Abstract
Objective
To understand the severity and potential impact of heterogeneity in definitions of hypoglycemia used in diabetes research, we aimed to review the hypoglycemia definitions adopted in randomized controlled trials (RCTs).
Methods
We reviewed 109 RCTs included in the Canadian Agency for Drugs and Technologies in Health reports for the second- and third-line therapy for the patients with type 2 diabetes (T2D).
Results
Nearly 60% (n=66) of the studies reviewed presented the definitions for overall hypoglycemia, and another 20% (n=22) of the studies reported the results for hypoglycemia but did not report a definition. Among these 66 studies, only 9 (14%) followed the American Diabetes Association/European Medicines Agency specified guidelines to define hypoglycemia, with an exact threshold of plasma glucose ≤3.9 mmol/L. Fifty-two of the 66 studies (79%) used a threshold considerably lower than the recommended ≤3.9 mmol/L, and 16 studies used a threshold between 3.8 and 4.0 mmol/L. The proportion of the trials that used a cutoff value of <3.1 mmol/L appeared to be slightly similar among the more commonly used non-insulin treatments, GLP-1s (7 of 18 [39%]), thiazolidinediones (TZDs; 6 of 11 [55%]), DPP-4s (12 of 19 [64%]), and sulfonylureas (11 of 20 [55%]). Among trials with intermediate-long-acting insulins (neutral protamine Hagedorn insulin, detemir, glargine), 7 of 26 trials (27%) used a cutoff of <3.1 mmol/L. The definition of severe hypoglycemia was also subject to substantial heterogeneity, in both the utilized threshold and accompanying soft definitions.
Conclusion
This review demonstrates that substantial heterogeneity exists in the definition of overall, severe/major, and nocturnal hypoglycemia across RCTs investigating T2D interventions.
Introduction
Type 2 diabetes (T2D) is a chronic, progressive disease caused by various pathological mechanisms such as impaired insulin secretion, increased peripheral insulin resistance, and increased hepatic glucose production, resulting in increased concentrations of glucose in blood.Citation1 According to the International Diabetes Federation’s 2014 update, the global prevalence of T2D is nearly 8.3%, with an estimated 387 million people living with T2D. It is also predicted that going at the current rate of incidence, an additional 205 million people will be diabetic by 2035.Citation2 With the advent of newer therapies to control diabetes and an increasing evidence base suggesting the importance of glycemic control in diabetics, the assessment of the risk of hypoglycemia with diabetes treatments is gaining importance.
Hypoglycemia is a common side effect of diabetes therapy, resulting in a lack of adequate cerebral glucose supply, leading to a range of neurogenic and neuroglycopenic symptoms, which in turn can lead to death, if not treated on time.Citation3 Therefore, addressing the issue of hypoglycemia is a very important aspect in both diabetic care and research. The American Diabetes Association (ADA), Canadian Diabetes Association (CDA), and the European Medicines Agency (EMA) have proposed guidelines to define hypoglycemia and its severity, by using plasma glucose (PG) or blood glucose (BG) values or symptoms of the patient.Citation4–Citation6 Symptomatic hypoglycemia is a condition with PG £3.9 mmol/L (£70 mg/dL) accompanied by typical adrenergic symptoms (e.g., sweating, palpitations, trembling, tingling). Asymptomatic hypoglycemia results when the PG measured is £3.9 mmol/L (£70 mg/dL) but not accompanied by the typical symptoms. Severe hypoglycemia is an event requiring the assistance of another person to administer carbohydrate and glucagon or to take other corrective actions.Citation4–Citation6 Physiologically, the first responses to a lowered PG concentration of <3.9 mmol/L (<70 mg/dL) are release of counter regulatory hormones such as glucagon and epinephrine. PG values <3.3 mmol/L (<60 mg/dL) can lead to the onset of autonomic and neuroglycopenic symptoms, and PG values of <2.9 mmol/L (<50 mg/dL) can lead to cognitive dysfunction.Citation7 To our knowledge, other national or international guidelines have not attempted to define hypoglycemia to similar quantitative granularity.
Clinical trials and studies evaluating antihyperglycemic drugs are heterogeneous in their definitions and therefore may not fully align with the criteria established by ADA or EMA guidelines. The heterogeneity in the definitions of hypoglycemia adopted by the investigators conducting these clinical trials hinders the comparison of it as a safety outcome across clinical trials.Citation7,Citation8 To our knowledge, no studies have examined and quantified the heterogeneity in the definitions of hypoglycemia in clinical trials. Therefore, an examination of the extent to which important heterogeneity exists across the adopted definitions of hypoglycemia in clinical trials of diabetes interventions is necessary. Differences in the definitions of the hypoglycemia may lead to faulty estimates in the assessment of comparative safety of various antihyperglycemic agents. Additionally, any differences in the definitions will also pose a challenge for new drug approvals, when compared to standard treatments in practice. To understand the severity of such heterogeneity in diabetes research, we aimed to review the extent of heterogeneity in the hypoglycemia definitions adopted in a sample of randomized controlled trials (RCTs) representative for general health technology assessment (HTA) purposes. Namely, we examined the differences in the definitions of hypoglycemia in RCTs included in a Canadian Agency for Drugs and Technologies in Health (CADTH) report that studied only the second- and third-line treatments for T2D, the most commonly studied populations in HTA of antihyperglycemic agents.
Materials and methods
Literature review
RCTs included in the CADTH reports for second- and third-line therapy for patients with T2D were reviewed. Two reviewers independently extracted definitions of overall, major, minor, severe, and nocturnal hypoglycemia from a total of 109 studies (Supplementary material). Here, second-line and third-line treatment refers to patients no longer being able to maintain adequate glycemic control on metformin and metformin plus sulfonylurea, respectively. The trials considered for the review included the following treatment classes: sulfonylureas, DPP-4 inhibitors (DPP-4), GLP-1 receptor agonists (GLP-1), thiazolidinediones (TZDs), alpha-glucosidase inhibitors (AGIs), glinides, premixed insulins, neutral protamine Hagedorn insulin, insulin glargine, insulin detemir, insulin lispro, and human insulin.
Analysis
The cutoff values for BG/PG used to define overall and severe hypoglycemia were obtained for each of the included trials, and descriptive statistics of the distribution of use of these values were produced. All the hypoglycemia definitions that were not severe/major and not nocturnal were included under overall hypoglycemia. BG/PG values were stratified into 3 categories, >3.8, <3.3–<3.8, and <3.1 mmol/L. To examine whether the use of cutoff values is different across treatment classes of oral antihyperglycemic agents, as well as injectables such as GLP-1 receptor agonists and insulins, the proportion of trials falling within each of the 3 categories were further stratified by treatment class and plotted accordingly. The definitions used for hypoglycemia in the individual RCTs were compared to the definitions of hypoglycemia according to the ADA and the EMA, in particular, examining whether guidelines have been used to define hypoglycemia. Heterogeneity in the definition of nocturnal hypoglycemia, and severe hypoglycemia, was also examined, and a select set of severe hypoglycemia definitions were reported as a representative sample. Again, where possible, comparisons to the ADA and EMA guidelines were performed.
Results
Overall hypoglycemia
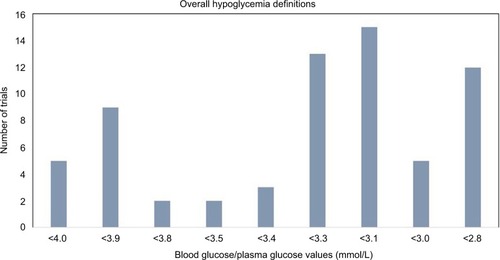
A total of 109 studies were reviewed from the reports of CADTH second-line and third-line treatments for T2D. Nearly 60% (n=66) of the studies reviewed presented the definitions for overall hypoglycemia, and another 20% (n=22) of the studies reported the results for hypoglycemia but did not report a definition. shows the number of studies with each of the cutoff value of BG/PG for the definition of overall hypoglycemia. Among these 66 studies, only 9 (14%) followed the ADA/EMA specified guideline to define hypoglycemia with an exact threshold of PG ≤3.9 mmol/L (16 studies used a threshold between 3.8 and 4.0 mmol/L). Fifty-two of the 66 studies (79%) used a threshold considerably lower than the recommended ≤3.9 mmol/L. Among the employed thresholds, <3.3 mmol/L (n=13), <3.1 mmol/L (n=15), and <2.8 mmol/L (n=12) were the most common.
Overall hypoglycemia according to treatment classes
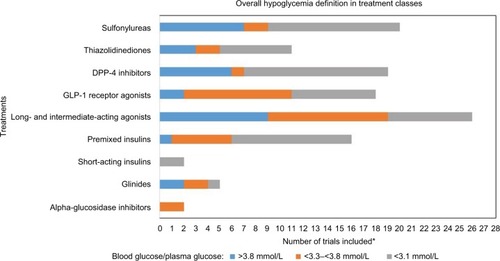
displays the distribution of employed hypoglycemia thresholds across the diabetes treatment classes. The proportion of the trials that used a cutoff value of <3.1 mmol/L appeared to be slightly similar among the more commonly used non-insulin treatments, GLP-1s (7 of 18 [39%]), TZDs (6 of 11 [55%]), DPP-4s (12 of 19 [64%]), and sulfonylureas (11 of 20 [55%]). However, the proportion of GLP-1 trials that used a cutoff between >3.3 and <3.8 mmol/L (9 of 18 [50%]) was considerably higher than the proportion of DPP-4 (1 of 19 [5%]), TZDs (2 of 11 [18%]), and sulfonylurea trials (2 of 20 [10%]) that used the same cutoff. Among trials that included intermediate-long-acting insulins (neutral protamine Hagedorn insulin, detemir, glargine), only 7 of 26 trials (27%) used a cutoff of <3.1 mmol/L, which stands in contrast to both trials including premixed insulin (10/16=63%) and trials including GLP-1s, DPP-4s, TZDs, and sulfonylureas (39%–64%).
Severe hypoglycemia
Fifty-two of the included studies reported results on severe/major hypoglycemia, and among these, 24 studies provided definitions. Considerable heterogeneity was also observed with regard to the definition of severe hypoglycemia. The employed BG/PG cutoff value spanned from <3.3 to <2.0 mmol/L. Additional criteria also differed substantially across trials. A summary of the different definitions of severe/major hypoglycemia used by the studies is presented in . Some of the trials defined severe hypoglycemia with neuroglycopenic symptom incidence with or without accompanied low BG/PG, while some others relied solely on the symptoms.
Table 1 Definitions of severe hypoglycemia
Nocturnal hypoglycemia
The definition of nocturnal hypoglycemia was not reported in a majority of the trials, with only 5 trials reporting the definition. Davies et alCitation9 defined it as an episode occurring between evening injection and breakfast. Diamant et alCitation10 defined it as an episode between bedtime and breakfast. Esposito et alCitation11 defined it as an episode between bedtime and being awake in the morning. Raskin et alCitation12 defined nocturnal hypoglycemia as an episode between 11 pm and 8 am. Rosenstock et alCitation13 defined nocturnal hypoglycemia as an episode occurring between evening injection and getting up.
Discussion
This review demonstrates that substantial heterogeneity exists in the definition of overall hypoglycemia, severe/major hypoglycemia, and nocturnal hypoglycemia across RCTs investigating T2D interventions. Only 66 of the 109 studies reviewed presented the definitions of hypoglycemia. Among these 66 studies, only 9 (14%) followed the ADA/EMA specified guidelines to define hypoglycemia, with an exact threshold of PG ≤3.9 mmol/L. Fifty-two of the 66 studies (79%) used a threshold considerably lower than the recommended ≤3.9 mmol/L, and 16 studies used a threshold between 3.8 and 4.0 mmol/L. The majority of the trials included in the review also did not adhere to the ADA/EMA guidelines for the definition of hypoglycemia
As with any literature review, this review has some strengths and limitations. First, to our knowledge, this is the first review of its kind examining the heterogeneity of hypoglycemia definitions across RCTs on T2D interventions. Second, the review is based on a recent comprehensive HTA performed by CADTH, and so, its foundation is strongly tied to medical decision making about T2D treatments. Third, the comprehensive examination focusing on heterogeneity across treatment classes, not just across trials, provides further insight on how heterogeneity in the definition of hypoglycemia may impact conclusions drawn from the available RCT literature and any systematic review of this. Interventional research for T2D is a rapidly evolving field, and so, not all newer agents available (January 2016) were included in this review. For example, the SGLT-2 inhibitors, long-acting GLP-1s, and ultra-long-acting insulins were not included in the evidence base of randomized trials. Among the trials included in this review, underreporting constitutes a serious concern. First, only 64 of 109 initially considered trials reported hypoglycemia definitions, whereas 24 trials reporting hypoglycemia did not report a definition. This is a limitation for both the strength and validity of the evidence base used in this review, as well as the transparency about hypoglycemia outcomes in the clinical trial literature. For the definition of severe hypoglycemia, another challenge is the fact that no events are observed (particularly in second-line trials). As such, where no events are observed, it is likely that manuscript authors chose to omit the trial definition of severe hypoglycemia. Among the largest concerns regarding the sparseness of evidence is the fact that only 5 trials reported nocturnal hypoglycemia with a definition. Additionally, there is always limitation around how the authors choose to report the glucose values, e.g., whole BG versus PG, as PG values are typically 10%–12% higher than whole BG samples for patients within the range of usual packed cell volumes. Although the data for this analysis were obtained from the RCTs included in the CADTH report (i.e., had a Canadian focus), the included RCTs were conducted across the globe. Further, the systematic literature practices used in the selected CADTH approach are of similar high standard found among well-established national HTA agencies across the world (e.g., National Institute for Health and Care Excellence and Scottish Medicines Consortium), and so, the selected sample is generalizable to the global setting.
As observed in our work, nearly 79% of the studies used a threshold lower than the value defined in the guidelines for the definition of hypoglycemia. This might lead to an underestimation of the incidence of hypoglycemia in the trials, thereby leading to faulty estimates when comparative safety assessments were done across treatments. There may be several reasons for the observed heterogeneity in definitions. Most RCTs were sponsored, and as such, many sponsors (i.e., pharmaceutical companies) may have engaged different groups of key opinion leaders, principal investigators, and contract research organizations to design and run their trial(s). To this end, certain groups of leading clinicians may have found some hypoglycemia definition thresholds more appropriate than others. Likewise, in some settings, leading statisticians may have been more adamant about hypoglycemia thresholds that would yield higher events counts, and thus, lower sample size requirements. This may have likely been the case for the first trials designed for the respective antihyperglycemic agents. However, as definitions have increasingly varied historically, regulatory authorities such as the FDA and EMA have likely requested that second confirmatory RCTs use alternative thresholds to bridge internal data and allow for more straightforward comparisons across trials. Regardless of the cause, the heterogeneity in the definition of hypoglycemia makes the comparison between the studies highly challenging. This problem has already been acknowledged in previous research. Zammit and Frier,Citation7 in their review about hypoglycemia in T2D, also acknowledged the heterogeneity in the definitions of hypoglycemia hindering the comparison between the studies. FrierCitation8 examined the epidemiology and impact of hypoglycemia on diabetic patients; he observed that, by whatever way hypoglycemic burden is measured, the different definitions of hypoglycemia used by investigators result in heterogeneity, which has a limited use in meta-analysis; this is a serious limitation when newer therapies such as insulins are compared in different studies. Severe hypoglycemia is an important safety signal in the treatment of diabetes; almost half of the trials that reported the definition of severe hypoglycemia used BG/PG values in addition to the neuroglycopenic symptoms of hypoglycemia, which adds to the heterogeneity across the trials and results in complexity in comparison to different treatments when the relative safety of interventions is estimated. It is important to note that, although, regulatory agencies are primarily concerned about superiority of the intervention over standard of care (i.e., metformin or metformin+sulfonylurea in the included trials) on the glycemic control outcomes, and with no severe hypoglycemia safety signals, major differences in the non-severe hypoglycemia (regardless of the threshold) are still relevant. Furthermore, in any systematic reviews or meta-analysis combining results from multiple RCTs that use heterogeneous set of definitions for hypoglycemia, statistical techniques such as sensitivity analyses should be used to examine the effects of differences in the definitions, and the definition of hypoglycemia may factor into the quality assessment for the hypoglycemia outcome(s) evidence base.
Conclusion
Defining hypoglycemia according to the guidelines is warranted in the design of the clinical trials that test the treatments for diabetes. All the future trials that consider hypoglycemia as a safety end point need to adhere to the ADA/EMA definition of hypoglycemia to make the trials comparable. Additionally, we recommend that any future review of antihyperglycemic drugs that quality assesses the individual RCTs should also take into account the quality of the outcome measures (e.g., appropriateness of hypoglycemia definition).
Disclosure
Funding for this study was granted by a Mitacs Elevate postdoctoral fellowship provided to CB. This fellowship allowed for the collaboration between researchers at Simon Fraser University and Precision Health Economics. ED, GS, KT, and EJM were employees of Precision Health Economics at the time of manuscript submission. MJ is a professor at Simon Fraser University. The authors report no other conflicts of interest in this work.
References
- KahnSECooperMEDel PratoSPathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and futureLancet201438399221068108324315620
- International Diabetes FederationIDF Diabetes Atlas6th ed2013 Available from: https://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdfAccessed December 5, 2016
- GroupUKHSRisk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their durationDiabetologia20075061140114717415551
- Canadian Diabetes Association Clinical Practice Guidelines Expert CClaytonDWooVYaleJFHypoglycemiaCan J Diabetes201337suppl 1S69S7124070966
- SeaquistERAndersonJChildsBHypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine SocietyDiabetes Care20133651384139523589542
- European Medicines AgencyGuideline on Clinical Investigation of Medicinal Products in the Treatment or Prevention of Diabetes Mellitus2012 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdfAccessed December 5, 2016
- ZammittNNFrierBMHypoglycemia in type 2 diabetes: pathophysiology, frequency, and effects of different treatment modalitiesDiabetes Care200528122948296116306561
- FrierBMHypoglycaemia in diabetes mellitus: epidemiology and clinical implicationsNat Rev Endocrinol2014101271172225287289
- DaviesMJThawarePKTringhamJRA randomized controlled trial examining combinations of repaglinide, metformin and NPH insulinDiabet Med200724771471917403126
- DiamantMVan GaalLStranksSSafety and efficacy of once-weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes over 84 weeksDiabetes Care201235468368922357185
- EspositoKCiotolaMMaiorinoMIAddition of neutral protamine lispro insulin or insulin glargine to oral type 2 diabetes regimens for patients with suboptimal glycemic control: a randomized trialAnn Intern Med2008149853153918936501
- RaskinPRHollanderPALewinAINITIATE Study GroupBasal insulin or premix analogue therapy in type 2 diabetes patientsEur J Intern Med2007181566217223044
- RosenstockJSugimotoDStrangePStewartJASoltes-RakEDaileyGTriple therapy in type 2 diabetes: insulin glargine or rosiglitazone added to combination therapy of sulfonylurea plus metformin in insulin-naive patientsDiabetes Care200629355455916505505
- RossSAZinmanBCamposRVStrackTCanadian Lispro Study GroupA comparative study of insulin lispro and human regular insulin in patients with type 2 diabetes mellitus and secondary failure of oral hypoglycemic agentsClin Invest Med200124629229811767232
- DeFronzoRARatnerREHanJKimDDFinemanMSBaronADEffects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetesDiabetes Care20052851092110015855572
- MarreMHowlettHLehertPAllavoineTImproved glycaemic control with metformin-glibenclamide combined tablet therapy (Glucovance) in type 2 diabetic patients inadequately controlled on metforminDiabet Med200219867368012147149
- BergenstalRMWyshamCMacconellLEfficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trialLancet2010376973943143920580422
- AschnerPChanJOwensDRInsulin glargine versus sitagliptin in insulin-naive patients with type 2 diabetes mellitus uncontrolled on metformin (EASIE): a multicentre, randomised open-label trialLancet201237998332262226922683131
- BarnettAHBurgerJJohnsDTolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trialClin Ther200729112333234818158075
- ChoYMKooBKSonHYEffect of the combination of mitiglinide and metformin on glycemic control in patients with type 2 diabetes mellitusJ Diabetes Investig201014143148
- GaoYYoonKHChuangLMEfficacy and safety of exenatide in patients of Asian descent with type 2 diabetes inadequately controlled with metformin or metformin and a sulphonylureaDiabetes Res Clin Pract2009831697619019476
- HeineRJVan GaalLFJohnsDGWAA Study GroupExenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trialAnn Intern Med2005143855956916230722
- JankaHUPleweGRiddleMCKliebe-FrischCSchweitzerMAYki-JarvinenHComparison of basal insulin added to oral agents versus twice-daily premixed insulin as initial insulin therapy for type 2 diabetesDiabetes Care200528225425915677775
- NauckMAEllisGCFleckPRWilsonCAMekkiQAlogliptin Study 008 GroupEfficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a multicentre, randomised, double-blind, placebo-controlled studyInt J Clin Pract2009631465519125992
- Yki-JarvinenHKauppinen-MakelinRTiikkainenMInsulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET studyDiabetologia200649344245116456680