Abstract
Background
It has been suggested that targeted human immunodeficiency virus (HIV) testing programs are cost-effective in populations with an HIV prevalence >0.1%. Several indicator diseases are known to be associated with increased risk of HIV infection, but estimates of HIV frequency in persons with relevant indicator diseases are nonexistent.
Methods
In a nationwide population-based cohort study encompassing all Danish residents aged 20–60 years during 1994–2013, we estimated the 5-year risk of an HIV diagnosis (FYRHD) after a first-time diagnosis of 147 prespecified potential indicator diseases. To estimate the risk of HIV diagnosis in the general population without any indicator diseases, we calculated the FYRHD starting at age 25, 35, 45, and 55 years.
Results
The risk in the male general population was substantially higher than the female general population, and the risk was lower in the older age categories. Individuals of African origin had a higher FYRHD than individuals of Danish origin. A number of diseases were identified with a FYRHD >0.1%, with infectious diseases, such as syphilis, hepatitis, and endocarditis, associated with a particularly high FYRHD. Other potential indicator diseases, such as most urologic, nephrologic, rheumatologic, and endocrine disorders were generally associated with a low FYRHD.
Conclusion
Our study identified a large number of indicator diseases associated with a FYRHD >0.1%. These data can be used as a tool for planning targeted HIV screening programs.
Introduction
Highly active antiretroviral therapy (HAART) has substantially reduced morbidity and mortality for human immunodeficiency virus (HIV)-infected individuals,Citation1 and the optimal timing of HAART initiation has recently been determined.Citation2 It is important to diagnose HIV early, as immunodeficiency resulting from late presentation is associated with increased mortalityCitation3,Citation4 and delaying HAART initiation to a very advanced stage of immunodeficiency is associated with increased mortality.Citation2 Moreover, early diagnosis and treatment decreases the risk of disease transmission.Citation5,Citation6
Several HIV case-finding strategies have been proposed to enhance early diagnosis.Citation7 While universal testing is recommended by the Center for Diseases Control and Prevention and the US Preventive Services Task Force,Citation8,Citation9 targeted testing was advocated by the “HIV in Europe 2007 Conference”.Citation10 It has been estimated that HIV testing in populations in whom the HIV prevalence is 0.1% or higher is cost-effective compared to interventions for other chronic conditions.Citation11,Citation12 A Danish case–control study identified several diseases associated with an increased relative risk of subsequent HIV diagnosis,Citation13 but due to its case–control design, it could not provide absolute risk estimates to guide targeted testing policies. The HIV indicator diseases across Europe study I demonstrated that patients with one of the eight preselected indicator diseases/conditions had an HIV prevalence of >0.1%, suggesting indicator disease-guided testing as a feasible way to identify occult HIV cases.Citation14
Ideally, nationwide estimates of HIV prevalence in persons with relevant indicator diseases should be obtained to guide HIV testing policies. However, as this is not possible, other measures have to be applied. In the present study, we used the high-quality national Danish registries to estimate the 5-year risk of HIV diagnosis (FYRHD) after a diagnosis of 147 prespecified indicator diseases.
Methods
We conducted a nationwide population-based cohort study encompassing all Danish residents aged 20–60 years during the period 1994–2013. The study outcome was FYRHD after a first-time diagnosis of 147 prespecified potential indicator diseases.
Setting
Among Denmark’s population of 5.5 million persons, an estimated 5,500 persons are infected with HIV,Citation15 yielding a prevalence of ~0.1%. Denmark’s tax-funded health care system provides treatment (including antiretroviral treatment) free of charge to all HIV-infected residents. During the period 1990–2012, 6,358 newly diagnosed HIV-infected patients were reported to the Danish authorities.Citation16
Data sources
We used the unique ten-digit personal identification number assigned to all Danish residents at birth or upon immigration to avoid multiple registrations and to track individuals in national registries. We extracted data from the Danish Civil Registration System, a national registry containing information on vital status, country of birth, and dates of immigration and emigration.Citation17 We also obtained information from the Danish National Patient Registry (DNPR) on all diagnoses made during hospital outpatient and emergency room contacts and during hospital stays in Danish nonpsychiatric hospitals. These diagnoses are coded by the attending physician according to the International Classification of Diseases, 10th revision.Citation18 From the DNPR, we extracted the first date of HIV diagnosis and the first date of any of the indicator diseases, grouped as described in the paper by Søgaard OS et al.Citation13
Study population
We included all Danish residents who were between the ages of 20 and 60 years at any time during the study period (January 1, 1994 to December 31, 2012) and who were born in Denmark or on the African continent. This restriction in age category was chosen to reflect the primary age of acquiring HIV diagnoses in Denmark. Patients from non-Danish Western countries and “other” countries (~14% of the potential study population) were excluded, as they represent a heterogeneous population from countries with very different HIV prevalences.
Statistical analysis
Follow-up time was calculated from the first diagnosis date of any of the indicator diseases to the date of HIV diagnosis, emigration, death, or January 1, 2013, whichever occurred first. We computed the FYRHD and 95% confidence intervals, considering death as a competing risk.Citation19 For indicator diseases with no cases of HIV within 5 years of observation, we computed the upper confidence limit using the exact method based on a binomial distribution.Citation20 Analyses were stratified according to place of birth (Denmark or Africa), sex, and age on the diagnosis date of the indicator disease at 10-year intervals from ages 20 to 60 years. To estimate the risk of HIV diagnosis in the general population, we calculated the FYRHD starting at age 25, 35, 45, and 55 years for all strata.
SPSS version 19.0 (IBM Corporation, Armonk, NY, USA) and R software, version 2.14.2 (Vienna, Austria), were used to perform the analyses.
Ethics
According to Danish law, registry-based studies do not require approval from an ethics board. The data were anonymized by Statistics Denmark prior to analyses.
Results
We identified 4,169,600 persons who fulfilled the study inclusion criteria. Among these, 2,098,773 were males of Danish origin, 2,039,271 were females of Danish origin, and 31,556 were of African origin (both sexes). Total observation time was >56 million years. During follow-up, 4,162 persons were diagnosed with HIV infection, which is 79.9% of the total number of HIV diagnoses (n=5,211) made in Demark during the study period. Characteristics of the study population are provided in .
Table 1 Characteristics of the study population
FYRHD in patient categories defined by age and origin
The FYRHD in the Danish population at age 25, 35, 45, and 55 years is shown in . The risk in the male general population was substantially higher than in the female general population, and the risk was lower in the older age categories. For males aged 35 years, the FYRHD was close to 0.1% (FYRHD: 0.064%, 95% confidence interval: 0.058%–0.071%). Individuals of African origin aged 35 years had an FYRHD >1%. In general, individuals of African origin had an FYRHD >0.1%. There were a total of 588 (147×4) categories defined by indicator diseases and age categories. For the Danish population, females more often than males had 0 events of HIV diagnosis in these 588 categories within the 5 years of observation. For persons of African origin, the median time from immigration to HIV diagnoses was 1.60 years (IQR: 0.34–5.34 years).
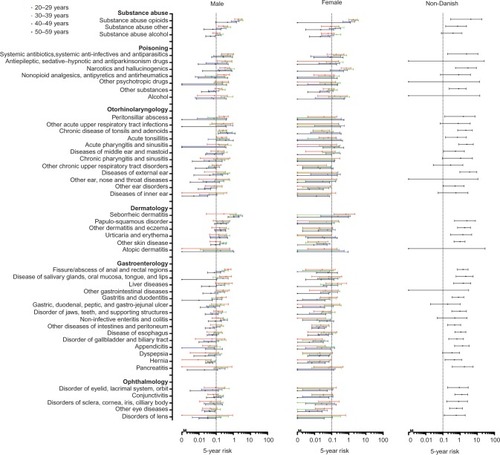
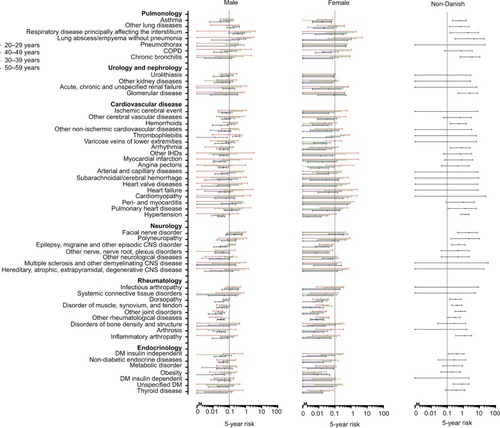
Figure 1 Five-year risk of human immunodeficiency virus in 147 indicator diseases for Danish males and females, and individuals of African origin.
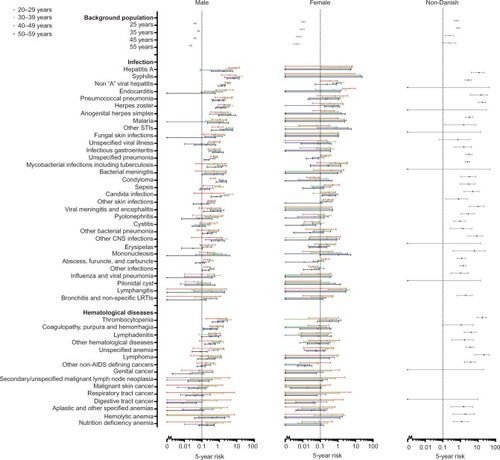
FYRHD in individuals of Danish origin
The FYRHD after a diagnosis of one of the predefined indicator diseases is illustrated in . For both sexes, almost all the potential indicator diseases in the infectious disease category were associated with an FYRHD >0.1% in one or more age categories.
Several hematological diseases, especially thrombocytopenia, were associated with an FYRHD >0.1%, particularly in males. Abuse of opioids was associated with an FYRHD >0.1% in both sexes and in all age categories, while for alcohol abuse, this was the case mainly in the groups aged 30–39 and 40–49 years.
Particularly in older males, gastrointestinal diseases were associated with an FYRHD >0.1%. Among females, the only gastrointestinal diseases associated with an FYRHD >0.1% were liver diseases.
In males, a number of skin diseases and ear, nose, and throat conditions were associated with an FYRHD >0.1%. The FYRHD was also close to or above 0.1% in males diagnosed with facial nerve disorder and neuropathy. Several vascular diseases also were associated with an FYRHD >0.1% in males. Except for asthma, pulmonary diseases were associated with an FYRHD >0.1% in one or more age and sex categories.
In some of the disease categories, such as urologic, nephrologic, rheumatologic and endocrine disorders, none or only a few indicator diseases were associated with an FYRHD >0.1%.
FYRHD in individuals of African origin
Individuals of African origin, irrespective of age category, had an FYRHD >0.1%. Furthermore, most disease categories were associated with an FYRHD >0.1% in individuals of African origin. Possible exceptions were cardiovascular, urologic, and nephrologic diseases, for which less than half of the categories were associated with an FYRHD >0.1%. Infectious diseases were associated with an especially high FYRHD.
Patient categories with a particularly high FYRHD
The ten combinations of indicator diseases, age, and sex with the highest FYRDH among individuals of Danish origin, as well as the ten indicator diseases with the highest FYRHD among individuals of African origin, are shown in .
Discussion
In this nationwide population-based cohort study encompassing the entire Danish population over a 19-year study period, we estimated the FYRHD following a diagnosis of one of 147 prespecified indicator diseases. A number of diseases were identified with an FYRHD >0.1%, with infectious diseases associated with a particularly high FYRHD.
Strengths
Central strengths of the study are its nationwide population-based design and long-term follow-up. To our knowledge, this study is the first to use the complete population of a nation to estimate risk of HIV diagnosis subsequent to a hospital contact for indicator diseases covering almost the whole spectrum of human diseases. The study also benefitted from the high quality of Danish registries.Citation17,Citation21–Citation23
Limitations
Our study has some limitations. First, in an ideal setting, all patients should have been HIV tested at diagnosis of an indicator disease, but this was not feasible in our population-based nationwide study. Instead, we estimated risk of an HIV diagnosis in the 5-year period following diagnosis of one of the 147 indicator diseases. Although the FYRHD could both overestimate and underestimate the true HIV prevalence, we assume that it is a good proxy for HIV infection. Any concerns arising from this approach, especially that not all patients are tested for HIV within the 5-year period and that FYRHD captures both prevalent and incident cases, are likely to be outweighed by the completeness of the population-based nationwide data used. Second, we used a hospital-based registry to identify indicator diseases. Some diseases, such as herpes zoster,Citation24 are primarily diagnosed and treated by general practitioners. Cases leading to hospitalization are usually more severe or complicated than those diagnosed and treated exclusively by general practitioners. Therefore, our estimates are more likely to reflect the risk of HIV infection in patients diagnosed with indicator diseases at hospital admission than during a consultation with a general practitioner. Furthermore, there might be a diagnostic delay from the time of diagnosis by general practitioners and the time of diagnoses in the hospital-based registry. Third, we did not account for indicator diseases possibly appearing in clusters, as this would result in very complex statistical models that would be difficult to interpret in daily clinical practice. Fourth, it must be noted that our study is based on data from a publicly funded health care system. Denmark’s hospital referral pattern might differ from that of countries with other health care systems. If the referral pattern does differ in other countries, the FYRHD associated with the 147 potential indicator diseases might also differ. Fifth, the FYRHD is highly dependent on the risk of HIV infection in the population under study, and HIV incidence is rather low in Denmark. Therefore, our results cannot readily be generalized to non-Western countries. Sixth, in the beginning of the study period, indicator diseases, to some extent, were a mixture of prevalent and incident diseases, as DNPR did not include hospital outpatient and emergency room contacts until 1995.
Finally, because FYRHD varies considerably according to age, sex, and country of origin, we stratified the analyses on these characteristics. In general, we lost statistical precision by providing estimates by population category.
Possible explanations for our findings
The increased FYRHD for some diseases, such as syphilis, condyloma, non-A hepatitis, or endocarditis, can be explained by shared routes of infection. The HIV risk associated with other diseases, such as malaria and hepatitis A, might mark immigration from or tourism to countries with a high prevalence of HIV infection. Increased susceptibility caused by the HIV infection and resulting immunodeficiency may lead to many of the AIDS-defining diseases. The association with HIV risk might also represent misdiagnosis of the indicator disease. For instance, Epstein–Barr virus-induced mononucleosis typically has a clinical presentation characterized by fever and lymphadenopathy, and the diagnosis is not always confirmed by virological testing. If a manifestation of acute HIV infection is diagnosed as mononucleosis, the FYRHD associated with mononucleosis will be high. Similar mechanisms likely explain the association of FYRHD with other indicator diseases, such as viral meningitis and lymphadenitis.
Policy implications
Our study has important implications for policies for HIV testing. We identified a number of indicator diseases, diagnosed in a nationwide population-based hospital system, which are associated with an FYRHD >0.1%. A 0.1% prevalence seems to be a reasonable threshold for cost-effectiveness, compared to other interventions in the health care system, and is associated with earlier HIV diagnosis.Citation11,Citation12,Citation14 Infectious diseases, in particular, seem to be associated with elevated FYRHD, so one simple recommendation is to perform HIV testing on all patients hospitalized for an infectious disease. As these diseases account for ~40% of all admissions to acute medical units,Citation25 they could be the target of a focused program. The rather high FYRHD found in certain categories of the background population, especially persons of African origin and Danish males aged 35 years, suggests that it also might be cost-effective to introduce universal testing in these groups irrespective of presentation of indicator diseases.
It must be emphasized that some diseases, such as most urologic, nephrologic, rheumatologic, and endocrine disorders, had an FYRHD <0.1%. Therefore, these diagnoses should not necessarily lead to an HIV test in the absence of other risk factors. When using FYRHD as a surrogate for prevalence in deciding which categories of patients to screen for HIV, it is important to consider that the FYRHD includes both cases of unknown HIV infection at the time of indicator disease diagnosis (prevalent cases) and HIV infections occurring during the 5-year follow-up period (incident cases). Incident cases would be missed if screening is performed close to indicator disease diagnosis. Therefore, screening ideally should be repeated after 5 years to ensure identification of all cases. Furthermore, although not supported by data in our study, persons with ongoing risk-taking behavior should receive counseling about safe sex practices and regular HIV testing.
Conclusion
Our study identified a large number of indicator diseases associated with an FYRHD >0.1%. These data can be used as a tool for planning targeted HIV screening programs.
Acknowledgments
NO has received research funding from Bristol-Myers Squibb, GlaxoSmithKline, Boehringer Ingelheim and Gilead.
This work was supported by Preben og Anne Simonsens Foundation, NOVO Nordisk Foundation, Rigshospitalet, Copenhagen University, The Danish AIDS Foundation and The Augustinus Foundation.
The funding sources had no role in data collection, analysis, or interpretation; the study design; the writing of the manuscript, any aspect pertinent to the study or the decision to submit it for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
- LohseNHansenA-BEPedersenGSurvival of persons with and without HIV infection in Denmark, 1995–2005Ann Intern Med20071462879517227932
- LundgrenJDBabikerAGGordinFINSIGHT START Study GroupInitiation of antiretroviral therapy in early asymptomatic HIV infectionN Engl J Med2015373979580726192873
- NakagawaFLodwickRKSmithCJProjected life expectancy of people with HIV according to timing of diagnosisAIDS201226333534322089374
- MocroftALundgrenJDSabinMLRisk factors and outcomes for late presentation for HIV-positive persons in Europe: results from the Collaboration of Observational HIV Epidemiological Research Europe Study (COHERE)PLoS Med2013109e100151024137103
- MarksGCrepazNJanssenRSEstimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USAAIDS200620101447145016791020
- VolzEMIonidesERomero-SeversonEOBrandtM-GMokotoffEKoopmanJSHIV-1 transmission during early infection in men who have sex with men: a phylodynamic analysisPLoS Med20131012e100156824339751
- BayerROppenheimerGMRoutine HIV screening – what counts in evidence-based policy?N Engl J Med2011365141265126821991946
- BransonBMHandsfieldHHLampeMARevised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settingsMMWR Recomm Rep200655RR-1411716988643
- MoyerVAU.S. Preventive Services Task Force*Screening for HIV: U.S. Preventive Services Task Force Recommendation StatementAnn Intern Med20131591516023698354
- GazzardBClumeckNd’Arminio MonforteALundgrenJDIndicator disease-guided testing for HIV – the next step for Europe?HIV Med20089Suppl 2344018557871
- SandersGDBayoumiAMSundaramVCost-effectiveness of screening for HIV in the era of highly active antiretroviral therapyN Engl J Med2005352657058515703422
- PaltielADWeinsteinMCKimmelADExpanded screening for HIV in the United States – an analysis of cost-effectivenessN Engl J Med2005352658659515703423
- SøgaardOSLohseNØstergaardLMorbidity and risk of subsequent diagnosis of HIV: a population based case control study identifying indicator diseases for HIV infectionPLoS One201273e3253822403672
- SullivanAKRabenDReekieJFeasibility and effectiveness of indicator condition-guided testing for HIV: results from HIDES I (HIV indicator diseases across Europe study)PLoS One201381e5284523341910
- SundhedsstyrelsenVejledning Om HIV (Human Immundefekt Virus) Og Hepatitis B Og C Virus Available from: http://sundhedsstyrelsen.dk/publ/Publ2013/11nov/HIVHEPvejl2udg.pdfAccessed June 1, 2015
- Statens Serum InstitutHIV 2012 Available from: http://www.ssi.dk/Aktuelt/Nyhedsbreve/EPI-NYT/2013/Uge%2044%20-%202013.aspxAccessed June 1, 2015
- SchmidtMPedersenLSørensenHTThe Danish Civil Registration System as a tool in epidemiologyEur J Epidemiol201429854154924965263
- LyngeESandegaardJLReboljMThe Danish National Patient RegisterScand J Public Health2011397 Suppl303321775347
- JepsenPVilstrupHAndersenPKThe clinical course of cirrhosis: The importance of multi-state models and competing risks analysisHepatology201562129230225376655
- JovanovicBDZalenskiRJSafety evaluation and confidence intervals when the number of observed events is small or zeroAnn Emerg Med19973033013069287891
- FrankLEpidemiology. When an entire country is a cohortScience200028754622398239910766613
- AndersenTFMadsenMJørgensenJMellemkjoerLOlsenJHThe Danish National Hospital Register. A valuable source of data for modern health sciencesDan Med Bull199946326326810421985
- ObelNReinholdtHOmlandLHEngsigFSørensenHTHansenA-BERetrivability in The Danish National Hospital Registry of HIV and hepatitis B and C coinfection diagnoses of patients managed in HIV centers 1995–2004BMC Med Res Methodol2008812518439245
- PierikJGJGumbsPDFortanierSACVan SteenwijkPCPostmaMJEpidemiological characteristics and societal burden of varicella zoster virus in the NetherlandsBMC Infect Dis20121211022574722
- HenriksenDPNielsenSLLaursenCBHallasJPedersenCLassenATHow well do discharge diagnoses identify hospitalised patients with community-acquired infections? – A validation studyPLoS One201493e9289124663388
