Abstract
Background
Both Alzheimer’s disease (AD) and cerebrovascular atherosclerosis (CA) contributed to dementia in an aged population. Whether they share the same mechanism is unknown.
Aim
Our goal was to explore the occurrence rates of CA in AD patients.
Method
Here we examined the degree of CA in different groups of AD patients with contrast angiography. Ninety-three AD patients were recruited to the present study. Contrast computed tomography scanning and contrast angiography were performed for CA analyses.
Result
We found that the cerebrovascular plaques were common in AD patients, which was partly correlated with the severity of AD (as determined by cognitive decline).
Conclusion
We concluded that vascular dementia may partly correlate with AD pathology.
Introduction
Both Alzheimer’s disease (AD) and cerebrovascular atherosclerosis (CA) contributed to dementia in an aged population.Citation1–Citation3 Whether the two diseases share a common mechanism or pathological progression is yet unclear. It is proposed that in AD the amyloid beta peptide mutates and accumulates to form abnormal aggregates that are resistant to internal clearance,Citation4–Citation6 and these aggregates lead to amyloid plaques (senile plaques) that cause neuronal death as well as other pathological changes inside the brain, with final functional loss. Conversely, for the CA multiple small infarcts (atheromatous plaque) formed inside microvasculature and interrupted the microperfusion.Citation7–Citation9 This also leads to cognitive decline and memory loss as the final result.
Given the two distinct theories explaining the pathogenesis of AD and CA, there are increasing studies that show some common risk factors for both diseases, such as apolipoprotein E, age, smoking, inflammation, and body metabolism.Citation2,Citation10–Citation15 In addition, the coronary atherosclerosis cases are increased in patients with AD compared to those without.Citation16–Citation18 Conceivably, the CA is higher in patients showing heart attack. Postmortem studies also reported the existence of CA in many AD patients. In the present study, we tried to employ radiological approach to investigate the occurrence of CA in AD patients.
Materials and methods
Clinical data
In the present study 93 AD patients were recruited in the Imaging Center, Nanfang Hospital of Southern Medical University, Guangzhou, People’s Republic of China (male, 61; female, 32; aged 59–82 years; average, 71.2 years old) from January 2012 to December 2012. The patients met the criteria of the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association for AD, with a clinical dementia rating of 0.5 ([early AD] 17 cases); or 1 ([mild AD] 26 cases); 2 ([moderate AD] 39 cases); or 3 ([severe AD] 11 cases) ().
Table 1 Clinical information for all patients
The study was approved by the ethics committee of medical research on human subjects at the General Hospital of Jinan Military Command, and the researchers obtained written consent from all subjects as well as their family members.
Contrast computed tomography scanning and contrast angiography were performed, atherosclerotic lesions were registered, and diameter stenosis degrees were calculated.
Statistics
The data were represented as mean ± standard deviation and analyzed with the Statistical Package for the Social Sciences 16.0 software (SPSS; IBM Corporation, Armonk, NY, USA) for statistics. The t-test was used to compare intergroup differences, and P < 0.05 was considered statistically significant.
Results
Detection of cerebrovascular atherosclerosis
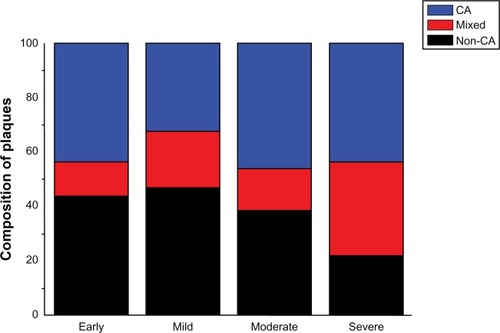
We found that atheromatous plaque is prevalent in AD patients: six of 17 (35.3%) in early AD; 14 of 26 (53.8%) in mild AD; 20 of 39 (51.3%) in moderate AD; and nine of eleven (81.8%) in severe AD. In addition, among these cases, both noncalcified and calcified atheromatous plaques were detected at different possibilities (). The percentage of noncalcified mixed and calcified atheromatous plaques were 44%, 12%, and 44% in early AD; 47%, 21%, and 32% in mild AD; 39%, 15%, and 46% in moderate AD; and 22%, 35%, and 43% in severe AD ().
Distribution of atheromatous plaques
We found that noncalcified atheromatous plaques were mainly distributed in the intracranial arteries (79.2%), mixed plaques in the intracranial arteries (19.1%), and intracranial internal carotid artery (ICA) (44.3%), calcified plaques in the intracranial ICA (55.2%), and extracranial arteries (24.7%) across the different AD groups. Interestingly, we found no obvious differences in plaque distribution across different staged AD patients (P > 0.05).
Results of atheromatous plaques as vessel occlusion
The differentially composed atheromatous plaques have been shown to be associated with different levels of vessel occlusion previously. The percentage of severe stenosis (>60%) and occlusion in the different AD groups were 3.1% (early), 6.7% (mild), 11.3% (moderate), and 10.7% (severe). The values were significantly different from the severe AD group to the early/mild AD groups.
Discussion
Few studies have examined the formation of CA plaques in AD patients, specifically.Citation1,Citation2,Citation7,Citation12 It has been shown that in an aged group with cognitive decline, atherosclerosis plaques could be used as the marker for vascular dementia.Citation4,Citation7,Citation19 In the present study, we found that cerebrovascular plaques leading to stenosis or occlusion existed in AD patients as well. Therefore, the mixed AD/CA pathology should be considered and referred in clinical diagnosis of certain aged patients with cognitive decline.
The mechanisms underlying such interactions are yet unclear. The common risk factors, such as apolipoprotein E, age, smoking, inflammation, and body metabolism, do not fully explain the increased atheromatous plaque occurrence in severe AD, rather than in early AD patients.Citation2,Citation4,Citation5,Citation20–Citation23 We believe it is possible that the neuronal death and neuroinflammation in late-stage AD triggered the atheromatous plaque formation. If so, the neuroinflammation would act as the share mechanism in pathogenesis and progression in patients with severe dementia, providing the common drug target for both syndromes.
There are certain limitations of the present study. Currently, it is possible to perform magnetic resonance imaging and positron emission tomography for the tracing of plaque formation in the vessels, as well as of the amyloid plaques for AD patients.Citation24–Citation26 It will be important to perform different tests on patients to correlate the changes of CA pathology with AD pathology (senile/amyloid plaque) progression. Additionally, we did not correlate other syndromes of vascular dementia into the clinical data of AD patients, taken together with the analysis of stenosis changes of the vessels.Citation12,Citation27 We expect to perform such studies in the future. Finally, it will be important to perform these examinations on aged-matched non-AD controls to see if AD pathology causes increased atheromatous plaque formation.
In conclusion, we believe that the vascular pathology may represent one common phenomenon in AD patients. The separation of AD and CA pathology might require reconsideration.
Acknowledgements
The authors received funding from the Guangzhou Baiyun Kexunju Grant 2011-KZ-92.
Disclosure
The authors report no conflicts of interest in this work.
References
- RoherAEDebbinsJPMalek-AhmadiMCerebral blood flow in Alzheimer’s diseaseVasc Health Risk Manag2012859961123109807
- AltmanRRutledgeJCThe vascular contribution to Alzheimer’s diseaseClin Sci (Lond)20101191040742120684749
- KennellySPLawlorBAKennyRABlood pressure and the risk for dementia: a double-edged swordAgeing Res Rev200982617019063999
- HonjoKBlackSEVerhoeffNPAlzheimer’s disease, cerebrovascular disease, and the β-amyloid cascadeCan J Neurol Sci201239671272823227576
- ChuLWAlzheimer’s disease: early diagnosis and treatmentHong Kong Med J201218322823722665688
- HooghiemstraAMEggermontLHScheltensPvan der FlierWMScherderEJExercise and early-onset Alzheimer’s disease: theoretical considerationsDement Geriatr Cogn Dis Extra2012213214522590474
- GrinbergLTThalDRVascular pathology in the aged human brainActa Neuropathol2010119327729020155424
- VeglioFPaglieriCRabbiaFBisbocciDBerguiMCerratoPHypertension and cerebrovascular damageAtherosclerosis2009205233134119100549
- OnyikeCUCerebrovascular disease and dementiaInt Rev Psychiatry200618542343117085361
- YarchoanMXieSXKlingMACerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementiasBrain2012135Pt 123749375623204143
- OrsucciDMancusoMIencoECSimonciniCSicilianoGBonuccelliUVascular factors and mitochondrial dysfunction: a central role in the pathogenesis of Alzheimer’s diseaseCurr Neurovasc Res2013101768023151073
- SilvestriniMViticchiGFalsettiLThe role of carotid atherosclerosis in Alzheimer’s disease progressionJ Alzheimer’s Dis201125471972621508532
- PalaciosHHYendluriBBParvathaneniKMitochondrion-specific antioxidants as drug treatments for Alzheimer diseaseCNS Neurol Disord Drug Targets201110214916221222631
- RocchiAOrsucciDTognoniGCeravoloRSicilianoGThe role of vascular factors in late-onset sporadic Alzheimer’s disease. Genetic and molecular aspectsCurr Alzheimer Res20096322423719519304
- JichaGAParisiJEDicksonDWAge and apoE associations with complex pathologic features in Alzheimer’s diseaseJ Neurol Sci20082731–2343918653200
- BhatNRLinking cardiometabolic disorders to sporadic Alzheimer’s disease: a perspective on potential mechanisms and mediatorsJ Neurochem2010115355156220807313
- BeeriMSRappMSilvermanJMCoronary artery disease is associated with Alzheimer disease neuropathology in APOE4 carriersNeurology20066691399140416682673
- KosunenOTalasniemiSLehtovirtaMRelation of coronary atherosclerosis and apolipoprotein E genotypes in Alzheimer patientsStroke19952657437487740560
- MorettiRTorrePAntonelloRMManganaroDVilottiCPizzolatoGRisk factors for vascular dementia: hypotension as a key pointVasc Health Risk Manag20084239540218561514
- WeinsteinGWolfPABeiserASAuRSeshadriSRisk estimations, risk factors, and genetic variants associated with Alzheimer’s disease in selected publications from the Framingham Heart StudyJ Alzheimers Dis201333Suppl 1S439S44522796871
- CechettoDFHachinskiVWhiteheadSNVascular risk factors and Alzheimer’s diseaseExpert Rev Neurother20088574375018457531
- RafaelHCerebral atherosclerosis and mild Alzheimer’s diseaseStroke2003348e10612855821
- PremkumarDRCohenDLHederaPFriedlandRPKalariaRNApolipoprotein E-epsilon4 alleles in cerebral amyloid angiopathy and cerebrovascular pathology associated with Alzheimer’s diseaseAm J Pathol19961486208320958669492
- MusiekESSabouryBMishraSFeasibility of estimation of brain volume and 2-deoxy-2-(18)F-fluoro-D-glucose metabolism using a novel automated image analysis method: application in Alzheimer’s diseaseHell J Nucl Med201215319019623106049
- WestmanEMuehlboeckJSSimmonsACombining MRI and CSF measures for classification of Alzheimer’s disease and prediction of mild cognitive impairment conversionNeuroimage201262122923822580170
- ManookAYousefiBHWilluweitASmall-animal PET imaging of amyloid-beta plaques with [11C]PiB and its multi-modal validation in an APP/PS1 mouse model of Alzheimer’s diseasePLoS One201273e3131022427802
- KalbackWEshCCastanoEMAtherosclerosis, vascular amyloidosis and brain hypoperfusion in the pathogenesis of sporadic Alzheimer’s diseaseNeurol Res200426552553915265270