Abstract
Introduction
Physical rehabilitation is commonly used in patients with Parkinson’s disease (PD) to improve their health and alleviate the symptoms.
Objective
We compared the effects of three programs, strength training (ST), aerobic training (AT), and physiotherapy, on motor symptoms, functional capacity, and electroencephalographic (EEG) activity in PD patients.
Methods
Twenty-two patients were recruited and randomized into three groups: AT (70% of maximum heart rate), ST (80% of one repetition maximum), and physiotherapy (in groups). Subjects participated in their respective interventions twice a week for 12 weeks. The assessments included measures of disease symptoms (Unified Parkinson’s Disease Rating Scale [UPDRS]), functional capacity (Senior Fitness Test), and EEG before and after 12 weeks of intervention.
Results
The PD motor symptoms (UPDRS-III) in the group of patients who performed ST and AT improved by 27.5% (effect size [ES]=1.25, confidence interval [CI]=−0.11, 2.25) and 35% (ES=1.34, CI=−0.16, 2.58), respectively, in contrast to the physiotherapy group, which showed a 2.9% improvement (ES=0.07, CI=−0.85, 0.99). Furthermore, the functional capacity of all three groups improved after the intervention. The mean frequency of the EEG analysis mainly showed the effect of the interventions on the groups (F=11.50, P=0.0001).
Conclusion
ST and AT in patients with PD are associated with improved outcomes in disease symptoms and functional capacity.
Introduction
Parkinson’s disease (PD) is currently considered the second most common neurodegenerative disorder, losing first place to Alzheimer’s disease by a small margin.Citation1,Citation2 Functional disability caused by PD has a major impact on the lives of patients and their families. The progression of PD leads to an increasing inability to perform daily activities, loss of independence, and a decreased quality of life, and it generates socioeconomic and occupational impairments.Citation3 Because of its complex and multifactorial etiology, which combines, in many cases, genetic, environmental, and lifestyle factors, PD is usually treated with pharmacological and surgical approaches, but these treatments are not always completely effective.Citation4
The motor impairment in PD patients caused by bradykinesia, rigidity, tremor, and postural instability accelerates the decline in functional capacity, especially when associated with decreased activity and with a sedentary lifestyle.Citation5,Citation6 Physical rehabilitation may be important for the maintenance and improvement of mobility, posture, and balance in PD patients.Citation7 Different modalities of nonpharmacological treatment such as physiotherapy (P), walking, running, strength training (ST), functional exercises, and whole body vibration significantly reduced the risk of falls and improved motor performance,Citation8,Citation9 balance and gait,Citation10 and executive functions.Citation11 However, further discussion is required regarding the levels of physical exertion to which PD patients may be subjected.Citation12 In this regard, P appears to have lower clinical effectsCitation13 when compared with aerobic training (AT)Citation14 and ST.Citation15 However, the possible differences among these interventions have not yet been investigated in a controlled study, to the best of our knowledge.
This trial aimed to compare the effects of P, AT, and ST on improvement of the symptoms of PD. The main hypothesis of this study was that an AT and/or an ST program would be superior to P in improving motor symptoms, based on the Unified Parkinson’s Disease Rating Scale (UPDRS) subscale UPDRS-III. The secondary outcomes studied were functional capacity (Senior Fitness Test), balance, walking speed, and electroencephalographic activity (EEG) to examine possible central nervous system changes (activity of the cerebral cortex).
Materials and methods
Standard protocols and patient consent
A prospective longitudinal, randomized, controlled intervention study was conducted from September 2010 to September 2012. Patients with idiopathic PD diagnosed by neurologist experts in movement disorders were recruited from an outpatient rehabilitation department of the Federal University of Rio de Janeiro, Brasil. The inclusion criteria were age between 45 years and 80 years, a diagnosis of PD, and stage 1–3 on the Hoehn and Yahr scale.Citation16 The exclusion criteria comprised any disease that hindered the application of an evaluation instrument, clinical comorbidities that make it impossible to use physical effort, individuals of New York Heart Association classes III and IV, significant physical limitations, and visual or hearing impairment. The patients were followed for 12 weeks. All subjects gave informed consent and the study was approved by the Ethics Committee of the Institute of Neurology Deolindo Couto (INDC) (protocol number 008-09-CEP).
Intervention
We decided to compare the effects of three approaches, AT, ST, and P, all of which are widely used for the prevention and treatment of a number of diseases. We applied specific programs for each method to determine if and how they were able to increase the functional capacity and reduce the symptoms of PD. The programs were equivalent with respect to the time of intervention, duration, and weekly frequency. The patients were randomized by a blinded investigator and controlled for age, motor score in terms of UPDRS-III,Citation17 and stage of the disease as defined by Hoehn and Yahr scale.Citation18 They participated in their respective interventions twice a week for 12 weeks. All patients performed exercise sessions supervised by trained coaches. Patients were advised not to perform additional exercises.
Aerobic training
The AT program consisted of 30 minutes of treadmill walking (Jog 500, Technogym®; Italy) preceded by 5 minutes of warming and 5 minutes of postexercise recovery. The training intensity corresponded to 60% of the maximum oxygen consumption (VO2max) or 70% of the maximum heart rate (HRmax) predicted by age and determined by the formula HRmax=208−(0.7× age).Citation19 The speed and treadmill inclination were changed so that the patients maintained the same intensity of training during the 12-week intervention. The VO2 during training was obtained by the walking equation described by the American College of Sports Medicine, VO2=[0.1 (velocity) +1.8 (velocity) (inclination/100) +3.5], where the velocity is given in m/min.Citation20
Strength training
The ST program consisted of exercises for large muscle groups using equipment for leg extensions, leg curls, leg presses, chest presses, and low row. All equipment was from the line Selection (Technogym®; Italy). The daily training volume was composed of two series of eight to 12 maximum repetitions for each exercise with rest intervals of 1 minute and 30 seconds between the sets. The initial training intensity was set between 70% and 80% of the value obtained in the one repetition maximum test, and the training load was constantly adjusted to maintain the maximum repetitions. The patients were instructed to perform a warm-up on each machine with minimum load before starting the training and to perform a stretching session at the end of the training.
Physiotherapy
The P group program consisted of calisthenics for the upper and lower limbs, stretching, and gait training in a 12 m hallway, led by a physiotherapist. The sessions lasted approximately 30–40 minutes and no type of overload was used in the proposed activities.
Study procedures
All patients were evaluated for the effect of dopaminergic medication during the training period. The motor symptoms were assessed using UPDRS-III by a single evaluator certified by the Movement Disorder Society (AC). The global cognitive status was assessed using the Mini-Mental State Examination.Citation21
To assess functional capacity, the following tests (from the Senior Fitness Test) were used:Citation22 the Chair Stand Test (CST), Arm Curl Test (ACT), 2-Minute Step Test (2-MST), Chair Sit and Reach Test (CSRT), Back Scratch Test (BST), and 8-Foot Up and Go Test (8-FT). In addition, we used the 10-Meter Walk Test (10-MWT)Citation23 and Berg Balance Scale (Berg).Citation24 All these tests have good reliability and validity values.Citation22
The EEG mean frequency (MF) was recorded and analyzed following the protocol used in a previous study from our laboratory.Citation25 The MF may also be modified by pharmacological and environmental interventions. Physical exercise increases the MF throughout the cortex;Citation26 however, there is no evidence in relation to patients with PD.
The patients returned to the laboratory after 3 months for follow-up. The whole process of the baseline assessment was repeated by the same examiner. Current medications and any adverse effects were recorded at each visit.
Statistical analysis
The mean and standard deviation were computed for parametric variables and the χ test was used for categorical variables for descriptive analysis. To test for the normality and homoscedasticity of the deltas (post and pre), the Shapiro-Wilk test and Levene’s tests were applied, respectively.
To compare the deltas (post and pre) between groups (AT, ST, and P), one-way analysis of variance (ANOVA) was performed for all physical tests (UPDRS, 8-FT, CST, 2-MST, ACT, BST, CSRT, Berg, 10-MWT). Significant differences in ANOVA were analyzed by the Bonferroni post hoc test.
Furthermore, we decided to use analysis of effect size (ES) in addition to traditional statistics, comparing the results obtained by all interventions to assess the clinical relevance of our findings. The ES analysis was performed using the formula proposed by Cohen.Citation27 The values of the ES were classified as trivial (0.20≤ES<0.50), moderate (0.50≤ES<0.80), and large (ES≥0.80).
For analyzing the values of EEG MF, the electrodes were divided into six areas (frontal pole, frontal, central, temporal, parietal, and occipital).Citation28 These areas are composed of electrodes Fp1, Fp2, and Fz (frontal pole); F4, F3, F7, and F8 (frontal); C3 and C4 (central); T3, T4, T5, and T6 (temporal); P3 and P4 (parietal); and O1 and O2 (occipital). The statistical model employed was a three-way ANOVA to obtain comparisons among moment (pre and post), the group (ST, AT, and P), and the area (frontal pole, frontal, central, parietal, temporal, and occipital). The statistical package SPSS® version 20.0 for Windows (IBM Corporation, Armonk, NY, USA) was used for all analyses, and the accepted significance level for this study was P≤0.05.
Results
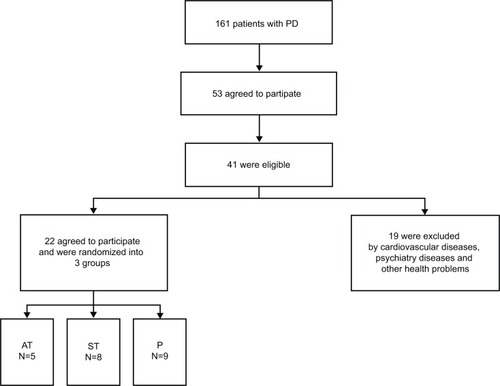
All variables investigated showed normality and homoscedasticity and therefore parametric analyzes were performed. Twenty-two patients were randomly assigned to the three groups (). The only difference among the groups was found in the use of dopamine agonists, most used by the P group ().
Table 1 Descriptive analysis of the three groups (physiotherapy [P], aerobic training [AT], and strength training [ST]) at baseline
Figure 1 Flow diagram for identification of eligible patients.
Symptoms of disease
Regarding the stages of PD (Hoehn and Yahr scale), 4.5% of the patients were in stage I, 54.5% in stage II, and 41% in stage III. There was no significant difference between the deltas of the three groups for the subscales of the UPDRS ().
Table 2 Comparison of symptoms of Parkinson’s disease and functional capacity (pre and post) of aerobic training, strength training, and physiotherapy
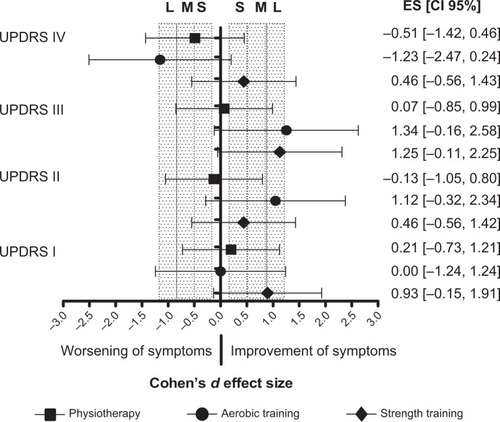
The ST group showed a large ES in UPDRS-I (ES=0.93) and UPDRS-III (ES=1.25), presenting improved behavioral and motor symptoms. The AT group presented a large ES in UPDRS-II (ES=1.12) and UPDRS-III (ES=1.34). There was no effect in UPDRS-I (ES=0.00) and worsening of symptoms in UPDRS-IV (ES=−1.23). The P group presented a trivial ES in UPDRS-III (ES=0.07) and UPDRS-I (ES=0.21) and worsening of symptoms in UPDRS-II (ES=−0.13) and UPDRS-IV (ES=−0.51) ().
Figure 2 Effect size (ES) of symptoms of Parkinson’s disease.
Functional capacity
In comparing the deltas of the functional capacity tests there was a significant difference among the groups (F=5.65, P=0.01) in their aerobic capacity assessed by the 2-MST. The Bonferroni post hoc test revealed that the ST and AT groups showed significant improvement after training, which was absent in the P group. There were no statistically significant differences among the groups for the analyses of the deltas of the other functional capacity tests ().
In the analysis of ES, the ST group had a large ES in the 8-FT (ES=−1.18) and CST (ES=1.81); a moderate ES in the ACT (ES=0.74), 2-MST (ES=0.72), and 10-MWT (ES=0.78); and a trivial ES in the BST (ES=0.38), CSRT (ES=0.28), and Berg (ES=0.44). The AT group showed a large ES in the 8-FT (ES=1.08), ACT (ES=1.16), CST (ES=0.86), and 10-MWT (ES=1.20); a moderate ES in the BST (ES=0.79) and 2-MST (ES=0.69); and a trivial ES in Berg (ES=0.38). The P group showed a large ES in the 8-FT (ES=1.35), a moderate ES in the CST (ES=0.57), a trivial ES in the BST (ES=0.41) and 10-MWT (ES=0.34), and worsening of symptoms in the 2-MST (ES=−0.73) ().
EEG MF
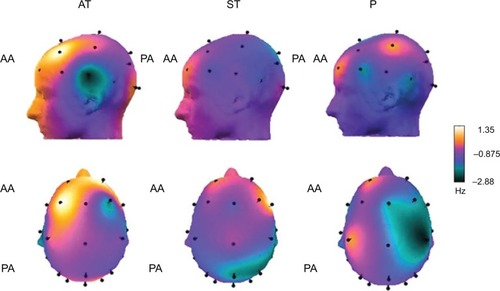
The results of the EEG MF analysis primarily demonstrated the effect of the interventions on the groups (F=11.50, P=0.00), without giving significance to area (F=1.98, P=0.97), moment (F=0.01, P=0.89), and interactions between moment × group (F=2.36, P=0.09), moment × area (F=0.27, P=0.93), group × area (F=0.21, P=0.99), and moment × group × area (F=0.23, P=0.93). The AT and ST groups showed higher MF compared with the P group, which is related to increased cortical activation. illustrates the cortical activity of the groups using cortical maps with the delta values. The yellow and orange colors represent increased cortical activity in the anterior and posterior areas of the cortex. The violet, black, and green colors represent decreased cortical activity in the same areas.
Discussion
This study aimed to compare the effects of ST, AT, and P as supplementary treatments for the motor symptoms of PD in a randomized controlled design. Our results showed that after 3 months of intervention, the improvement in PD symptoms was similar between the groups that performed AT and ST, and this improvement was greater than that of the group that was on P sessions. To the best of our knowledge, this is the first study to compare two types of exercise (AT and ST) with a group that was submitted to P as a control group. We found that the effects in the exercise groups are not only due to physical activity but possibly associated with physiological changes in response to training with a controlled overload and progression of intensity. This inference is further strengthened by the observation that the largest ES was found in the groups that had undergone AT and ST.
The groups of patients that underwent AT and ST showed significant clinical improvements in the motor symptoms of PD, as well as in functional capacity, indicating that prescribing exercise with controlled intensity, duration, and frequency may improve the physical health of patients with PD. There was a 27.5% and 35% improvement in motor symptoms (UPDRS-III) for the ST and AT groups, respectively, in contrast to a 2.9% improvement for the P group. These results demonstrate that pharmacological treatment associated with physical exercise (AT and/or ST) may promote better results for symptoms such as rigidity and bradykinesia than just abiding to the conventional P usually prescribed to PD patients. Our hypothesis was also recently confirmed by Tambosco et alCitation12 who illustrated that the inclusion of AT and ST was effective in rehabilitation programs for PD patients. Our results also corroborate the findings of Dibble et alCitation29 Ridgel et alCitation30 and Alberts et alCitation14 who showed that AT and ST associated with pharmacological treatment are capable of promoting positive responses in the cardinal symptoms of PD. In a preliminary study, Dibble et alCitation29 submitted ten PD patients for 12 weeks of an ST program with high intensity (80% HRmax), lasting 45–60 minutes, three times a week. The patients showed an improvement in bradykinesia assessed by speed walking tests (ES=0.68) and agility (ES=0.59), compared with an active control group (ES=0.07).Citation29 Ridgel et alCitation30 and Alberts et alCitation14 observed a 35% improvement in motor symptoms after submitting PD patients for 3 weeks of forced training on a cycloergometer.Citation14,Citation30 In contrast to the conclusion of Alberts et alCitation14 that forced aerobic exercise would be more effective than voluntary exercise for improving motor symptoms of PD, our results showed that there was a 35% improvement in motor symptoms on UPDRS-III in the volunteer subjects who walked on a treadmill with due control of workout intensity.
In relation to functional capacity, ST and AT significantly improved the patients’ aerobic capacity, measured by the 2-MST. Moreover, by studying the ES, we found that the ST and AT groups improved in agility, strength of the upper and lower limbs, lower limb endurance, walking speed, and dynamic balance. The AT group also showed an improvement in the flexibility of the upper limbs. The group submitted to P also improved in agility and lower limb strength. However, the flexibility and endurance of the lower limbs worsened after the intervention period. These results demonstrate the effect of exercise on improving variables such as strength, flexibility, and especially aerobic endurance.Citation12 There was no difference between exercise and P in improving variables such as agility and balance.
Concerning the EEG, the ST and AT groups showed an increase in MF compared with the P group. As far as we know, our study is also the first to use EEG results to evaluate the effect of exercise and P in the treatment of PD. In PD patients, diffuse slowing of EEG was present according to progression of disease, and this phenomenon implies an impairment of neural activity, with implications on cognition, executive function, and motor function.Citation31,Citation32 The MF is a measure comprising all frequency bands of the EEG (delta, theta, alpha, beta, and gamma) and is positively correlated with cerebral blood flow and glucose metabolism.Citation28 Herz et alCitation33 observed that dopaminergic treatment appears to increase the beta and gamma frequency bands of the EEG in prefrontal and premotor areas. Our results suggest that exercise positively influences cortical activity in PD patients. During the execution of physical exercise, more cortical areas are activated, and the main hypothesis of this phenomenon is the increase in cerebral blood flow during exercise.Citation28 Studies on other diseases such as major depression demonstrated that AT promotes a change in asymmetryCitation34 and an increase in the MF of the EEG.Citation34,Citation35 Therefore, our results corroborate the evidence on the positive effect of exercise on the brain and on mental health. Likewise, Alberts et alCitation14 demonstrated increased activity in the cortical and subcortical areas through functional magnetic resonance imaging immediately after AT.
Studies performed in animal models suggest that the physiological adaptations promoted by exercise with intensity seem to promote structural changes in the brain, mediated by increased expression of trophic factors such as brain-derived neurotrophic factor, glial cell-derived neurotrophic factor, vascular endothelial growth factor, and insulin-like growth factor 1, positively influencing brain functions (motor, cognitive, and behavioral).Citation14,Citation36,Citation37 Further translational studies in humans are warranted to find out whether this is also the fact in PD patients.
The present study has some limitations that should be considered. The use of a small number of subjects in each group may be associated with type II error. This limited sample size has to be taken into account when interpreting to what extent the results can be generalized, particularly given the well-known heterogeneity among PD patients. Moreover, patients with PD present several comorbidities (eg, cardiovascular and mood disorders) that were not investigated in the present study, and it is important to observe the effects of exercise in these patients. Due to this fact, we did not limit our analysis to the test of statistical significance by rejecting the null hypothesis. Instead, we looked into the magnitude of the effect, which demonstrated the clinical application of our findings. For future research, we suggest applying and controlling variables such as other training intensities, combining different approaches (combined AT and ST), and analyzing hormones and trophic factors of the patients.
Conclusion
Aerobic and strength training, controlled by parameters of intensity, duration, and frequency, showed a greater improvement than conventional physical therapy in symptoms and cortical activity of PD patients, confirming the need for a systematic program of training with controlled intensity. Therefore, both strength and aerobic training associated with pharmacological treatment may contribute to improving response to the treatment of PD and must be prescribed to improve the physical and mental health of patients.
Acknowledgments
This work was fully supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brasil). Special thanks go to all members of the Lanex Laboratory for academic support and mutual cooperation, in particular to friends Heitor Silveira, Thiago Guimarães, Paulo de Tarso, and José Vicente. JL and EC were supported by Conselho Nacional de Pesquisa (CNPq, Brasil), JL was supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) – Prioridade Rio (E-26/112.631/2012), AC was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brasil), and ACD was supported by FAPERJ – Jovem Cientista do Nosso Estado (E26/102.174/2013).
Disclosure
The authors report no conflicts of interest in this work.
References
- SchapiraAHOlanowCWNeuroprotection in Parkinson disease: mysteries, myths, and misconceptionsJAMA2004291335836414734599
- SchapiraAHNeurobiology and treatment of Parkinson’s diseaseTrends Pharmacol Sci2009301414719042040
- MorrisMEWattsJJIansekRQuantifying the profile and progression of impairments, activity, participation, and quality of life in people with Parkinson disease: protocol for a prospective cohort studyBMC Geriatr20099219152709
- ConnollyBSLangAEPharmacological treatment of Parkinson disease: a reviewJAMA2014311161670168324756517
- van HiltenJJHooglandGvan der VeldeEAMiddelkoopHAKerkhofGARoosRADiurnal effects of motor activity and fatigue in Parkinson’s diseaseJ Neurol Neurosurg Psychiatry19935688748778350103
- van NimwegenMSpeelmanADHofman-van RossumEJPhysical inactivity in Parkinson’s diseaseJ Neurol2011258122214222121614433
- FoxSHKatzenschlagerRLimSYThe Movement Disorder Society evidence-based medicine review update: treatments for the motor symptoms of Parkinson’s diseaseMov Disord201126Suppl 3S2S4122021173
- AshburnAFazakarleyLBallingerCPickeringRMcLellanLDFittonCA randomised controlled trial of a home based exercise programme to reduce the risk of falling among people with Parkinson’s diseaseJ Neurol Neurosurg Psychiatry200778767868417119004
- CaglarATGursesHNMutluayFKKiziltanGEffects of home exercises on motor performance in patients with Parkinson’s diseaseClin Rehabil200519887087716323386
- EbersbachGEdlerDKaufholdOWisselJWhole body vibration versus conventional physiotherapy to improve balance and gait in Parkinson’s diseaseArch Phys Med Rehabil200889339940318295614
- TanakaKQuadrosACJrSantosRFStellaFGobbiLTGobbiSBenefits of physical exercise on executive functions in older people with Parkinson’s diseaseBrain Cogn200969243544119006643
- TamboscoLPercebois-MacadreLRapinANicomette-BardelJBoyerFCEffort training in Parkinson’s disease: a systematic reviewAnn Phys Rehabil Med20145727910424582335
- TomlinsonCLPatelSMeekCPhysiotherapy versus placebo or no intervention in Parkinson’s diseaseCochrane Database Syst Rev20128CD00281722895932
- AlbertsJLLinderSMPenkoALLoweMJPhillipsMIt is not about the bike, it is about the pedaling: forced exercise and Parkinson’s diseaseExerc Sport Sci Rev201139417718621799425
- CorcosDMRobichaudJADavidFJA two-year randomized controlled trial of progressive resistance exercise for Parkinson’s diseaseMov Disord20132891230124023536417
- HoehnMMYahrMDParkinsonism: onset, progression and mortalityNeurology19671754274426067254
- Movement Disorder Society Task Force on Rating Scales for Parkinson’s DiseaseThe Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendationsMov Disord200318773875012815652
- GoetzCGPoeweWRascolOMovement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendationsMov Disord20041991020102815372591
- TanakaHMonahanKDSealsDRAge-predicted maximal heart rate revisitedJ Am Coll Cardiol200137115315611153730
- American College of Sports MedicineACSM’s Guidelines for Exercise Testing and PrescriptionPhiladelphia, PALippincott Williams & Wilkins2005
- BertolucciPHBruckiSMCampacciSRJulianoYThe Mini-Mental State Examination in a general population: impact of educational statusArq Neuropsiquiatr199452117 Portugeuse8002795
- RikliREJonesCJSenior Fitness Test ManualChampaign, ILHuman Kinetics2001
- SalbachNMMayoNEHigginsJAhmedSFinchLERichardsCLResponsiveness and predictability of gait speed and other disability measures in acute strokeArch Phys Med Rehabil20018291204121211552192
- ScalzoPLNovaICPerraciniMRValidation of the Brazilian version of the Berg balance scale for patients with Parkinson’s diseaseArq Neuropsiquiatr2009673B83183519838513
- SilveiraHDeslandesACde MoraesHEffects of exercise on electroencephalographic mean frequency in depressed elderly subjectsNeuropsychobiology201061314114720110739
- LardonMTPolichJEEG changes from long-term physical exerciseBiol Psychol199644119308906355
- CohenJStatistical Power Analysis for the Behavioral Sciences2nd Edition2aNew York, NYRoutledge1988
- IngvarDHRosenIJohannessonGEEG related to cerebral metabolism and blood flowPharmakopsychiatr Neuropsychopharmakol1979122200209461506
- DibbleLEHaleTFMarcusRLGerberJPLaStayoPCHigh intensity eccentric resistance training decreases bradykinesia and improves quality of life in persons with Parkinson’s disease: a preliminary studyParkinsonism Relat Disord2009151075275719497777
- RidgelALVitekJLAlbertsJLForced, not voluntary, exercise improves motor function in Parkinson’s disease patientsNeurorehabil Neural Repair200923660060819131578
- MoritaAKameiSSerizawaKMizutaniTThe relationship between slowing EEGs and the progression of Parkinson’s diseaseJ Clin Neurophysiol200926642642919952568
- MoritaAKameiSMizutaniTRelationship between slowing of the EEG and cognitive impairment in Parkinson diseaseJ Clin Neurophysiol201128438438721811128
- HerzDMSiebnerHRHulmeOJFlorinEChristensenMSTimmermannLLevodopa reinstates connectivity from prefrontal to premotor cortex during externally paced movement in Parkinson’s diseaseNeuroimage201490152324269570
- DeslandesACMoraesHAlvesHEffect of aerobic training on EEG alpha asymmetry and depressive symptoms in the elderly: a 1-year follow-up studyBraz J Med Biol Res201043658559220464340
- SilveiraHDeslandesAde MoraesHEffects of exercise on electroencephalographic mean frequency in depressed elderly subjectsNeuropsychobiology201061314114720110739
- DeslandesAMoraesHFerreiraCExercise and mental health: many reasons to moveNeuropsychobiology200959419119819521110
- DeslandesAThe biological clock keeps ticking, but exercise may turn it backArq Neuropsiquiatr201371211311823392323