Abstract
Age-related macular degeneration (AMD) is a degenerative ocular disease primarily affecting central vision in the elderly. Its pathogenesis is complex, involving cellular senescence and immune homeostasis dysregulation. This review investigates the interaction between these two critical biological processes in AMD pathogenesis and their impact on disease progression. Initially, cellular senescence is analyzed, with particular emphasis on retinal damage induced by senescent retinal pigment epithelial cells. Subsequently, the occurrence of immune homeostasis dysregulation within the retina and its mechanistic role in AMD areis explored. Furthermore, the paper also discusses in detail the interplay between cellular senescence and immune responses, forming a vicious cycle that exacerbates retinal damage and may influence treatment outcomes. In summary, a deeper understanding of the interrelation between cellular senescence and immune dysregulation is vital for the developing innovative therapeutic strategies for AMD.
Introduction
Age-related macular degeneration (AMD) is a major cause of blindness among the elderly in developed countries, and its prevalence is escalating due to an aging global population. It is estimated that 8.69% of the global population is affected by AMD, impacting 196 million people in 2020. This prevalence is projected to increase to 288 million by 2042, significantly contributing to the global disease burden.Citation1 The pathogenesis of AMD is complex, involving genetics, environmental influences, metabolic processes, and lifestyle choices.Citation2 Recent research has highlighted the pivotal roles of cellular senescence and the dysregulation of immune homeostasis in AMD pathophysiology.
Cellular senescence represents a complex biological phenomenon, extensively present within the retina. In particular, senescence of retinal pigment epithelial (RPE) cells significantly impacts the structural and functional integrity of the retina, thereby contributing to AMD progression.Citation3 The homeostasis of the immune system plays an essential role in maintaining tissue health. In AMD, immune homeostasis disruption is characterized by chronic low-grade inflammation, a critical factor accelerating damage to both the retina and RPE.Citation4
The interplay between cellular senescence and the dysregulation of immune homeostasis constitutes a critical characteristic of the pathophysiological process in AMD. In this paper, we will thoroughly examine how these two processes interact via complex molecular and cellular mechanisms, collaboratively contributing to the progression of AMD.
AMD and Cellular Senescence
Cellular Senescence
Cellular senescence (also termed “senescence”) was first proposed by Hayflick and Moorhead in 1961. It refers to irreversible cell cycle arrest during mitosis, leading to the emergence of senescent cells (SNCs).Citation5 SNCs play crucial roles in various biological processes, including embryonic development, tissue remodeling, wound repair, tumorigenesis, aging, and age-related diseases.Citation6 However, alterations in immune system function and the resistance of SNCs to apoptosis result in their accumulation, thereby triggering a range of age-related diseases, including Alzheimer’s disease, osteoarthritis, pulmonary fibrosis, and AMD.Citation7–9 The mechanism of cellular senescence involves two tumor suppressor pathways: p16INK4A -pRB and p53-p21CIP1/WAF1. These pathways inhibit the expression of cell cycle-related proteins and proliferation-promoting transcription factors, resulting in cell cycle arrest at G1 or G2 phase.Citation10 Although SNCs stop replicating and proliferating, they remain in a metabolically active state, accompanied by the upregulation of numerous inflammatory factors, chemokines, growth factors, etc., collectively termed the Senescence-Associated Secretory Phenotype (SASP).Citation11
Cellular senescence can be categorized into three types: replicative senescence (RS), stress-induced early senescence (SIPS) and developmentally programmed senescence (DPS). RS is caused by telomere shortening during the cell replication process. To prevent genomic instability caused by telomere shortening, the DNA damage response activates a series of cascade reactions, including the activation of ATM/ATR-mediated p53-p21CIP1/WAF1 and p16INK4A -pRB pathways, cell cycle arrest and apoptosis. SIPS is induced by factors such as oxidative stress, oncogene activation, genotoxic damage, chemotherapy, and viral infections. DPS primarily occurs during the formation of mammalian embryos and plays a role in development and morphogenesis.Citation10
SNCs undergo significant and profound changes in both morphology and function.Citation10,Citation12 These changes manifest in various ways: (i) morphological changes: the cell’s cytosol and nucleus increase in size, the cell becomes flattened and multivacuolated, and intracytoplasmic granules increase; (ii) organelle changes: mitochondria increase in number and dysfunction; lysosomal proteins are up-regulated and increase in content (increased activity of β-galactosidase (SA-β-Gal), accumulation of lipofuscin); endoplasmic reticulum stress, and dysregulation of protein homeostasis; (iii) nuclear changes: chromosomal instability (deletion of the nuclear lamina structural protein LaminB1, release of the high mobility group box 1 protein; accumulation of chromatin modifications (enrichment of senescence-associated heterochromatin foci in the transcriptionally silenced histone H2A variant); decrease in DNA content; (iv) metabolic changes: increased glycolysis and SASP release; (v) increased expression of senescence-associated proteins: p38 mitogen-activated protein kinase, p53, p21CIP1, p16INK4A, RB and cyclin kinase inhibitors.
These features may reflect senescence triggers or consequences, but are not specific to SNCs. Currently, no single biomarker has been identified that can uniquely recognize SNCs. Furthermore, not all SNCs exhibit all biological markers of cellular senescence. Therefore, SNC identification requires simultaneous detection of multiple senescence biomarkers.
Cellular Senescence in AMD
Clinically, AMD is divided into two forms: dry (also known as atrophic or non-exudative) AMD, which may progress to geographic atrophy (GA) and wet (exudative) AMD, characterized by choroidal neovascularization (CNV). In dry AMD, the initial stages are marked by the appearance of large confluent drusen and pigmentary changes due to RPE dysfunction. This can progress to drusen reabsorption and pigmentary attenuation following the loss of RPE cells. Dysfunction and loss of photoreceptors (PRs) and the choriocapillaris (CC) are secondary to RPE cells loss. () In wet AMD, the initial damage in the PR/RPE/Bruch’s membrane (BM)/CC complex is the loss of choroidal vasculature. As AMD progresses, the accumulation of pro-inflammatory factors creates an inflammatory environment. Although the RPE remains intact, hypoxia leads to the production of angiogenic substances such as VEGF, stimulating the formation of CNV. These events result in the death of PRs due to nutrient deprivation. Both dry and wet AMD are characterized by dysfunction and/or death of the PR/RPE/BM/CC complex, which functionally interact as a whole ().Citation13
Figure 1 Physiologic and Pathologic Images of the Retina. (a) Structure diagram of retinal layers and immunosuppressive mechanism in the retina; (b) Pathological changes in dry AMD: drusen formation, RPE dysfunction; (c) Pathological changes in wet AMD: CNV formation, photoreceptor/RPE/BM/CC atrophy and dysfunction.
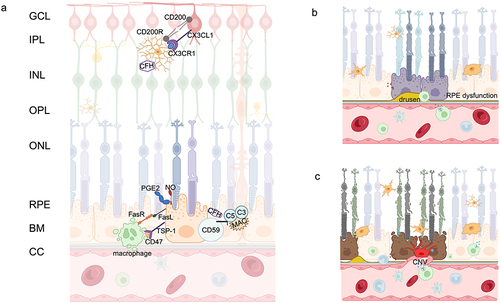
RPE plays an important role in the pathogenesis of both forms of AMD, whether dry or wet AMD, and the role of RPE cellular senescence in the pathogenesis of AMD has been substantiated by extensive research.Citation14 RPE cells isolated from donor eyes of AMD patients showed up-regulation of genes characteristic of senescence, such as p16INK4A, p21CIP1, p53 and bone morphogenetic protein 4 (BMP4).Citation15,Citation16 A subset of cells within the human RPE, capable of activating RPE stem cells in vitro, has been identified. Lazzarini et al discovered that these RPE stem cells can undergo RS, impacting their proliferative and differentiation capacities. Additionally, they release SASP, creating an inflammatory environment that contributes to the progression of AMD.Citation17
Genome-wide association studies (GWAS) have identified single nucleotide polymorphisms in the high-temperature requirement protein A1 (HTRA1) gene as closely associated with AMD. As a serine protease, HTRA1 regulates protein function by cleaving specific peptide bonds, crucial for maintaining extracellular matrix stability and functionality.Citation18 Elevated HTRA1 protein levels in the RPE of AMD patients correlate with the upregulation of several senescence markers, including p21CIP1/WAF1, p16INK4a and SA-β-gal.Citation19 The role of HTRA1 in AMD cellular senescence is multifaceted, involving oxidative stress response, mitochondrial function, and regulation of hypoxia-inducible factor-1 and p38 mitogen-activated protein kinase signaling pathways, inducing RPE senescence, extracellular matrix deposition, and AMD-related choroidal vasculopathy.Citation20,Citation21 The differential expression of bone morphogenetic protein 4 (BMP4) across AMD subtypes is intimately linked to cellular senescence. In dry AMD patients, high BMP4 expression activates p53 via the Smad and p38 signaling pathways, increases p21WAF1/CIP1 expression, and decreases pRb levels, thereby mediating RPE senescence.Citation22 Conversely, in wet AMD, BMP4 expression is reduced, regulated by the SASP such as TNF.Citation23 Due to the loss of BMP4-mediated inhibition of cellular senescence, neovascularization proliferates extensively. However, when the lesion progresses to scarring, with degeneration and loss of neovascular endothelial cells, BMP4 expression subsequently increases.Citation22 Transforming growth factor-β-activated kinase 1 (TAK1) is highly expressed in RPE cells, and its expression varies in response to oxidative stress. Inhibition of TAK1 leads to reduced proliferation and cell cycle arrest in RPE cells, characteristics indicative of cellular senescence. Abnormal TAK1 activity also triggers the secretion of factors by RPE, such as matrix metalloproteinase (MMP) 9, which induce hypertrophy and fibrotic changes in neighboring cells. Thus, TAK1 plays a pivotal role in the pathogenesis of AMD.Citation24
In AMD, the formation of Drusen and lipofuscin is a direct result of senescence and metabolic disorders in RPE. These deposits locally trigger inflammatory responses, increase oxidative stress, and damage RPE cells and surrounding tissues, leading to further retinal damage. Drusen is an early marker of AMD with a complex composition containing lipids, proteins, trace metals, pro-inflammatory factors, etc. amyloid-β in drusen promotes RPE cellular senescence, secretes higher concentrations of IL-8 and MMP-9, and contributes to the disruption of the RPE barrier and chronic inflammation, which is an important pathologic alteration in AMD.Citation25 7-ketocholesterol (7KC), an oxidative cholesterol, accumulates in AMD-associated Drusen. 7KC mediates RPE senescence and SASP via the mTOR signaling pathway, disrupting the BRB and attracting choroidal endothelial cells (ECs) to the RPE. Furthermore, 7KC induces serine phosphorylation of multi-domain proteins in RPE, which is an important factor in the progression of AMD fibrosis.Citation26 Lipofuscin accumulation in the RPE increases with age. N-retinylidene-N-retinylethanolamine (A2E), generated from the chemical reaction of retinaldehyde, is the primary fluorescent component of lipofuscin in the RPE. Photosensitized A2E can induce DNA damage, including telomere loss and deprotection, accelerating RPE senescence and SASP, contributing to AMD progression.Citation27 Additionally, Sun et al discovered that A2E-induced RPE senescence links to the upregulated Caveolin-1. Elevated Caveolin-1 inhibits RPE epithelial-mesenchymal transition, reducing sub-retinal fibrosis. Concurrently, Caveolin-1 blocks the cell cycle, accelerating RPE cell senescence, thus promoting GA progression.Citation28
As a consequence of cellular senescence, the SASP also plays a role in the pathophysiological changes of AMD. The SASP drives the senescence of neighboring cells through autocrine or paracrine mechanisms and exerts deleterious effects in the tissue microenvironment.Citation11 In the oxygen-induced retinopathy (OIR) mice model, cellular senescence observed in neurons can spread to other cell populations in the retina (such as microglia(MG) cells and ECs), further exacerbating retinal pathology.Citation29 The SASP produced by SNCs is a major and persistent source of age-related chronic inflammation, with its components varying depending on the disease, triggering factors, and cell type. RPE cells primarily undergo RS or SIPS, involving a series of changes of SASP, including interleukin-6 (IL-6), IL-8, IL-10, IL-12, IL-17, monocyte chemotactic protein 1 (MCP-1), tumor necrosis factor-α (TNF-α), VEGF, MMP-9, IFN-γ, complement factor B (CFB), and TGF-β.Citation17,Citation25 In addition to damaging RPE cells and surrounding tissues, the inflammatory response resulting from RPE cell senescence will trigger abnormal activation of the immune system. Senescent mononuclear-phagocytes within the retina also express higher levels of inflammatory factors, including IL-1α, IL-1β, IL-6, IL-8, IL-12, TNF-α, C3, CFB, CXCL1, TGF-β, nitric oxide (NO), and superoxide anion.Citation8,Citation30
In the pathophysiological alterations of AMD, cellular senescence is not limited to RPE cells. Neuronal cells, immune cells, and vascular endothelial cells also undergo senescence. The specific processes and mechanisms of senescence in these cell types in AMD will be elaborated in the subsequent sections of this paper.Citation22,Citation29,Citation31–33
AMD and Immune Homeostasis Dysregulation
GWAS identified 52 independent variants associated with AMD across 34 genetic loci in a comprehensive genomic variant analysis conducted on a large cohort of AMD patients and control subjects. These variants contribute to the majority of the genetic risk for AMD. Several of these genes are expressed in retinal immune cells and play roles in various inflammatory pathways. The association between AMD risk and these inflammatory genes suggests that alterations in the immune system might be a primary factor leading to the onset of AMD.Citation18
Historically, adaptive immunity was not considered a major trigger for AMD, primarily due to the lack of lymphatic systems in the retina and choroid, and the scarcity of AMD-related target antigens in the retina. However, recent studies have found an increase in the CD56+ and CD28− subgroups of CD8+ T cells in the peripheral blood of AMD patients,Citation34 and the presence of CD8+ T cells in the macula.Citation35 Additionally, levels of anti-retinal autoantibodies in the peripheral blood of AMD patients are elevated,Citation36 but there is no conclusive evidence linking B cells with the induction or progression of AMD. Therefore, while current research is limited, these studies suggest a correlation between the adaptive immune system and the pathogenesis of AMD. Given the current ambiguity in the research on the adaptive immune system’s mechanisms in AMD, this paper focuses on elucidating the role of innate immune system dysregulation in AMD pathogenesis.
Immune Privilege of the Retina
When discussing the immune homeostasis of the retina, it is essential to mention its unique feature: immune privilege. This refers to specific mechanisms reducing immune responses to protect vision. This privilege is mainly achieved through two mechanisms. First, the blood-retinal barrier (BRB), composed of ECs and RPE cells, effectively limits immune cell and macromolecule entry into the retina.Citation37 Second, the expression of immunosuppressive molecules like Fas ligand and CD200 reduce immune responses and maintain retinal stability. While retinal immune responses are suppressed (),Citation38,Citation39 in disease states, this privilege can be compromised, leading to retinal lesions and vision decline.
Innate Immune Cell
The Mononuclear Phagocyte System (MPs) in the retina is derived from two main sources: resident microglia and circulating macrophages. Microglia and macrophages are often difficult to distinguish, and are thus frequently conflated in many studies. In the following sections, they are not differentiated unless specifically indicated in the research. The accumulation of MPs in the subretinal space (SRS) is a key step in early AMD,Citation40 involving mainly two chemokine pathways: CCL2-CCR2 and CX3CL1-CX3CR1. These pathways mediate the recruitment and activation of systemic and local myeloid cell populations in response to exogenous and endogenous inflammatory stimuli.Citation41
MG cells, the primary tissue-resident macrophages in the retina, express high levels of CX3CR1 and are regularly distributed in the inner layers of the retina, with fewer numbers in the outer layers.Citation42 MG cells are involved in processes such as removal of apoptotic neurons and maintenance of synaptic structure and function.Citation43 Upon tissue injury, MG cells are activated, increasing in number, changing morphology, releasing inflammatory mediators, phagocytosing, and clearing cellular debris to repair lesions.Citation44 However, the activation of MG cells can also lead to detrimental effects. In dry AMD, reactive microglia accumulate on the apical surface of RPE, covering and residing within drusen, promoting the formation of drusen and secreting inflammatory factors that contribute to PRs and RPE cells death.Citation42,Citation45 In nAMD, MG cells migrating to the SRS is associated with CNV. MGs secrete VEGF and other angiogenic factors, such as platelet-derived growth factor-β, fibroblast growth factor-1, fibroblast growth factor-2, and TGF-β1, promoting pathological choroidal vascular growth.Citation46 Under normal conditions, macrophages are absent from the retina but are distributed in the choroid. The choroid contains proliferative resident macrophages and inflammatory macrophages recruited from the blood monocytes.Citation39 In AMD, macrophages are recruited from the choroid or systemic circulation into the retina, where they perform phagocytic functions, clearing lipid-rich membranous debris and drusen.Citation47 Macrophages can exhibit different polarization states, including pro-inflammatory M1 phenotypes and anti-inflammatory reparative M2 phenotypes. In GA patients, the proportion of M1 macrophages is higher, while in nAMD patients, the proportion of M2 macrophages is higher.Citation48 In the early stages of AMD, increased and overactivated M1 macrophages lead to an increase in proinflammatory cytokines, which damage RPE cells and PRs and promote disease progression. M2-type macrophages play a protective role by limiting inflammation through the removal of cellular debris and inhibiting the production of inflammatory factors. In the later stages, an increase in IL-10 expression leads to a shift from M1 to M2 phenotype, with M2 macrophages participating in neovascularization. Therefore, the transition of macrophages from M1 to M2 phenotype is a driving factor in AMD progression.Citation49
Neutrophils are one of the most abundant leukocyte types in the immune system and play multiple roles in immune defense, inflammation regulation, and tissue repair.Citation50 In AMD, the amyloid β component in drusen can activate neutrophils via TLR4 and NADPH oxidase-dependent pathways. Activated neutrophils produce a large amount of Neutrophil Extracellular Traps (NETs), and inhibiting NETs formation can effectively reduce retinal inflammatory responses.Citation51 François et al found that in OIR mice, neutrophils promote retinal vascular remodeling by clearing senescent vascular cells through the release of NETs.Citation52 Increased levels of interferon-λ (IFN-λ) in early AMD also trigger neutrophil activation and upregulation of lipocalin-2 (LCN-2). LCN-2, by modulating levels of integrin β1, stimulates the adherence and migration of activated neutrophils to the retina and choroid, thereby promoting inflammation.Citation53,Citation54 Therefore, neutrophils play a significant role in the pathological changes of AMD, and targeting NETs could be a potential strategy for treating AMD.
Natural Killer cells (NKs) are an important component of the innate immune system and play a key role in the recognition and elimination of infected and tumor cells.Citation55 Goverdhan et al found that the combination of the HLA-Cw*0701 allele and the inhibitory receptor AA haplotype of NK cells was significantly associated with AMD, suggesting that NK cells may contribute to AMD pathogenesis through interactions with specific HLA-I class molecules.Citation56 Zeng et al identified complement C1s, adrenomedullin, and immediate early response 5-like as AMD diagnostic biomarkers, and these genes are associated with infiltration of NK cells.Citation57 Lee et al found that NKs facilitate the expression of VEGF in macrophages through the secretion of IFN-γ, thereby playing a promotive role in the CNV process.Citation58
Mast cells (MCs) are key inflammation effector cells, and the choroid contains numerous MCs.Citation59 In AMD patients, there is an increased and activated MCs population in the macular region and the sub-macular choroid. These activated MCs undergo degranulation, releasing substantial cytokines and enzymes. In GA, proteases released by MCs cause choroidal thinning and BM degradation, consequently killing RPE and causing CC degeneration. In nAMD, MC degranulation releases factors such as heparin and VEGF, interacting with ECs to promote their proliferation and CNV formation.Citation59 Moreover, MC activation can facilitate macrophage recruitment and inflammation onset.Citation60
Voigt et al identified a Dendritic cells (DCs) population via choroidal transcriptomics. Their analysis of choroidal AMD genetic risk factors found DCs highly express VEGF and MMP9 genes, associated with nAMD.Citation61 However, in laser-induced nAMD models, DCs show no significant role.Citation62 Therefore, the specific role of DCs in AMD pathogenesis requires further investigation.
Complement System
The complement system, comprising over 50 plasma proteins, membrane-bound receptors, and regulatory proteins, is the principal humoral component of the innate immunity, performing diverse functions including pathogen clearance and immune cell recruitment. Most complement components are inactive proenzymes, which can be activated via the classical pathway, alternative pathway, or lectin pathway. These three pathways converge at C3, initiating a cascade reaction through C3 convertase, leading to the formation of immunostimulatory byproducts like C3a, C5a, and the membrane attack complex (MAC, C5b-9).Citation63 In retina, MG cells and RPE cells can locally synthesize certain complement components, such as complement C1q, C3, and complement regulatory factors like CFH, CFB, and CD59.Citation64
The notion that complement plays a major role in AMD has been supported by genetic studies.Citation18 The complement system is a double-edged sword for the retina. Low levels of complement activation facilitate maintenance of immune privilege, whereas overactivation damages retinal tissue and elicits chemotactic aggregation of immune cells. As AMD progresses, complement activation levels gradually increase, especially in individuals with a heavier genetic burden of complement genes.Citation65 In AMD patient plasma, there is an increased concentration of activated products such as C3a, C3b, Ba, Bb, C5a, and CFH.Citation66 Elevated complement C5a levels significantly stimulate T cells to produce IL-22 and IL-17, promoting the onset of AMD-related inflammation.Citation67 The presence and activation of C3 and C5 in drusen lead to chronic inflammation and exacerbate subretinal damage.Citation68 MAC-mediated lysis of ECs is a key factor in the formation of CNV in nAMD.Citation31,Citation69
The Interplay of Cellular Senescence and Immune Homeostasis Dysregulation in AMD
In the retina, PRs, ECs, ganglion cells, MG cells, and RPE cells all actively participate in suppressing immune cell activation.Citation70 For instance, neurons exert immunosuppressive functions through the CX3CL1-CX3CR1 axis or various cell surface molecule interactions (such as CD200-CD200R, CD47-signal regulatory proteins).Citation71 RPE cells may regulate the antigen-presenting properties of MG cells by secreting immunomodulators like Prostaglandin E2 and NO, thereby protecting the retina.Citation70 RPE cell senescence often triggers abnormal activation of immune cells and complement. Senescent RPE cells release SASP, causing a substantial increase in proinflammatory and immunologic factors in the extracellular environment. Moreover, senescence-induced autophagy defects result in reduced phagocytic function of RPE cells towards PR outer segments, accumulated lipofuscin within cells, and increased SRS waste.Citation3 All these factors trigger the activation and migration of MG cells. Additionally, neuronal cell senescence leads to decreased expression of CX3CL1 and CD200, weakening their inhibitory effect on MGs and further amplifying their activation ().Citation72
MG cells transition from a branched to an amoeboid morphology, migrating from the neural retina to the SRS. On the apical side of the RPE, MG cells digest and phagocytose deposits, exerting a protective role on the aging retina.Citation42 The presence of MG cells in the SRS induce RPE cells to express more chemokines and adhesion molecules (such as CCL2, CCL5, vascular cell adhesion molecular-1, intercellular cell adhesion molecular-1, etc.), creating a chemotactically attractive environment in the outer retina and recruiting more immune cells from the choroidal and systemic circulation.Citation73 Recruited monocytes partly enter the retina to replenish the MG cells population, while others differentiate into macrophages, MCs, DCs, etc, infiltrating the basal side of RPE cells.Citation74 Activated MPs express inducible NO synthase, which is deposited near drusen and waste productsCitation75 that exert scavenging and phagocytic functions through P2X7 receptors.Citation76 Immune cell-mediated clearance of senescent retinal cells is crucial for maintaining retinal homeostasis. Studies in skin tissue have shown that senescent fibroblasts evade immune clearance by upregulating HLA-E expression, thereby inhibiting NK and CD8+ T cell responses.Citation77 The relevance of HLA and NK cells to the pathogenesis of AMD is significant, and whether retinal senescent cells also use HLA for immune evasion is an intriguing topic that warrants further research.
The overactivation and accumulation of MPs have neurotoxic effects. To prevent damage, RPE cells can induce apoptosis in immune cells. RPE cells possess Fas Ligand (FasL) which interacts with the Fas receptor on MPs, leading to the clearance of MPs from the SRS.Citation78 The CD47/Thrombospondin-1 (TSP-1) signaling pathway is crucial for MPs elimination beneath the retina.Citation79 RPE cells produce TSP-1, which interacts with CD47 on macrophages, reducing their phagocytic ability and increasing FasL sensitivity ().Citation80 However, in AMD, RPE cell senescence decrease FasL and TSP-1 expression,Citation81 diminishing the clearance of MPs. Different CFH variants also affect MPs clearance efficiency, CFH binding to CD11b hinders the CD47/TSP-1 pathway, prolonging MPs lifespan.Citation79 The continuous accumulation of MPs promotes degenerative changes in the retina.
It is important to note that immune cells also undergo cellular senescence. Following senescence in MG cells, there is an accumulation of lipofuscin within cells, and a decrease in their capacity to respond to injury and phagocytic ability.Citation82 In senescent MG cells, key genes controlling inflammatory responses (eg nuclear transcription factor-κB (NF-κB), C3, CFB) exhibit significant changes, promoting complement activation and immune dysregulation, driving chronic inflammation in the retina.Citation30,Citation44 In senescent macrophages, the cholesterol efflux regulators ATP-binding cassette transporter A1 (ABCA1) and ABCG1 are downregulated, leading to impaired lipid efflux and cholesterol-rich drusen deposition in early AMD.Citation33 Furthermore, elevated lipid levels promote macrophage polarization to M2, and senescence itself also induces polarization to M2. These factors collectively contribute to the development of CNV in AMD.Citation33 Additionally, studies have found that senescent macrophages express high miRNA-33 and 150, causing abnormal lipid metabolism and mediate inflammation and pathologic angiogenesis by down-regulating ABCA1 and stearoyl coa desaturase 2 expression.Citation83
Under pathological AMD conditions, retinal cells contact and interact with immune cells, amplifying the inflammatory response and exacerbating retinal damage. MPs secrete IL-1β, which is toxic to neuronal cells and can induce PRs death, especially cone cells.Citation84 TNF-α secreted by MPs inhibits the key transcription factor Orthodenticle homeobox 2 in RPE cells, disrupting RPE homeostasis and damaging the visual cycle.Citation85 IL-6 secreted by MPs further reduces FasL expression in senescent RPE, interfering with the immunosuppressive ability of RPE to clear macrophages.Citation78 IL-1β and TNF-α synergistically stimulate RPE cells to produce recruitment factors (MCP-1 and IL-8), continuously recruiting monocytes to the CNV site, amplifying pathological angiogenesis.Citation86 Additionally, MPs are associated with late-stage fibrotic lesions in nAMD. MPs can trigger the expression of integrins α1 and α5 on the RPE surface through TNF-α, promoting RPE cells migration on fibronectin and type I collagen, leading to fibrotic scarring.Citation87 Furthermore, Little et al found that C3a and TGF-β can induce MPs transformation to myofibroblasts, directly promoting fibrosis progression, with 20–30% of infiltrating macrophages at nAMD lesion sites expressing the myofibroblast marker α-smooth muscle actin ().Citation88 The AKT2/NF-κB/LCN-2 signaling axis in AMD reveals the dynamic interactions among RPE cells, MG cells and neutrophils. Senescent RPE cells release cytokines and chemokines that activate MG cells pro-inflammatory transformation via Akt2. Activated MG cells release pro-inflammatory mediators NF-κB, upregulating CD14. CD14, an important ligand for integrins, binds with adhesion proteins on neutrophils (eg integrins β1 and α4), causing neutrophil activation. Activated neutrophils exhibit increased LCN-2 and NETs, triggering inflammatory responses in AMD ().Citation89
Figure 2 Vicious Cycle. Mechanisms of interaction between cellular senescence and dysregulation of immune homeostasis in retina.
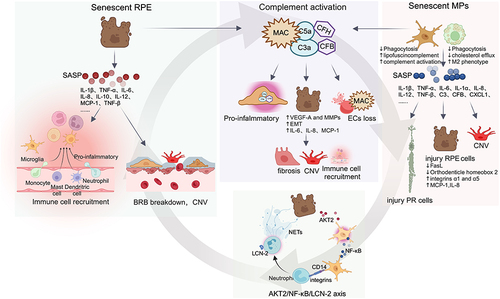
The interaction between immune cells and the complement system also drives the progression of AMD. On the one hand, inflammatory cytokines from activated MG cells or infiltrating macrophages increase complement genes expression. For instance, the accumulation of senescent MG cells decrease CFH and CFB expression, leading to complement activation.Citation90 On the other hand, complement activation releases complement fragments C3a and C5a, further affecting MPs activation and migration (). In addition to immune cells, RPE cells also interact with the complement system. RPE cells can promote the expression of complement regulators C1INH and CD59a in macrophages, exerting an inhibitory effect on the complement system. However, this ability is reduced when RPE cells undergo senescence, promoting complement activation and deposition at the retina-choroid interface.Citation91 Complement fragments C3a and C5a signal through their respective G-protein coupled receptors to upregulate VEGF-A and MMPs expression in RPE cells, promoting CNV formation.Citation92,Citation93 C5a also mediates the epithelial-mesenchymal transition of RPE cells, playing a significant role in subretinal fibrosis secondary to nAMD.Citation94 Generally, the MAC does not deposit on RPE cells. Apart from being directly inhibited by complement regulatory factors expressed by RPE cells (such as CD59), RPE cells also process surface-bound MAC through endocytic pathways and lysosomal degradation. However, in senescence, RPE cells are more susceptible to MAC attack due to autophagy dysfunction.Citation95 MAC promotes RPE cells to produce IL-6, IL-8, and MCP-1, creating a pro-inflammatory environment in early AMD and recruiting immune cells; it also increases MMPs and VEGF expression, associated with neovascularization.Citation96 As previously discussed, MAC disrupts choriocapillaris ECs. With the senescence of ECs, local production of CFH may decrease, leading to excessive complement activation and MAC deposition. Additionally, senescent ECs exhibit increased stiffness, associated with elevated Rho activity, which in turn increases their sensitivity to MAC damage, contributing to choroidal degeneration in AMD ().Citation31,Citation97
Conclusion
The interplay of cellular senescence and immune homeostasis dysregulation creates a chronically worsening inflammatory environment in the retina, driving AMD progression. Therapies targeting these two mechanisms could ameliorate retinal pathological changes. Current strategies for reversing cellular senescence primarily include two aspects. First, senolytics, inducing apoptosis of SNCs to eliminate them, such as dasatinib+quercetin, Bcl-2/BET protein inhibitors. In the OIR mice model, the BCL-xL inhibitor UBX1967 effectively eliminates senescent ECs and inhibits neovascularization.Citation98 Second, senostatics, reducing SASP levels by modulating the NF-κB, mTOR, and AMPK pathways. In the OIR mice model, metformin (an AMPK activator) can reduce destructive retinal neovascularization by inhibiting SASP.Citation29 The clearance of SNCs mediated by immune cells demonstrates the potential of immunotherapy in anti-senescence strategies. For instance, NETs can clear senescent ECs;Citation52 LXR agonists or miR-33 inhibitors can restore cholesterol homeostasis in macrophages, reversing retinal senescence in mice.Citation33 However, several important issues need consideration when applying these therapies clinically. How can drugs be delivered effectively to the SRS? Which cells in the retina are targeted by the drugs? Does the removal of SNCs impact function due to tissue loss?
With immunology research deepening in AMD, targeted complement therapy has become a new focus.Citation99 However, several clinical trials of complement inhibitors yielded unsatisfactory results, with poor visual acuity improvement and disease progression control. The failure of lampalizumab, a CFD inhibitor, to prevent GA progression in a Phase 3 study exemplifies this.Citation100 AMD pathogenesis is complex and involves persistent local inflammation and reduced retinal injury repair. AMD treatment should not be solely anti-inflammatory, but rather create a permissive environment for repair, possibly including promoting the complement system alongside systemic immunotherapy.Citation101 The goal should be developing effective treatments optimizing aging immune system performance to enhance tissue repair. Panmacular subthreshold diode micropulse (SDM) laser shows promise, regular SDM significantly reducing risk of dry AMD converting to wet form and improving vision.Citation102 SDM preserves RPE cells, normalizes VEGF and chemokine levels, and promotes retinal repair, showing great therapeutic potential.Citation103
In summary, the interaction between cellular senescence and immune homeostasis dysregulation reveals a complex yet crucial pathological mechanism in AMD. This interplay not only deepens our understanding of AMD pathology but also offers potential directions for developing new therapeutic strategies.
Abbreviations
AMD, age-related macular degeneration; RPE, retinal pigment epithelial; SNCs, senescent cells; SASP, Senescence-Associated Secretory Phenotype; RS, replicative senescence; SIPS, stress-induced early senescence; DPS, developmentally programmed senescence; SA-β-Gal, senescence-associated β-galactosidase; GA, geographic atrophy; CNV, choroidal neovascularization; PRs, photoreceptors; CC, choriocapillaris; BM, Bruch’s membrane; BMP4, bone morphogenetic protein 4; GWAS, Genome-wide association studies; HTRA1, high-temperature requirement protein A1; TAK1, Transforming growth factor-β-activated kinase 1; MMP, matrix metalloproteinase; 7KC, 7-ketocholesterol; ECs, endothelial cells; A2E, N-retinylidene-N-retinylethanolamine; OIR, oxygen-induced retinopathy; MG, microglia; IL-6, interleukin-6; MCP-1, monocyte chemotactic protein; TNF-α, tumor necrosis factor-α; CFB, complement factor B; NO, nitric oxide; BRB, blood-retinal barrier; MPs, Mononuclear Phagocyte System; SRS, subretinal space; IFN-λ, interferon-λ; NF-κB, nuclear transcription factor-κB; ABCA1, ATP-binding cassette transporter A1; NETs, Neutrophil Extracellular Traps; LCN-2, lipocalin-2; NKs, Natural Killer cells; MCs, Mast cells; DCs, Dendritic cells; MAC, membrane attack complex; FASL, Fas Ligand; TSP-1, Thrombospondin-1; SDM, subthreshold diode micropulse.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors appreciate the help of Dr. Ming Ming Yang in providing editorial assistance and support in the preparation of the manuscript. At the same time, we thank Shenzhen Science and Technology Program and Shenzhen Natural Science Foundation for their support.
Additional information
Funding
References
- Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116. doi:10.1016/S2214-109X(13)70145-1
- Heesterbeek TJ, Lorés‐Motta L, Hoyng CB, Lechanteur YTE, Den Hollander AI. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol Opt. 2020;40(2):140–170. doi:10.1111/opo.12675
- Blasiak J. Senescence in the pathogenesis of age-related macular degeneration. Cell Mol Life Sci. 2020;77(5):789–805. doi:10.1007/s00018-019-03420-x
- Wang S, Wang X, Cheng Y, et al. Autophagy dysfunction, cellular senescence, and abnormal immune-inflammatory responses in AMD: from mechanisms to therapeutic potential. Oxid Med Cell Longev. 2019;2019:1–13.
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25(3):585–621. doi:10.1016/0014-4827(61)90192-6
- Von Kobbe C. Cellular senescence: a view throughout organismal life. Cell Mol Life Sci. 2018;75(19):3553–3567. doi:10.1007/s00018-018-2879-8
- Zhang L, Pitcher LE, Yousefzadeh MJ, Niedernhofer LJ, Robbins PD, Zhu Y. Cellular senescence: a key therapeutic target in aging and diseases. J Clin Invest. 2022;132(15):e158450. doi:10.1172/JCI158450
- Sreekumar PG, Hinton DR, Kannan R. The emerging role of senescence in ocular disease. Oxid Med Cell Longev. 2020;2020:1–19. doi:10.1155/2020/2583601
- Soleimani M, Cheraqpour K, Koganti R, Djalilian AR. Cellular senescence and ophthalmic diseases: narrative review. Graefes Arch Clin Exp Ophthalmol. 2023;261(11):3067–3082. doi:10.1007/s00417-023-06070-9
- Roger L, Tomas F, Gire V. Mechanisms and Regulation of Cellular Senescence. Int J Mol Sci. 2021;22(23):13173. doi:10.3390/ijms222313173
- Giroud J, Bouriez I, Paulus H, Pourtier A, Debacq-Chainiaux F, Pluquet O. Exploring the communication of the SASP: dynamic, interactive, and adaptive effects on the microenvironment. Int J Mol Sci. 2023;24(13):10788. doi:10.3390/ijms241310788
- Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018;28(6):436–453. doi:10.1016/j.tcb.2018.02.001
- Deng Y, Qiao L, Du M, et al. Age-related macular degeneration: epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes Dis. 2022;9(1):62–79. doi:10.1016/j.gendis.2021.02.009
- Kozlowski MR. RPE cell senescence: a key contributor to age-related macular degeneration. Med Hypotheses. 2012;78(4):505–510. doi:10.1016/j.mehy.2012.01.018
- Chaum E, Winborn CS, Bhattacharya S. Genomic regulation of senescence and innate immunity signaling in the retinal pigment epithelium. Mamm Genome. 2015;26(5–6):210–221. doi:10.1007/s00335-015-9568-9
- Zhu D, Wu J, Spee C, Ryan SJ, Hinton DR. BMP4 mediates oxidative stress-induced retinal pigment epithelial cell senescence and is overexpressed in age-related macular degeneration. J Biol Chem. 2009;284(14):9529–9539. doi:10.1074/jbc.M809393200
- Lazzarini R, Nicolai M, Pirani V, Mariotti C, Di Primio R. Effects of senescent secretory phenotype acquisition on human retinal pigment epithelial stem cells. Aging. 2018;10(11):3173–3184. doi:10.18632/aging.101624
- Fritsche LG, Igl W, Bailey JNC, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48(2):134–143. doi:10.1038/ng.3448
- Lin MK, Yang J, Hsu CW, et al. HTRA 1, an age‐related macular degeneration protease, processes extracellular matrix proteins EFEMP 1 and TSP 1. Aging Cell. 2018;17(4):e12710. doi:10.1111/acel.12710
- Supanji SM, Hasan M, Kawaichi M, Oka C, Oka C. HtrA1 is induced by oxidative stress and enhances cell senescence through p38 MAPK pathway. Exp Eye Res. 2013;112:79–92. doi:10.1016/j.exer.2013.04.013
- Xu W, Liu X, Han W, et al. Inhibiting HIF-1 signaling alleviates HTRA1-induced RPE senescence in retinal degeneration. Cell Commun Signal. 2023;21(1):134. doi:10.1186/s12964-023-01138-9
- Zhu D, Deng X, Xu J, Hinton DR. What determines the switch between atrophic and neovascular forms of age related macular degeneration? The role of BMP4 induced senescence. Aging. 2009;1(8):740–745. doi:10.18632/aging.100078
- Xu J, Zhu D, He S, Spee C, Ryan SJ, Hinton DR. Transcriptional regulation of bone morphogenetic protein 4 by tumor necrosis factor and its relationship with age-related macular degeneration. FASEB J. 2011;25(7):2221–2233. doi:10.1096/fj.10-178350
- Dvashi Z, Green Y, Pollack A. TAK1 inhibition accelerates cellular senescence of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2014;55(9):5679–5686. doi:10.1167/iovs.14-14349
- Cao L, Wang H, Wang F, et al. Aβ-induced senescent retinal pigment epithelial cells create a proinflammatory microenvironment in AMD. Invest Ophthalmol Vis Sci. 2013;54(5):3738–3750. doi:10.1167/iovs.13-11612
- Wang H, Ramshekar A, Cung T, et al. 7-ketocholesterol promotes retinal pigment epithelium senescence and fibrosis of choroidal neovascularization via IQGAP1 phosphorylation-dependent signaling. Int J Mol Sci. 2023;24(12):10276. doi:10.3390/ijms241210276
- Wang J, Feng Y, Han P, et al. Photosensitization of A2E triggers telomere dysfunction and accelerates retinal pigment epithelium senescence. Cell Death Dis. 2018;9(2):178. doi:10.1038/s41419-017-0200-7
- Shimizu H, Yamada K, Suzumura A, et al. Caveolin-1 promotes cellular senescence in exchange for blocking subretinal fibrosis in age-related macular degeneration. Invest Opthalmol Vis Sci. 2020;61(11):21. doi:10.1167/iovs.61.11.21
- Oubaha M, Miloudi K, Dejda A, et al. Senescence-associated secretory phenotype contributes to pathological angiogenesis in retinopathy. Sci Transl Med. 2016;8(362):ra144. doi:10.1126/scitranslmed.aaf9440
- Ma W, Cojocaru R, Gotoh N, et al. Gene expression changes in aging retinal microglia: relationship to microglial support functions and regulation of activation. Neurobiol Aging. 2013;34(10):2310–2321. doi:10.1016/j.neurobiolaging.2013.03.022
- Cabrera AP, Bhaskaran A, Xu J, et al. Senescence increases choroidal endothelial stiffness and susceptibility to complement injury: implications for choriocapillaris loss in AMD. Invest Opthalmol Vis Sci. 2016;57(14):5910. doi:10.1167/iovs.16-19727
- López-Luppo M, Catita J, Ramos D, et al. Cellular senescence is associated with human retinal microaneurysm formation during aging. Invest Opthalmol Vis Sci. 2017;58(7):2832. doi:10.1167/iovs.16-20312
- Sene A, Khan AA, Cox D, et al. Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell Metab. 2013;17(4):549–561. doi:10.1016/j.cmet.2013.03.009
- Faber C, Singh A, Krüger Falk M, Juel HB, Sørensen TL, Nissen MH. Age-related macular degeneration is associated with increased proportion of CD56+ T cells in peripheral blood. Ophthalmology. 2013;120(11):2310–2316. doi:10.1016/j.ophtha.2013.04.014
- Ezzat MK, Hann CR, Vuk-Pavlovic S, Pulido JS. Immune cells in the human choroid. Br J Ophthalmol. 2008;92(7):976–980. doi:10.1136/bjo.2007.129742
- Morohoshi K, Patel N, Ohbayashi M, et al. Serum autoantibody biomarkers for age-related macular degeneration and possible regulators of neovascularization. Exp Mol Pathol. 2012;92(1):64–73. doi:10.1016/j.yexmp.2011.09.017
- O’Leary F, Campbell M. The blood–retina barrier in health and disease. FEBS J. 2023;290(4):878–891. doi:10.1111/febs.16330
- Chen M, Luo C, Zhao J, Devarajan G, Xu H. Immune regulation in the aging retina. Prog Retin Eye Res. 2019;69:159–172. doi:10.1016/j.preteyeres.2018.10.003
- McMenamin PG, Saban DR, Dando SJ. Immune cells in the retina and choroid: two different tissue environments that require different defenses and surveillance. Prog Retin Eye Res. 2019;70:85–98. doi:10.1016/j.preteyeres.2018.12.002
- Fletcher EL. Contribution of microglia and monocytes to the development and progression of age related macular degeneration. Ophthalmic Physiol Opt. 2020;40(2):128–139. doi:10.1111/opo.12671
- Raoul W, Auvynet C, Camelo S, et al. CCL2/CCR2 and CX3CL1/CX3CR1 chemokine axes and their possible involvement in age-related macular degeneration. J Neuroinflammation. 2010;7(1):87. doi:10.1186/1742-2094-7-87
- Silverman SM, Wong WT. Microglia in the retina: roles in development, maturity, and disease. Annu Rev Vis Sci. 2018;4(1):45–77. doi:10.1146/annurev-vision-091517-034425
- Wang X, Zhao L, Zhang J, et al. Requirement for microglia for the maintenance of synaptic function and integrity in the mature retina. J Neurosci. 2016;36(9):2827–2842. doi:10.1523/JNEUROSCI.3575-15.2016
- Ma W, Wong WT. Aging changes in retinal microglia and their relevance to age-related retinal disease. Adv Exp Med Biol. 2016;854:73–78.
- Dietrich L, Lucius R, Roider J, Klettner A. Interaction of inflammatorily activated retinal pigment epithelium with retinal microglia and neuronal cells. Exp Eye Res. 2020;199:108167. doi:10.1016/j.exer.2020.108167
- Li L, Heiduschka P, Alex AF, Niekämper D, Eter N. Behaviour of CD11b-positive cells in an animal model of laser-induced choroidal neovascularisation. Ophthalmologica. 2017;237(1):29–41. doi:10.1159/000453550
- Saban DR. New concepts in macrophage ontogeny in the adult neural retina. Cell Immunol. 2018;330:79–85. doi:10.1016/j.cellimm.2018.04.008
- Cao X, Shen D, Patel MM, et al. Macrophage polarization in the maculae of age-related macular degeneration: a pilot study. Pathol Int. 2011;61(9):528–535. doi:10.1111/j.1440-1827.2011.02695.x
- Ardeljan D, Chan CC. Aging is not a disease: distinguishing age-related macular degeneration from aging. Prog Retin Eye Res. 2013;37:68–89. doi:10.1016/j.preteyeres.2013.07.003
- Liew PX, Kubes P. The neutrophil’s role during health and disease. Physiol Rev. 2019;99(2):1223–1248. doi:10.1152/physrev.00012.2018
- Chen J, Zhao L, Ding X, et al. Aβ1–40 oligomers trigger neutrophil extracellular trap formation through TLR4- and NADPH oxidase-dependent pathways in age-related macular degeneration. Oxid Med Cell Longev. 2022;2022:1–15.
- Binet F, Cagnone G, Crespo-Garcia S, et al. Neutrophil extracellular traps target senescent vasculature for tissue remodeling in retinopathy. Science. 2020;369(6506):eaay5356. doi:10.1126/science.aay5356
- Ghosh S, Padmanabhan A, Vaidya T, et al. Neutrophils homing into the retina trigger pathology in early age-related macular degeneration. Commun Biol. 2019;2(1):348. doi:10.1038/s42003-019-0588-y
- Ghosh S, Shang P, Yazdankhah M, et al. Activating the AKT2 –nuclear factor‐ κB –lipocalin‐2 axis elicits an inflammatory response in age‐related macular degeneration. J Pathol. 2017;241(5):583–588. doi:10.1002/path.4870
- Prager I, Watzl C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J Leukoc Biol. 2019;105(6):1319–1329. doi:10.1002/JLB.MR0718-269R
- Goverdhan SV, Khakoo SI, Gaston H, Chen X, Lotery AJ. Age-related macular degeneration is associated with the HLA-Cw*0701 genotype and the natural killer cell receptor AA haplotype. Invest Opthalmol Vis Sci. 2008;49(11):5077. doi:10.1167/iovs.08-1837
- Zeng Y, Yin X, Chen C, Xing Y. Identification of diagnostic biomarkers and their correlation with immune infiltration in age-related macular degeneration. Diagnostics. 2021;11(6):1079. doi:10.3390/diagnostics11061079
- Lee H, Schlereth SL, Park EY, Emami-Naeini P, Chauhan SK, Dana R. A novel pro-angiogenic function for interferon-γ-secreting natural killer cells. Invest Ophthalmol Vis Sci. 2014;55(5):2885–2892. doi:10.1167/iovs.14-14093
- Bhutto IA, McLeod DS, Jing T, Sunness JS, Seddon JM, Lutty GA. Increased choroidal mast cells and their degranulation in age-related macular degeneration. Br J Ophthalmol. 2016;100(5):720–726. doi:10.1136/bjophthalmol-2015-308290
- Mcharg S, Booth L, Perveen R, et al. Mast cell infiltration of the choroid and protease release are early events in age-related macular degeneration associated with genetic risk at both chromosomes 1q32 and 10q26. Proc Natl Acad Sci. 2022;119(20):e2118510119. doi:10.1073/pnas.2118510119
- Voigt AP, Mullin NK, Mulfaul K, et al. Choroidal endothelial and macrophage gene expression in atrophic and neovascular macular degeneration. Hum Mol Genet. 2022;31(14):2406–2423. doi:10.1093/hmg/ddac043
- Droho S, Perlman H, Lavine JA. Dendritic cells play no significant role in the laser-induced choroidal neovascularization model. Sci Rep. 2021;11(1):17254. doi:10.1038/s41598-021-96704-x
- Merle NS, Noe R, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, Roumenina LT. Complement system part II: role in immunity. Front Immunol. 2015;6:257. doi:10.3389/fimmu.2015.00257
- Anderson DH, Radeke MJ, Gallo NB, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010;29(2):95–112. doi:10.1016/j.preteyeres.2009.11.003
- Heesterbeek TJ, Lechanteur YTE, Lorés-Motta L, et al. Complement activation levels are related to disease stage in AMD. Invest Opthalmol Vis Sci. 2020;61(3):18. doi:10.1167/iovs.61.3.18
- Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Opthalmol Vis Sci. 2009;50(12):5818. doi:10.1167/iovs.09-3928
- Liu B, Wei L, Meyerle C, et al. Complement component C5a promotes expression of IL-22 and IL-17 from human T cells and its implication in age-related macular degeneration. J Transl Med. 2011;9(1):111. doi:10.1186/1479-5876-9-111
- Kim BJ, Mastellos DC, Li Y, Dunaief JL, Lambris JD. Targeting complement components C3 and C5 for the retina: key concepts and lingering questions. Prog Retin Eye Res. 2021;83:100936. doi:10.1016/j.preteyeres.2020.100936
- Whitmore SS, Sohn EH, Chirco KR, et al. Complement activation and choriocapillaris loss in early AMD: implications for pathophysiology and therapy. Prog Retin Eye Res. 2015;45:1–29. doi:10.1016/j.preteyeres.2014.11.005
- Rathnasamy G, Foulds WS, Ling EA, Kaur C. Retinal microglia – a key player in healthy and diseased retina. Prog Neurobiol. 2019;173:18–40. doi:10.1016/j.pneurobio.2018.05.006
- Zhang H, Li F, Yang Y, Chen J, Hu X. SIRP/CD47 signaling in neurological disorders. Brain Res. 2015;1623:74–80. doi:10.1016/j.brainres.2015.03.012
- Mecca C, Giambanco I, Donato R, Arcuri C. Microglia and aging: the role of the TREM2-DAP12 and CX3CL1-CX3CR1 axes. Int J Mol Sci. 2018;19(1):318. doi:10.3390/ijms19010318
- Ma W, Zhao L, Fontainhas AM, Fariss RN, Wong WT. Microglia in the mouse retina alter the structure and function of retinal pigmented epithelial cells: a potential cellular interaction relevant to AMD. PLoS One. 2009;4(11):e7945. doi:10.1371/journal.pone.0007945
- Ma W, Zhang Y, Gao C, Fariss RN, Tam J, Wong WT. Monocyte infiltration and proliferation reestablish myeloid cell homeostasis in the mouse retina following retinal pigment epithelial cell injury. Sci Rep. 2017;7(1):8433. doi:10.1038/s41598-017-08702-7
- Cherepanoff S, McMenamin P, Gillies MC, Kettle E, Sarks SH. Bruch’s membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br J Ophthalmol. 2010;94(7):918–925. doi:10.1136/bjo.2009.165563
- Déchelle-Marquet PA, Guillonneau X, Sennlaub F, Delarasse C. P2X7-dependent immune pathways in retinal diseases. Neuropharmacology. 2023;223:109332. doi:10.1016/j.neuropharm.2022.109332
- Pereira BI, Devine OP, Vukmanovic-Stejic M, et al. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8+ T cell inhibition. Nat Commun. 2019;10(1):2387. doi:10.1038/s41467-019-10335-5
- Levy O, Calippe B, Lavalette S, et al. Apolipoprotein E promotes subretinal mononuclear phagocyte survival and chronic inflammation in age‐related macular degeneration. EMBO Mol Med. 2015;7(2):211–226. doi:10.15252/emmm.201404524
- Calippe B, Augustin S, Beguier F, et al. Complement factor H inhibits CD47-mediated resolution of inflammation. Immunity. 2017;46(2):261–272. doi:10.1016/j.immuni.2017.01.006
- Gautier EL, Ivanov S, Lesnik P, Randolph GJ. Local apoptosis mediates clearance of macrophages from resolving inflammation in mice. Blood. 2013;122(15):2714–2722. doi:10.1182/blood-2013-01-478206
- König S, Hadrian K, Schlatt S, Wistuba J, Thanos S, Böhm MRR. Topographic protein profiling of the age-related proteome in the retinal pigment epithelium of Callithrix jacchus with respect to macular degeneration. J Proteomics. 2019;191:1–15. doi:10.1016/j.jprot.2018.05.016
- Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT. Age-related alterations in the dynamic behavior of microglia. Aging Cell. 2011;10(2):263–276. doi:10.1111/j.1474-9726.2010.00660.x
- Lin JB, Moolani HV, Sene A, et al. Macrophage microRNA-150 promotes pathological angiogenesis as seen in age-related macular degeneration. JCI Insight. 2018;3(7):e120157. doi:10.1172/jci.insight.120157
- Eandi CM, Charles Messance H, Augustin S, et al. Subretinal mononuclear phagocytes induce cone segment loss via IL-1β. eLife. 2016;5:e16490. doi:10.7554/eLife.16490
- Mathis T, Housset M, Eandi C, et al. Activated monocytes resist elimination by retinal pigment epithelium and downregulate their OTX 2 expression via TNF ‐α. Aging Cell. 2017;16(1):173–182. doi:10.1111/acel.12540
- Lechner J, Chen M, Hogg RE, et al. Peripheral blood mononuclear cells from neovascular age-related macular degeneration patients produce higher levels of chemokines CCL2 (MCP-1) and CXCL8 (IL-8). J Neuroinflammation. 2017;14(1):42. doi:10.1186/s12974-017-0820-y
- Tenbrock L, Wolf J, Boneva S, et al. Subretinal fibrosis in neovascular age-related macular degeneration: current concepts, therapeutic avenues, and future perspectives. Cell Tissue Res. 2022;387(3):361–375. doi:10.1007/s00441-021-03514-8
- Little K, Llorián-Salvador M, Tang M, et al. Macrophage to myofibroblast transition contributes to subretinal fibrosis secondary to neovascular age-related macular degeneration. J Neuroinflammation. 2020;17(1):355. doi:10.1186/s12974-020-02033-7
- Boyce M, Xin Y, Chowdhury O, et al. Microglia–neutrophil interactions drive dry AMD-like pathology in a mouse model. Cells. 2022;11(22):3535. doi:10.3390/cells11223535
- Ma W, Coon S, Zhao L, Fariss RN, Wong WT. A2E accumulation influences retinal microglial activation and complement regulation. Neurobiol Aging. 2013;34(3):943–960. doi:10.1016/j.neurobiolaging.2012.06.010
- Luo C, Zhao J, Chen M, Xu H. The expression of C1 inhibitor (C1INH) in macrophages is upregulated by retinal pigment epithelial cells – implication in subretinal immune privilege in the aging eye. Aging. 2018;10(6):1380–1389. doi:10.18632/aging.101474
- Rohrer B. Anaphylatoxin signaling in retinal pigment and choroidal endothelial cells: characteristics and relevance to age-related macular degeneration. Adv Exp Med Biol. 2018;1074:45–51.
- Bandyopadhyay M, Rohrer B. Matrix metalloproteinase activity creates pro-angiogenic environment in primary human retinal pigment epithelial cells exposed to complement. Invest Opthalmol Vis Sci. 2012;53(4):1953. doi:10.1167/iovs.11-8638
- Llorián-Salvador M, Byrne EM, Szczepan M, Little K, Chen M, Xu H. Complement activation contributes to subretinal fibrosis through the induction of epithelial-to-mesenchymal transition (EMT) in retinal pigment epithelial cells. J Neuroinflammation. 2022;19(1):182. doi:10.1186/s12974-022-02546-3
- Georgiannakis A, Burgoyne T, Lueck K, Futter C, Greenwood J, Moss SE. Retinal pigment epithelial cells mitigate the effects of complement attack by endocytosis of C5b-9. J Immunol. 2015;195(7):3382–3389.
- Lueck K, Wasmuth S, Williams J, et al. Sub-lytic C5b-9 induces functional changes in retinal pigment epithelial cells consistent with age-related macular degeneration. Eye. 2011;25(8):1074–1082. doi:10.1038/eye.2011.109
- Mulfaul K, Mullin NK, Giacalone JC, et al. Local factor H production by human choroidal endothelial cells mitigates complement deposition: implications for macular degeneration. J Pathol. 2022;257(1):29–38. doi:10.1002/path.5867
- Crespo-Garcia S, Tsuruda PR, Dejda A, et al. Pathological angiogenesis in retinopathy engages cellular senescence and is amenable to therapeutic elimination via BCL-xL inhibition. Cell Metab. 2021;33(4):818–832.e7. doi:10.1016/j.cmet.2021.01.011
- Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat Rev Immunol. 2013;13(6):438–451. doi:10.1038/nri3459
- Holz FG, Sadda SR, Busbee B, et al. Efficacy and safety of lampalizumab for geographic atrophy due to age-related macular degeneration: chroma and spectri Phase 3 randomized clinical trials. JAMA Ophthalmol. 2018;136(6):666–677. doi:10.1001/jamaophthalmol.2018.1544
- Kent D. The pathogenesis of age-related macular degeneration is not inflammatory mediated but is instead due to immunosenescence-related failure of tissue repair. Med Hypotheses. 2021;146:110392. doi:10.1016/j.mehy.2020.110392
- Luttrull JK, Gray G. Real world data comparison of standard care vs SDM laser vision protection therapy for prevention of neovascular AMD. Clin Ophthalmol. 2022;16:1555–1568. doi:10.2147/OPTH.S366150
- Luttrull JK, Sinclair SH, Elmann S, Chang DB, Kent D. Slowed progression of age-related geographic atrophy following subthreshold laser. Clin Ophthalmol. 2020;14:2983–2993. doi:10.2147/OPTH.S268322
