes and other cells involved in the innate immunity system, as well as T, B, and natural killer (NK) lymphocytes (the major components of the adaptive immune system); and the interactions between these cells are modulated by cytokines, chemokines and other molecules.Citation18 Uncontrolled acute or chronic inflammation can lead to tissue damage. For example, altered levels of a wide spectrum of IIR mediators in blood, the aqueous humor, the vitreous body, and eye tissues support the idea that abnormal activity of the immune system is involved in both anterior and posterior ocular segment disorders.Citation19–Citation22 Moreover, altered immune response regulation may shift the physiological equilibrium over a chronic-cumulative period, leading to a low-grade inflammatory degenerative process, known as para-inflammation, with the oxidative stress acting as a local trigger for retinal para-inflammatory responses.Citation23
Advances in biotechnology, such as multiplexed flow cytometry assays, may improve our understanding of the pathogenesis and progression of eye diseases.Citation24,Citation25
Antioxidants (AOXs) and anti-inflammatory compounds, such as essential polyunsaturated fatty acids (EPUFAs), may have potential for the treatment of eye diseases. EPUFAs, such as omega-3 (ω-3) and omega-6 (ω-6) fatty acids have important effects in the body. Omega fatty acids provide energy and perform important functions in the body; enhance appropriate prenatal and postnatal development (mainly of the central and peripheral nervous systems), lower cholesterol and triglyceride levels, reduce acute and chronic inflammation, help in the management of emotional distress and depression, benefit patients with neurodegenerative disorders, reduce respiratory and asthma-like symptoms, regulate the blood pressure, and reduce the odds of developing cancer, heart disease, and stroke.Citation26–Citation32 The ω-3-derived eicosanoids exert anti-inflammatory effects, whereas the ω-6-derived eicosanoids are pro-inflammatory.Citation28,Citation30 The regulation of the biochemical mechanisms of acute inflammation, which is at least in part performed by endogenous EPUFA-derived autacoids (including pro-resolving mediators such as lipoxins, resolvins, protectins, and maresins), has recently been the subject of diverse studies.Citation31–Citation38 EPUFAs are expected to be useful molecules for studies of ocular health and disease, including DEDs and POAG.
In this study, our main goals were the following: (1) to assess the expression of IIR molecules in tears of patients diagnosed with DEDs or POAG and (2) to evaluate the effect of oral supplementation with a combined formulation of AOXs and EPUFAs on the signs and symptoms of dry eyes in patients diagnosed with nonsevere DEDs or POAG, by comparing the supplemented DED or POAG groups with the corresponding unsupplemented groups, as well as with healthy controls.
Materials and methods
This prospective, open-label, randomized study was approved by the Institutional Review Board of the University Hospital (Valencia, Spain), as a nonsignificant risk investigational device study (Ethics Committee approved reference 59/10), and all tenets of the Declaration of Helsinki for the protection of human subjects in medical research were strictly observed.
Study design
Using the main inclusion and exclusion criteria (), we enrolled a total of 97 participants of both sexes, aged 25–80 years, during ophthalmologic appointments at the study centers University and Polytechnic Hospital La Fe (Valencia, Spain), Ophthalmic Research Unit Santiago Grisolia (Valencia, Spain), and Hospital of Jerez (Jerez de la Frontera, Cadiz, Spain), between March 2012 and November 2012.
Table 1 Main inclusion and exclusion criteria among the study participants
Prior to the baseline visit, participants were required to discontinue, for at least 1 month, use of nutritional supplements, systemic antihistamines, and treatments related to dry eyes (or meibomian gland disorder) such as antibiotics, nonsteroidal and anti-inflammatory drugs, and corticosteroids, as well as artificial tears containing vitamins. In addition, participants were asked to strictly follow the recommendations of the ophthalmologists throughout the duration of study. Ocular lubricants without nutraceuticals were permitted and their use or nonuse was recorded. Antihypertensive eye drops were also allowed and their use or nonuse was also recorded. Patients with obvious infection or significant eyelid inflammation were excluded from the study, as were patients with advanced glaucoma and patients with a previous diagnosis of coexisting POAG and DEDs. We wanted to analyze the ocular surface characteristics and the expression of IIR molecules in tears for two specific groups of patients: those with a diagnosis of a nonsevere DED and those with a diagnosis of nonadvanced POAG.
Suitable participants (97 participants, 194 eyes) were assigned to one of the following groups: (1) patients diagnosed with nonsevere DEDs (DEDG; n = 30), (2) patients diagnosed of nonadvanced POAG (POAGG; n = 31), and (3) a control group of healthy participants (CG; n = 36). Two homogeneous subgroups were selected: one group was prescribed an oral supplement containing AOXs and EPUFAs (AOX/EPUFA, two capsules per day, +S) and the other received no supplement (−NS). The oral supplement was Brudysec 1.5® (Brudy Laboratories, Barcelona, Spain), each capsule of which contains the following components in a combined nutraceutical formulation: docosahexaenoic acid (350 mg), eicosapentaenoic acid (42.5 mg), docosapentaenoic acid (30 mg), vitamin A (133 μg), vitamin C (26.7 mg), vitamin E (4 mg), tyrosine (10.8 mg), cysteine (5.83 mg), glutathione (2 mg), zinc (1.6 mg), copper (0.16 mg), manganese (0.33 mg), selenium (9.17 μg).
Ophthalmologists were instructed to emphasize, during an initial personal interview with each participant, compliance with the oral supplementation regimen, to maximize the effectiveness of the supplements and in turn, the reliability of the study data. After the initial visit, all participants were followed every 4 weeks for a total follow-up period of 3 months.
Participant management
At the beginning of the study, all participants underwent an interview during which information about their personal and familial background and the characteristics of their eye disease (DED or POAG) was collected; emphasis was placed on the signs and symptoms of dry eyes and the participants’ subjective sensations. For each group of participants, the effectiveness of AOX/EPUFA supplementation was evaluated in terms of clinical and molecular changes from baseline to 3 months.
A systematized ophthalmologic examination was conducted on all participants: the examination included measurement of the best corrected visual acuity in each eye, the Schirmer’s test, slit lamp examination of the eye adnexa and anterior eye segment, measurement of tear breakup time (BUT) with fluorescein, and examination of corneal surface details with fluorescein. All of the ophthalmologists were instructed to fill a specifically designed a fullsheath to enclose all data and were advised to strictly follow the study protocol. The primary outcome ophthalmologic measures of the effectiveness of the oral nutraceutical formulation were the Schirmer’s test and fluorescein tear BUT, and the secondary outcome measures were dry eye symptoms and subjective sensations.
Tear sampling
We used the gentle rubbing method to obtain reflex tears from all participants, and we assayed the expression of a specific set of cytokines and chemokines in the tear samples by means of the Luminex® R-200 multiplex system (Luminex, Austin, TX, USA). Polystyrene beads coupled covalently to specifically directed antibodies (human cytokine/chemokine panel) were allowed to react with 30–40 μL of each tear sample containing unknown amounts of cytokines and chemokines, or with a standard solution containing known amounts of cytokines and chemokines, at room temperature for 1 hour, as previously described.Citation39 The specific set of IIR molecules that were determined by means of this assay, as standardized by the manufacturers, were interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, and IL-12; tumor necrosis factor alpha (TNF-α); vascular endothelial growth factor (VEGF); granulocyte-macrophage colony-stimulating factor (GM-CSF); and interferon gamma. Briefly, a series of washes were carried out to remove unbound protein. Then a biotinylated detection antibody specific for a different epitope on the cytokine was added to the beads and incubated at room temperature for 30 minutes. Streptavidin-phycoerythrin, which binds to the biotinylated detection antibodies, was used to detect the reaction mixture. We used a flow-based Bio-Plex® suspension array system (Bio-Rad Laboratories, Hercules, CA, USA) to identify and quantify each particular antigen–antibody reaction. Identification of the assayed molecules was based on bead color and fluorescence, using fluorescently labeled reporter molecules associated with each target protein. Unknown cytokine and chemokine concentrations were calculated automatically by the Bio-Plex Manager software using a standard curve derived from a recombinant cytokine standard. Cytokine and chemokine concentrations were corrected for the initial total protein concentration of each human tear sample during analysis.
All the results are shown as means ± standard deviations for two or three determinations and are expressed in picograms per milliliters per milligram.
Statistical procedures
Demographic, clinical, and biochemical data were recorded in a previously designed Excel sheet (Microsoft Corporation, Redmond, WA, USA). A nonparametric Mann–Whitney U-test was selected for comparing two independent sample groups by means of the SPSS software (version 15.0, SPSS Inc, Chicago, IL, USA). All results were statistically analyzed to detect differences between groups. For our purposes, P < 0.05 was established as statistically significant.
Results
The median ages of the participant groups were 52 ± 15 years (DEDG), 64 ± 17 years (POAGG), and 50 ± 12 years (CG). When the participants were aged over 40 years (pivotal risk factor for both the DEDs and POAG) the percentages attending each group were 64% (DEDG), 67% (POAGG), and 60% (CG). Regarding sex, the percentages of men and women in all groups were 28% and 72% (DEDG), 29% and 71% (POAGG), and 32% and 68% (CG) respectively. Patient characteristics and risk factors for DEDs are reflected in the .
Table 2 Patient characteristics
All the DEDG patients reported a history of dry eyes before the start of the study; they suffered from at least one of the following signs and symptoms: scratchy sensation, soreness, itchiness, grittiness, foreign body sensation, dryness, burning, photophobia, eye fatigue and/or blurred vision, and redness. Most of the DEDG patients (89%) used eye drops regularly for treating their ocular surface alteration. None of the DEDG patients suffered from severe dryness or Sjögren’s syndrome.
None of the POAGG patients had a previous diagnosis of a DED or used tear substitutes. Of the POAGG patients who were under treatment with antihypertensive drops treatment, 52% reported at least one of the dry eye signs and symptoms listed above for the DEDG patients. The POAGG patients mainly presented with a burning, dryness, photophobia, and/or conjunctival hyperemia, usually in both eyes.
The three groups of participants were examined under a slit lamp in relation to their anterior eye segment, and almost every DEDG patient displayed ocular surface disorder morphological alterations such as marginal blepharitis and stinging of the cornea. Most of the POAGG patients showed DED-related morphological changes (38%). However, neither the interviews nor the ocular surface examination revealed significant DED-related manifestations in the healthy participants.
The Schirmer’s test scores were significantly lower in the DEDG (4.26 ± 0.59 mm) than in the POAGG (7.82 ± 1.92 mm) or the CG (13.25 ± 2.46 mm; P = 0.0002), reflecting the altered tear secretion in both the DEDG and POAGG patients. DEDG and POAGG patients who were younger than 40 normally moistened 7–12 mm of each paper strip with 5 minutes of contacting the paper strip, whereas patients older than 40 usually moistened about 4–9 mm of each strip.
The fluorescein tear BUT was much shorter in the DEDG patients (4.35 ± 1.23 seconds) than in the POAGG and CG patients (6.14 ± 2.32 seconds and 14.24 ± 3.22 seconds, respectively), which strongly reflects the altered tear film stability in the DEDG and the POAGG patients as compared to that in the CG patients (P = 0.0001).
None of the three groups showed significant differences in best corrected visual acuity from baseline to 3 months, and no changes in this parameter was also noticed in the oral supplementation subgroups from pre- to postsupplementation.
Results from the tear-sampling procedure were calculated by subtracting background cytokine and chemokine concentrations from cytokine concentrations in the tear samples. The standard curves for both the kit assay and extraction buffers were similar for the 12 analyzed molecules. The set of assayed molecules showed a wide variety of expression levels in the tear samples, and the precision of the values as measured by the Luminex multianalyte profiling bioassay system was acceptable. The amounts of tear samples obtained from the study participants permitted detection in up to 92% of the samples.
Noticeable increases in the collected amount of tears (30% or more) were observed in the DEDG and POAGG subgroups taking the AOX/EPUFA supplement with respect to the amounts in the same groups not taking the supplement.
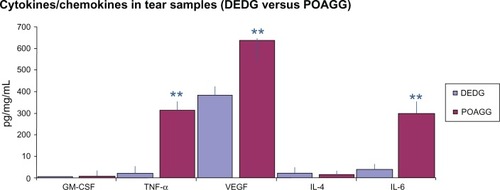
Comparison of the results from the DEDG and POAGG patients showed significant differences in tear levels of GM-CSF (P = 0.008), TNF-α (P = 0.005), VEGF (P = 0.038), IL-4 (P = 0.030), and IL-6 (P = 0.044). These results are shown in .
Figure 1 Comparison of the expression of cytokines/chemokines in tears from the DEDG and POAGG patients.
Abbreviations: DEDG, dry eye disorder group; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL-4, interleukin-4; IL-6, interleukin-6; POAGG, primary open-angle glaucoma group; TNF-α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor.
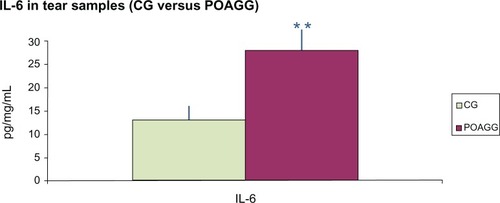
Comparison of the data for the POAGG and CG patients demonstrated significant differences in the tear levels of IL-6 (P = 0.014) ().
Figure 2 Comparison of the expression of IL-6 in tears from the CG and POAGG patients.
Abbreviations: CG, control group; IL-6, interleukin-6; POAGG, primary open-angle glaucoma group.
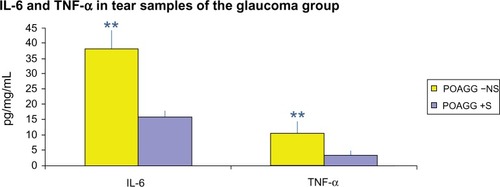
The POAGG +S patients showed a significant reduction in the tear concentrations of IL-6 (P = 0.44) and TNF-α (P = 0.00001) compared to the POAGG −NS patients ().
Figure 3 Comparison of the expression of IL-6 and TnF-α in tears from the POAGG +S compared to the POAGG −NS patients.
Abbreviations: IL-6, interleukin-6; POAGG −NS, primary open-angle glaucoma group without oral supplements; POAGG +S, primary open-angle glaucoma group with oral supplements; TNF-α, tumor necrosis factor alpha.
The main signs and symptoms of dry eyes were significantly improved in the DEDG +S and POAGG +S patients compared to the corresponding patients who did not receive the supplement. At 3 months, an overall improvement in both the objective and the subjective manifestations was observed in the DEDG +S patients as compared to the DEDG −NS patients, and in the POAGG +S patients as compared to the POAGG −NS patients. Patients reported amelioration of at least 68% of ocular signs such as dryness, burning, photophobia, eye heaviness, and blurred vision, as well as noticeable improvements in eyelashes, hair, nails and skin.
Discussion
Dry eyes are usually due to environmental irritants and topical or systemic medications.Citation3–Citation5,Citation39,Citation40 Because most patients with glaucoma are not aware of suffering any type of ocular surface disorder, special attention has to be paid to patients with chronic glaucoma who may develop DED signs and symptoms during the course of their treatment. The main goal of this study was to determine whether there were any differences in tear expression of IIR mediators in patients with nonsevere DEDs and patients with POAG as compared to expression in healthy controls. We used the Luminex multianalyte profiling assay system, a new technique that allows simultaneous measurement of various molecules in a single microplate well.Citation41 We also evaluated the effect on patients with POAG or DED of oral supplementation with AOX/EPUFA over the course of 3 months for each group of participants.
Human tears contain a wide spectrum of cytokines and chemokines, the main function of which is to maintain the morphologic and physiologic properties of the ocular surface.Citation41–Citation46 We found that tears collected from patients with DEDG and POAG showed differential expression of the assayed cytokines and chemokines compared to the tears of healthy controls. Specifically, the concentrations of IL-6 and TNF-α were significantly higher in the DEDG and POAGG than in the CG. Other investigators have also detected higher IL-6 and TNF-α levels in the conjunctival epithelium and in tear samples of patients with DEDs.Citation43,Citation47,Citation48 However, it has also been reported that the concentration of IL-6 is not higher in tear samples from patients with moderately dry eyes.Citation49 Because inflammatory processes mediated by cytokines and chemokines are commonly associated with ocular surface disorders, independently of their etiology, our data strongly suggest that the augmented expression of IL-6 and TNF-α in tears is worth noting, because these proinflammatory cytokines are most probably the underlying cause of most clinical manifestations of the ocular surface alterations in both the DEDG and POAGG patients.
Aging is a relevant factor in both DEDs and POAG. When all the participants were assembled as being aged over 40 years, each group (DEDG, POAGG and CG) and subgroup (patients taking or not taking oral supplements) was homogeneous in their percentages.
Percentages of men and women in all groups were 28% and 72% (DEDG), 29% and 71% (POAGG), and 32% and 68% (CG) respectively, reflecting the differences in gender of both diseases in the general population.
Daily dietary intake of EPUFAs (ω-3 and ω-6) is necessary so that they can exert their effects in the body (eg, on the immune system) at both the cellular and the humoral levels.Citation30,Citation31 Furthermore, the ω-3 EPUFA intake and the ω-3 EPUFA/ω-6 EPUFA intake ratio affect the expression of inflammatory biomarkers.Citation50 Therefore, it has been shown that ω-3 EPUFA supplementation can protect the ocular surface in patients at risk of DEDs.Citation33,Citation51–Citation53 In the present work, we determined whether the oral supplementation of a combined formulation of AOXs and EPUFAs (Brudysec 1.5®) influenced the progression of DED, and our data suggest that supplement intake significantly changed the expression patterns of various cytokines and chemokines in tears collected from patients with DEDs or POAG compared to the patterns in the corresponding unsupplemented patients. Other investigators previously reported similar results for AOX and EPUFA supplementation in patients with dry eyes.Citation51–Citation53
Patients with POAG are generally not aware of having ocular surface dysfunction, probably because they do not recognize some of the ocular signs and symptoms. As reported by GilbardCitation54 other possible causes (and their symptoms) have to be considered for a patient’s chronic eye irritation, as follows: iatrogenic, non-specific ocular irritation, tarsal foreign body, anterior blepharitis, obstruction of the lacrimal drainage system, meibomian gland dysfunction, allergic conjunctivitis, nocturnal lagophthalmos, superior limbic keratoconjunctivitis, superficial punctate Thygenson’s keratitis, dry eyelid skin, normal eyes responding to abnormal environment, and blepharospasm. In these cases, careful questioning and specific clinical examination may reveal that patients are experiencing DED signs and symptoms rather than eye irritation.
All of the DED patients in our study reported a history of dry eyes before starting the study, whereas only half of the patients with POAG suffered symptoms related to dry eyes, mainly dryness, photophobia, burning, or conjunctival hyperemia. Furthermore, examination of the POAG patients revealed significant reductions in Schirmer’s test scores and fluorescein tear BUT compared to those of the controls. In agreement with our data, other authors have also reported that around 60% of patients with POAG have dry eye signs and symptoms.Citation9,Citation10 We speculate that POAG patients needing long-term treatment with antihypertensive eye drops are at risk of developing a DED (or exacerbating a latent DED). The significant role of aging in both DEDs and POAG may increase the probability of POAG and DED comorbidity.Citation55–Citation57 Therefore, we strongly recommend that patients with POAG be carefully evaluated for dry eye symptoms prior to starting any topical antiglaucomatous therapy. Our results suggest that in addition to the use of eye drops to protect the ocular surface, oral supplementation with a combination of AOXs and EPUFAs, such as in the present study (Brudysec 1.5®), should be considered for elderly glaucomatous patients at risk for DEDs.
Disclosure
The authors report no conflicts of interest in this work. Part of this work was presented to the Meeting of the Society of Research in Retina and Visual Sciences (SIRCOVA) in Valencia, Spain (2013).
References
- CaspariRLeeSHIs human longevity a consequence of cultural change or modern biology?Am J Phys Anthropol200612951251716342259
- United Nations, Department of Economic and Social Affairs, Population DivisionWorld Population Prospects: The 2010 RevisionCD-ROM Edition – Extended Dataset in Excel and ASCII FormatsUnited Nations publication sales no 11.XIII.7
- PerryHDDry eye disease: pathophysiology, classification, and diagnosisAm J Manag Care200814Suppl 3S79S8718452371
- TavaresF de PFernandesRSBernardesTFDry eye diseaseSemin Ophthalmol201025849320590418
- LabbéABrignole-BaudouinFBaudouinCOcular surface investigations in dry eyeJ Fr Ophthalmol2007307697
- KwonYHFingertJHKuehnMHAlwardWLPrimary open-angle glaucomaN Engl J Med2009360111113112419279343
- MunozBWestSKRubinGSCauses of blindness and visual impairment in a population of older Americans: The Salisbury Eye Evaluation StudyArch Ophthalmol200011881982510865321
- FriedmanDSWolfsRCO’ColmainBJPrevalence of open-angle glaucoma among adults in the United StatesArch Ophthalmol2004122453253815078671
- TsaiJHDerbyEHollandEJKhatanaAKIncidence and prevalence of glaucoma in severe ocular surface diseaseCornea200625553053216783140
- LeungEWMedeirosFAWeinrebRNPrevalence of ocular surface disease in glaucoma patientsJ Glaucoma200817535035518703943
- HalliwellBFree radicals and antioxidants: Updating a personal viewNutr Rev20127025726522537212
- Zanon-MorenoVMarco-VenturaPLleó-PerezAVOxidative stress in primary open-angle glaucomaJ Glaucoma20081726326818552610
- Pinazo-DuránMDZanon-MorenoVGarcia-MedinaJJGallego-PinazoREvaluation of presumptive biomarkers of oxidative stress, immune response and apoptosis in primary open-angle glaucomaCurrent Opin Pharmacol201313198107
- BerthoudVMBeyerECOxidative stress, lens gap junctions, and cataractsAntioxid Redox Signal20091133935318831679
- Garcia-MedinaJJPinazo-DuránMDGarcia-MedinaMAntioxidant supplementation on diabetic retinopathy over a 5-year follow-up periodEur J Ophthalmol20112163764321218388
- MancinoRDi PierroDVaresiCLipid peroxidation and total antioxidant capacity in vitreous, aqueous humor, and blood samples from patients with diabetic retinopathyMol Vis2011171298130421633716
- SuzukiMTsujikawaMItabeHChronic photo-oxidative stress and subsequent MCP-1 activation as causative factors for age-related macular degenerationJ Cell Sci20121252407241522357958
- MedzhitovRJanewayCJrInnate immunityN Engl J Med200034333834410922424
- SuzukiMTsujikawaMItabeHChronic photo-oxidative stress and subsequent MCP-1 activation as causative factors for age-related macular degenerationJ Cell Sci20121252407241522357958
- ZoukhriDEffect of inflammation on lacrimal gland functionExp Eye Res20068288589816309672
- KrabbeKSPedersenMBruunsgaardHInflammatory mediators in the elderlyExp Gerontol20043968769915130663
- StevensonWChauhanSKDanaRAn immune-mediated ocular surface disorderArch Ophthalmol20121309010022232476
- XuHChenMForresterJVPara-inflammation in the aging retinaProg Retin Eye Res20092834836819560552
- YoshidaNIkedaYNotomiSLaboratory evidence of sustained chronic inflammatory reaction in retinitis pigmentosaOphthalmology20131201e5e1222986110
- Gallego-PinazoRMarsigliaMMrejenSOuter retinal tubulations in chronic central serous chorioretinopathyGraefes Arch Clin Exp Ophthalmol2012
- SwenorBKBresslerSCaulfieldLThe impact of fish and shellfish consumption on age-related macular degenerationOphthalmology20101172395240120630597
- ChongEWTKreisAJWongJYDietary ω-3 fatty acid and fish intake in the primary prevention of age-related macular degeneration: a systematic review and meta-analysisArch Ophthalmol200812682683318541848
- LicinioJWongMLThe role of inflammatory mediators in the biology of major depression: Central nervous system cytokines modulate the biological substrate of depressive symptoms, regulate stress-responsive systems, and contribute to neurotoxicity and neuroprotectionMol Psychiatry1999431732710483047
- RenHMagulikeNGhebremeskelKPrimary open-angle glaucoma patients have reduced levels of blood docosahexaenoic and eicosapentaenoic acidsProstaglandins Leukot Essent Fatty Acids20067415716316410047
- Pinazo-DuránMDBoscá-GomarLAnti-inflammatory properties of essential fatty acids. EditorialArch Soc Esp Oftalmol20128720320522732118
- SapiehaPStahlAChenJ5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of ω-3 polyunsaturated fatty acidsSci Transl Med2011369ra12
- RosenbergESAsbellPAEssential fatty acids in the treatment of dry eyeOcul Surf20108182820105404
- Pinazo-DuránMDGalbis-EstradaCCantu-DibildoxJPons-VazquezSBenítez-del-CastilloJEffects of a nutraceutical formulation based on the combination of antioxidants and ω-3 essential fatty acids in the expression of inflammation and immune response mediators in tears from patients with dry eye disordersClin Int Aging20138110
- RonconeMBartlettHEperjesiFEssential fatty acids for dry eye: a reviewCont Lens Anterior Eye201033495420031476
- Brignole-BaudouinFBaudouinCAragonaPA multicentre, double-masked, randomized, controlled trial assessing the effect of oral supplementation of omega-3 and omega-6 fatty acids on a conjunctival inflammatory marker in dry eye patientsActa Ophthalmol201189e591e59721834921
- RandALAsbellPANutritional supplements for dry eye syndromeCurr Opin Ophthalmol20112227928221597374
- CortinaMSBazanHEDocosahexaenoic acid, protectins and dry eyeCurr Opin Clin Nutr Metab Care20111413213721157308
- LiNHeJSchwartzCEResolvin E1 improves tear production and decreases inflammation in a dry eye mouse modelJ Ocul Pharmacol Ther20102643143920874497
- MossSEKleinRKleinBEPrevalence of and risk factors for dry eye syndromeArch Ophthalmol20001181264126810980773
- BaudouinCA new approach for better comprehension of diseases of the ocular surfaceJ Fr Ophthalmol200730239246
- VanDerMeidKRSuSPKrenzerKLA method to extract cytokines and matrix metalloproteinases from Schirmer strips and analyse using LuminexMol Vis2011171056106321552500
- Enríquez-de-SalamancaACastellanosESternMETear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye diseaseMol Vis20101686287320508732
- BaudouinCBrignoleFBecquetFFlow cytometry in impression cytology specimens: a new method for evaluation of conjunctival inflammationInvest Ophthalmol Vis Sci19978145814649191610
- VignaliDAMultiplexed particle-based flow cytometric assaysJ Immunol Methods200024324325510986418
- MassingaleMLLiXVallabhajosyulaMAnalysis of inflammatory cytokines in the tears of dry eye patientsCornea2009281023102719724208
- BrignoleFPisellaPHGoldschildMFlow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyesInvest Ophthalmol Vis Sci2001413561363
- HiguchiAKawakitaTTsubotaKIL-6 induction in desiccated corneal epithelium in vitro and in vivoMol Vis2011172400240621976951
- BoehmNRiechardtAIWiegandMPfeifferNGrusFHProinflammatory cytokine profiling of tears from dry eye patients by means of antibody microarraysInvest Ophthalmol Vis Sci2011527725773021775656
- NarayananSMillerWLMcDermottAMConjunctival cytokine expression in symptomatic moderate dry eye subjectsInvest Ophthalmol Vis Sci2006472445245016723455
- HibbelnJRNieminenLRGBlasbalgTLRiggsJALandsWEMHealthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversityAm J Clin Nutrition2006831483S1493S16841858
- SullivanBDCermakJMSullivanRMCorrelations between nutrient intake and the polar lipid profiles of meibomian gland secretions in women with Sjogrens syndromeSullivanDLacrimal Gland, Tear Film, and Dry Eye SyndromeKluwer Academic/Plenum Publishers2002441447
- ChiaEMMitchellPRochtchinaEPrevalence and associations of dry eye syndrome in an older population: the Blue Mountains Eye StudyClin Experiment Ophthalmol (Australia)200331229232
- MiljanovićBTrivediKADanaMRGilbardJPBuringJESchaumbergDARelation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in womenAm J Clin Nutr20058288789316210721
- GilbardJ PDry Eye DisordersAlbertDMJakobiecFAPrinciples and Practice of OphthalmologyPhiladelphia: WB Saunders Company1994257276 ScheinODMunozBTielschJMPrevalence of dry eye among the elderlyAm J Ophthalmol19971247237289402817
- RossiGCTinelliCPasinettiGMDry eye syndrome-related quality of life in glaucoma patientsEur J Ophthalmol20091957257919551671
- RüferFErbCInfluence of dry eye syndrome on glaucoma diagnostic proceduresOphthalmologe20121091082108623179813
- LabbéATerryOBrasnuEVan WentCBaudouinCTear film osmolarity in patients treated for glaucoma or ocular hypertensionCornea20123199499922710490