Abstract
Peripheral arterial disease (PAD) is frequently diagnosed after permanent damage has occurred, resulting in a high rate of morbidity, amputation, and loss of life. Early and ongoing diagnosis and treatment is required for this progressive disease. Lifestyle modifications can prevent or delay disease progression and improve symptoms. Limb-sparing endovascular interventions can restore circulation based on appropriate diagnostic testing to pinpoint vascular targets, and intervention must occur as early as possible to ensure optimal clinical outcomes. An algorithm for the diagnosis and management of PAD was developed to enable a collaborative approach between the family practice and primary care physician or internist and various specialists that may include a diabetologist, endocrinologist, smoking cessation expert, hypertension and lipid specialist, endovascular interventionalist, vascular surgeon, orthopedist, neurologist, nurse practitioner, podiatrist, wound healing expert, and/or others. A multidisciplinary team working together has the greatest chance of providing optimal care for the patient with PAD and ensuring ongoing surveillance of the patient’s overall health, ultimately resulting in better quality of life and increased longevity for patients with PAD.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Peripheral arterial disease (PAD) is the progressive stenosis or occlusion of the arteries of the extremities.Citation1 The resulting deficiency of oxygenated blood results in degeneration of the vasculature, nerves, and other tissues. PAD can result in intermittent claudication (pain on exertion or impairment walking), pain at rest, and loss of sensation in the extremities, progressing to critical limb ischemia with persistent wounds and infections and ultimately a gangrenous lesion requiring amputation of digits or an extremity.Citation2 PAD is associated with a higher risk of coronary artery disease, myocardial infarction, and cerebrovascular disease,Citation3 and is a marker for cardiovascular death and disability with a 22% mortality rate at 4.4 years.Citation4 PAD is typically caused by atherosclerosis of the peripheral arteries. Symptoms of this pathology may not be apparent for years, and the majority of individuals with PAD are asymptomaticCitation5 or ascribe their symptoms to innocuous causes. Consequently, the majority of cases of PAD are undiagnosed, and greater awareness of early signs and symptoms is needed.
Patients with PAD suffer a double burden of delayed diagnosis of their condition and infrequent use of limb-sparing treatments. PAD often progresses to a point of irreversible damage due to lack of awareness on the part of both patients and health care providers, limited availability of diagnostic tests in some primary care centers, and delayed referral for endovascular evaluation.Citation6 A 2012 review of Medicare patients who underwent a major amputation for critical limb ischemia (n=20,464), showed that 71% had no revascularization attempts, and 46% had no diagnostic angiogram prior to a major amputation.Citation7 Another study showed that primary amputation was the first procedure for the treatment of critical limb ischemia in 67% of Medicare patients.Citation8 The progressive, heterogeneous nature of PAD requires ongoing diagnostic testing to ensure optimal clinical outcomes.
The incidence of PAD has grown to epidemic proportions and is expected to increase further due to the continued growth in prevalence of diabetes and the general aging of the US population. Approximately 8–14 million people in the USA and an estimated 202 million people worldwide had PAD as of 2010.Citation9,Citation10 More than 1 million people in the USA have lost a limb due to vascular disease, including diabetes, PAD, and critical limb ischemia,Citation11 and approximately half the individuals with limb loss due to vascular disease die within 5 years of the amputation.Citation12 Up to 85% of these amputations could have been delayed or prevented through patient education, lifestyle modification, early diagnosis, and endovascular intervention.Citation13–Citation19
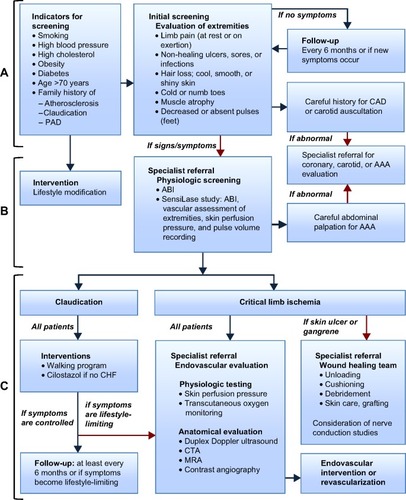
This paper describes an algorithm for the screening and ongoing diagnosis of PAD by a primary care physician in partnership with a team of specialty health care providers (). The goal of this algorithm is early diagnosis, appropriate referrals, and establishment of a multidisciplinary treatment strategy including early angiography, appropriate use of endovascular interventions, and ultimately decreased limb loss, morbidity, and mortality.
Figure 1 Algorithm for the diagnosis and management of patients with PAD.
Abbreviations: AAA, abdominal aortic aneurysm; ABI, ankle–brachial index; CAD, coronary artery disease; CHF, congestive heart failure; CTA, computed tomographic angiography; MRA, magnetic resonance angiography; PAD, peripheral arterial disease.
Initial screening
PAD screening should be performed in adults over the age of 50 years with risk factors such as high cholesterol, hypertension, diabetes, smoking, obesity, family history of atherosclerosis, PAD or claudication, neuropathic leg pain, or a non-healing wound or infection on an extremity, in addition to anyone over the age of 70 years.Citation10,Citation20 The initial screening for PAD includes an evaluation of the extremities and a detailed medical history (). Claudication occurs in approximately 60% of individuals with PAD,Citation21 and other signs and symptoms of PAD may be present in patients without pain. Critical limb ischemia can occur with or without prior occurrence of less severe symptoms and is present in 5%–10% of patients at the time of PAD diagnosis.Citation3 Patients with critical limb ischemia have a 20% mortality rate in the first year after presentation, adding to the urgency of diagnosis and treatment.Citation22 Coronary artery or cerebrovascular disease is present in 40%–60% of patients with PAD.Citation22 Consequently, all patients with PAD are high-risk patients.
Lower limb evaluation
Assess the lower limbs for signs and symptoms of PAD including: leg pain (at rest or on exertion); non-healing ulcers, sores, or infections; hair loss; cool, smooth or shiny skin; muscle atrophy; decreased or absent pulses (feet); and cold or numb toes.Citation21,Citation23 Claudication includes pain, ache, or cramp in the buttock, hip, thigh, or calf and, in 10%–35% of PAD patients, occurs only during exercise such as walking and resolves within 10 minutes of resting.Citation3 Claudication is atypical in 40%–50% of PAD patients, such that leg pain does not resolve at rest and/or the pain begins while the patient is at rest.Citation3 Comorbidities can influence the experience of leg pain and further complicate diagnosis.Citation24
Careful medical history for coronary or carotid artery disease
Risk factors for coronary artery disease include those for PAD as well as radiation therapy to the chest, physical inactivity, and stress. Intermittent pain, pressure or tightness of the chest, and shortness of breath on exertion are symptoms of coronary artery disease. Risk factors for carotid artery disease include those for PAD as well as physical inactivity and sleep apnea. There are no signs or symptoms of carotid artery disease until a stroke or transient ischemic attack occurs, which causes sudden numbness, weakness, dizziness, severe headache, difficulty speaking, and/or trouble seeing. Carotid auscultation may reveal bruits suggestive of carotid artery disease.
All screened individuals should be provided with education regarding lifestyle modifications to prevent or delay progression of PAD (). If the patient has signs or symptoms of PAD, then the individual should undergo physiological screening with an endovascular interventionalist or vascular specialist. If the screening evaluation for coronary or carotid artery disease is suggestive, then the patient should be referred to a cardiologist or vascular specialist for further evaluation and treatment.
Table 1 Lifestyle modifications
Physiological screening
Physiological screening () should be performed in all patients with any signs or symptoms of PAD and must include an ankle–brachial index (ABI). Clinics with a SensiLase® system may conduct other assessments with the ABI. Clinical sensory testing should also be performed. Detailed descriptions of each assessment are provided in Anderson et alCitation25 and a guideline to differential diagnoses for intermittent claudication may be found in the practice guidelines for atherosclerotic occlusive disease of the lower extremities from the Society for Vascular Surgery.Citation5
The ABI compares the systolic blood pressure of the ankle with that of the arm both at rest and after exercise. PAD generally produces discrepancies between the blood pressure of the ankles and that of the arms. If an ABI is 0.9 or less at rest, or greater than 1.3, the patient should be referred to a specialist familiar with endovascular intervention for a complete endovascular evaluation. This test is not reliable in patients with long-term diabetes or advanced renal disease, who may have severely calcified arteries, preventing an accurate result reflective of the disease.Citation26,Citation27 The SensiLase study provides an ABI as well as a noninvasive upper and lower extremity vascular assessment including skin perfusion pressure and pulse volume recording.Citation6 Quantitative clinical sensory testing assesses nerve sensitivity with regard to hot, cold, and vibration, quantifying abnormalities in pain and sensory pathways that can suggest deafferentation and/or sensitization.Citation28
Careful abdominal palpation and auscultation should be performed for abdominal aortic aneurysm. Symptoms of an abdominal aortic aneurysm include abdominal or back pain as well as a pulsating feeling near the navel. Palpation of the abdomen for a pulsating bulge may reveal an aortic aneurysm, and auscultation may reveal a bruit. Smoking and family history are the most common risk factors for abdominal aortic aneurysm and a history of vasculitis is a strong predictor. If the evaluation is suggestive of abdominal aortic aneurysm, then the patient is referred to a cardiologist or vascular specialist for further evaluation and treatment.
If physiological screening results are not suggestive and there are no symptoms, the individual may be re-evaluated every 6 months or sooner if symptoms arise. If the patient has claudication but other tests are not suggestive, then a walking program or supervised exercise program and anti-platelet medication may be added to the lifestyle modifications (). Many individuals require an organized program or trainer while others do well with a timer or chart. In patients diagnosed with PAD, aspirin (starting at 75–100 mg once daily) or other antiplatelet medications such as clopidogrel (75 mg once daily) may be prescribed, if not contraindicated, for preventing stroke, myocardial infarction, and death. In patients who do not have congestive heart failure, cilostazol, an antiplatelet and vasodilator agent, may be prescribed.Citation29 These individuals should be followed on a 6-monthly basis.
If the patient has symptoms that are lifestyle-limiting or the results of any evaluations are suggestive, then the individual is considered to be at high risk for critical limb ischemia and should undergo physiologic testing with an endovascular interventionalist or vascular specialist. Early intervention with endovascular techniques can provide functional improvement and increase quality of life.Citation2 The combination of an exercise program and endovascular intervention has shown better results than either of these alone in patients with intermittent claudication.Citation2,Citation30
Endovascular evaluation
Endovascular evaluation should be performed when claudication or other symptoms of PAD are lifestyle-limiting or critical limb ischemia is suspected (). Endovascular evaluation includes physiological testing (skin perfusion pressure and transcutaneous oxygen monitoring) as well as anatomic evaluation (duplex Doppler ultrasound, various types of angiography).Citation9,Citation21,Citation23,Citation25 These assessments provide verification of PAD, location of arteries that may be blocked, and the level of severity of the disease.Citation5,Citation26 In a study of over one million Medicare inpatients with critical limb ischemia, an angiogram alone was found to reduce the odds of amputation by 90%.Citation31 The appropriate type of angiogram can pinpoint the vascular targets for procedural treatment.Citation32 Selection of the imaging study is based on availability and expertise in the local region, patient characteristics, and potential treatment options for a given patient. Generally, the least invasive tests are conducted first. The results of these tests will direct the intervention(s) most appropriate for the patient. Referral to a specialist familiar with endovascular intervention should happen prior to these tests, allowing the specialist to conduct and interpret the tests appropriate to the individual. The results of the tests should be shared with the multidisciplinary team, and a coordinated treatment and surveillance plan should be developed.
Duplex Doppler ultrasound
Duplex Doppler ultrasound is the most important evaluation for confirming a diagnosis of PAD and assessing the severity of arterial stenosis. It combines hemodynamic evaluation with imaging and provides information on pressure and flow.Citation5,Citation25
Magnetic resonance angiography
Magnetic resonance angiography and computed tomographic angiography are imaging studies, and one of these is the next step after duplex Doppler ultrasound in planning an intervention for PAD. Magnetic resonance angiography is prohibited in patients with pacemakers or numerous other implantable devices.Citation5,Citation25
Computed tomographic angiography
Computed tomographic angiography is an imaging study considered to be the next step after duplex Doppler ultrasound in planning an intervention for PAD. Generally, image clarity is improved over magnetic resonance angiography; however, the contrast material interacts with arterial calcification, producing artifacts that can interfere with image quality. In addition, an allergy to the contrast material may be present, and this must be ruled out prior to use. Consequently, contrast angiography may be unsuitable in some patients.Citation5,Citation25
Contrast or catheter angiography
This is considered the gold standard for evaluating arterial anatomy due to the superior image quality. Although more invasive, catheter angiography is essential for revascularization planning. However, similar issues exist with regard to injecting contrast material, and arterial access site complications may present a substantial risk to some patients.Citation5,Citation25 Angiographic testing may not be considered in planning the care of the patient with PAD by some practitioners due to the risk of contrast-induced nephropathy caused by iodinated contrast. Contrast-induced nephropathy is defined as an increase of 25% or more, or an absolute increase of 0.5 mg/dL or more, in serum creatinine from the baseline value at 48–72 hours following exposure to contrast medium.Citation33 Mehran and Nikolsky developed a simple risk scoring system for evaluating the risk of contrast-induced nephropathy that can help identify when alternatives to contrast medium should be used.Citation34 An alternative to iodinated contrast is carbon dioxide for angiographic assessment and interventional treatment of PAD. With the use of a commercially available system, carbon dioxide provides high quality imaging of the peripheral vasculature. Gaseous carbon dioxide displaces the blood, then is rapidly dissolved in the blood and excreted within several breaths via the lungs. Since there is no increased risk for contrast-induced nephropathy without the use of iodinated contrast, angiography using carbon dioxide is a viable option for patients with PAD.Citation35
Skin perfusion pressure test
The skin perfusion pressure test measures tissue perfusion (microcirculation) and reflects the metabolic state of the lower limbs for diagnosis of critical limb ischemia as well as giving an indication of wound healing and severity of PAD. A skin perfusion pressure measurement greater than 40 mmHg predicts sufficient blood flow for wound healing.Citation36
Transcutaneous oxygen monitoring
This noninvasive test measures tissue perfusion (microcirculation), reflects the metabolic state of the lower limbs for diagnosis of critical limb ischemia, and is a predictor of wound healing. Generally, a transcutaneous oxygen measurement over 30 mmHg predicts sufficient blood flow for wound healing. The occasional patient will experience sufficient pain during this test as to prevent completion of the test.Citation36
Podiatry/wound healing
Patients with non-healing ulcers or infections on their feet should be referred to a podiatrist or wound healing team for complete foot care and regular follow-up (). Skin ulcers often go undetected in individuals with a loss of feeling in their feet and limited flexibility to observe foot health. Patient education, regular foot care, and checkups have been shown to save limbs. The Comprehensive Diabetic Foot Exam program of the American Diabetes Association screens diabetic patients with no obvious wounds, which allows prevention programs and intervention to occur earlier.Citation37
Wounds may require unloading, cushioning, debridement, and skin grafting by a wound care team or podiatrist. Nerve conduction studies may be useful. Non-healing wounds on an extremity may be treated with a prolonged duration of antibiotics and/or hyperbaric therapy; however, these therapies are only effective if the circulation of the injured extremity is sufficient. Thus, knowledge of circulatory status is mandatory for treatment of ulcers and non-healing wounds, and yet some patients are treated with these therapies with no assessment of the foot.Citation38 A multidisciplinary team approach to foot and wound care will ensure that wounds are prevented, wounds are treated properly, and blood flow to and within the foot is monitored. A significant decrease in amputation rates will rely on a team approach.Citation39
Management of PAD
Recent advances in endovascular interventions have provided numerous limb-sparing options for the treatment of PAD. Improvements in technique and equipment have expanded the use of endovascular interventions to more severe patients as well as less severe cases.Citation40 However, utilization of these interventions varies considerably according to region and the health of the individual.Citation7 Comorbid conditions, such as arthritis, spinal disease, neuropathy, diabetes, and spinal stenosis can impact the presentation of PAD and influence the selection of treatment.Citation24 Patients with comorbidities that prevent them from being candidates for surgical procedures may be able to undergo endovascular interventions, and yet these limb-sparing procedures may be overlooked. Patients with decreased mobility at the time of diagnosis may also be overlooked for limb-sparing interventions.
Critical limb ischemia and lifestyle-limiting claudication can be treated with an endovascular intervention such as angioplasty to open the artery or with vascular surgery to bypass the arterial blockage. In some patients, a combination of endovascular and surgical interventions may be beneficial. Angioplasty includes the use of a catheter and balloon to expand a blocked or narrow artery; placement of stents, which may be medicated, within the artery; bypass grafting surgery to shunt blood around the blockage using an artery from elsewhere in the patient’s body; and atherectomy to reduce the plaque build-up within the artery using a cutting device or laser. Both percutaneous transluminal intervention and bypass surgery have shown a limb salvage rate of 80% at 3 years.Citation41
A multidisciplinary team provides an optimal approach to the diagnosis and treatment of PAD and the prevention of unnecessary amputations. The PAD management team may include the family practice and primary care physician, an internist, diabetologist, endocrinologist, hypertension and lipid specialist, endovascular interventionalist, vascular surgeon, orthopedist, neurologist, nurse practitioner, podiatrist, wound healing expert, smoking cessation expert, and others. Each patient has a unique combination of symptoms, comorbidities, genetics, and vasculature, and over time comorbidities change, the patient ages, and the disease progresses, revealing new symptoms and pathophysiologies. Prompt referral and due consideration of the particular details of a given patient will encourage appropriate and effective management with the greatest chance of longevity, quality of life, and prevention of unnecessary amputations.
Acknowledgments
The authors acknowledge the collaborative support of the Horizons International Peripheral Group and the contributions of Leslie Todd, Eminence Clinical Research Inc, to this manuscript.
Disclosure
Funding for the coordination of this manuscript and assistance with medical writing was provided by Covidien. This research was presented in part at the New Cardiovascular Horizons Annual Conference, New Orleans, LA, USA, June 5–7, 2013. The authors report no other conflicts of interest.
References
- HiattWRGoldstoneJSmithSCJrAtherosclerotic Peripheral Vascular Disease Symposium II: nomenclature for vascular diseasesCirculation20081182826282919106403
- JonesWSSchmitKMVemulapalliSTreatment strategies for patients with peripheral artery diseaseComparative Effectiveness Review No. 118. (Prepared by the Duke Evidence-based Practice Center under Contract No. 290-2007-10066-I.) AHRQ Publication No. 13-EHC090-EFRockville, MD, USAAgency for Healthcare Research and Quality52013 Available from: www.effectivehealthcare.ahrq.gov/reports/final.cfmAccessed April 21, 2015
- HirschATHaskalZJHertzerNRACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic)Circulation2006113e463e65416549646
- PandeRLPerlsteinTSBeckmanJACreagerMASecondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004Circulation2011124172321690489
- Society for Vascular Surgery Lower Extremity Guidelines Writing Group; ConteMSPomposelliFBClairDGSociety for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudicationJ Vasc Surg2015613 Suppl2S41S25638515
- SerenaTSensiLase studycast system: a platform for critical limb diagnostics and electronic referral programAdv Wound Care (New Rochelle)2012114214524527295
- GoodneyPPTravisLLNallamothuBKVariation in the use of lower extremity vascular procedures for critical limb ischemiaCirc Cardiovasc Qual Outcomes201259410222147886
- AllieDEHebertCJLirtzmanMDCritical limb ischemia: a global epidemic. A critical analysis of current treatment unmasks the clinical and economic costs of CLIEuro Intervention200516069
- RogerVLGoASLloyd-JonesDMHeart Disease and Stroke Statistics. 2011 update: a report from the American Heart AssociationCirculation2011123e18e20921160056
- FowkesFGRudanDRudanIComparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysisLancet20133821329134023915883
- Ziegler-GrahamKMacKenzieEJEphraimPLTravisonTGBrookmeyerREstimating the prevalence of limb loss in the United States: 2005 to 2050Arch Phys Med Rehabil20088942242918295618
- RobbinsJMStraussGAronDLongJKubaJKaplanYMortality rates and diabetic foot ulcersJ Am Podiatr Med Assoc20089848949319017860
- FrykbergRGDiabetic foot ulcers: pathogenesis and managementAm Fam Phys20026616551662
- MaloneJMSnyderMAndersonGBernhardVMHollowayGABuntTJPrevention of amputation by diabetic educationAm J Surg19891585205242589581
- EdmondsMDiabetic foot ulcers: practical treatment recommendationsDrugs20066691392916740006
- SandersLJRobbinsJMEdmondsMEHistory of the team approach to amputation prevention: pioneers and milestonesJ Vasc Surg2010523 Suppl3S16S20804927
- OrtegonMMRedekopWKNiessenLWCost-effectiveness of prevention and treatment of the diabetic footDiabetes Care20042790190715047646
- Centers for Disease Control and PreventionNational Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011 Available from: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdfAccessed August 27, 2014
- DriverVRMadsenJGoodmanRAReducing amputation rates in patients with diabetes at a military medical center: the limb preservation service modelDiabetes Care20052824825315677774
- AllisonMAHoEDenenbergJOEthnic-specific prevalence of peripheral arterial disease in the United StatesAm J Prevent Med200732328333
- CreagerMALoscalzoJVascular diseases of the extremitiesFauciASBraunwaldEKasperDLHarrison’s Principles of Internal Medicine17th edNew York, NY, USAMcGraw Hill2008
- NorgrenLHiattWRDormandyJANehlerMRHarrisKAFowkesFGRon behalf of the TASC II Working GroupInter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II)J Vasc Surg2007451 Suppl S5A67A
- RookeTWWennbergPWDiagnosis and management of diseases of the peripheral arteries and veinsWalshRASimonDIHoitBDHurst’s The Heart12th edNew York, NY, USAMcGraw Hill2007
- McDermottMMGreenlandPLiuKLeg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairmentJAMA20012861599160611585483
- AndersonJLHalperinJLAlbertNMManagement of patients with peripheral artery disease (Compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice GuidelinesCirculation20131271425144323457117
- CaoPEcksteinHHDe RangoPChapter II: Diagnostic methodsEur J Vasc Endovasc Surg201142Suppl 2S13S3222172470
- AboyansVCriquiMHAbrahamPMeasurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart AssociationCirculation20121262890290923159553
- LangPMSchoberGMRolkeRSensory neuropathy and signs of central sensitization in patients with peripheral arterial diseasePain200612419020016716518
- ReillyMPMohlerER3rdCilostazol: treatment of intermittent claudicationAnn Pharmacother200135485611197586
- FransFABipatSReekersJASystematic review of exercise training or percutaneous transluminal angioplasty for intermittent claudicationBr J Surg201299162821928409
- HenryAJHeveloneNDBelkinMNguyenLLSocioeconomic and hospital-related predictors of amputation for critical limb ischemiaJ Vasc Surg20115333033921163610
- PalenaLMBroccoEManziMThe clinical utility of below-the-ankle angioplasty using “transmetatarsal artery access” in complex cases of CLICatheter Cardiovasc Interv20148312312923696069
- YamadaTOhtaTIshibashiHClinical reliability and utility of skin perfusion pressure measurement in ischemic limbs – comparison with other noninvasive diagnostic methods200847318323
- MehranRNikolskyEContrast-induced nephropathy: definition, epidemiology, and patients at riskKidney Int Suppl2006100S11S1516612394
- MehranRAymongEDNikolskyEA simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention development and initial validationJ Am Coll Cardiol2004441393139915464318
- MicariASbarzagliaPMeeksMENew imaging modalities in peripheral interventionsEur Heart J Suppl201517Suppl AA18A22
- BoultonAJArmstrongDGAlbertSFComprehensive foot examination and risk assessment: A report of the Task Force of the Foot Care Interest Group of the American Diabetes Association, with endorsement by the American Association of Clinical EndocrinologistsDiabetes Care2008311679168518663232
- YamadaTOnishiKUtsunomiyaMNakamuraMOur treatment strategy for critical limb ischemiaInt J Vasc Med2013201343747124386568
- AlaviASibbaldRGMayerDDiabetic foot ulcers: Part I. Pathophysiology and preventionJ Am Acad Dermatol2014701.e1e1824355275
- JaffMRCahillKEYuAPBirnbaumHGEngelhartLMClinical outcomes and medical care costs among Medicare beneficiaries receiving therapy for peripheral arterial diseaseAnn Vasc Surg20102457758720579582
- SchampKBMeerwaldtRReijnenMMGeelkerkenRHZeebregtsCJThe ongoing battle between infrapopliteal angioplasty and bypass surgery for critical limb ischemiaAnn Vasc Surg2012261145115322835563
