Abstract
Background
Chronic hepatitis C (CHC) causes liver cirrhosis in 5%–20% of patients, leading to increased morbidity and mortality. This study aimed to estimate liver-related morbidity and mortality among patients with CHC and cirrhosis in Denmark with and without antiviral treatment and sustained virologic response (SVR). Furthermore we aimed to estimate the rate of hepatocellular carcinoma (HCC) and decompensation associated with certain prognostic factors.
Materials and methods
Patients with CHC and cirrhosis registered in the Danish Database for Hepatitis B and C were eligible. Cirrhosis was based on liver biopsy, transient elastography, and clinical cirrhosis. Data were extracted from nationwide registries. The study period was from 2002 until 2013.
Results
Of 1,038 patients included, 716 (69%) were male and the median age was 52 years. Median follow-up was 3.8 years, 360 patients died, and 233 of 519 treated patients achieved SVR. Alcohol overuse and hepatitis C virus genotype 3 were associated with an increased incidence rate (IR) of HCC, whereas diabetes and alcohol overuse were associated with increased IRs of decompensation. Achieving SVR reduced all-cause mortality (adjusted mortality rate ratio 0.68 [95% CI 0.43–1.09]) and liver-related mortality (mortality rate ratio 0.6 [95% CI 0.36–1]), as well as liver-related morbidity with adjusted IR ratios of 0.37 (95% CI 0.22–0.62) for HCC and 0.31 (95% CI 0.17–0.57) for decompensation. The IRs of HCC and decompensation remained elevated in patients with alcohol overuse after SVR.
Conclusion
Alcohol overuse, hepatitis C genotype 3, and diabetes were associated with liver-related morbidity in patients with CHC and cirrhosis. SVR markedly reduced liver-related morbidity and mortality; however, special attention to patients with alcohol overuse should continue after SVR.
Plain-language summary
Chronic infection with hepatitis C virus (HCV) can cause liver cirrhosis in 5%–20% of patients. With cirrhosis, the risk of liver cancer and complications increases dramatically; however, successful antiviral treatment of chronic hepatitis C (CHC) reduces this risk. Little is known about the risk of liver cancer, complications, and death in patients with CHC and cirrhosis in Denmark. Therefore, this study aimed to estimate the incidence of liver cancer, liver complications, and liver-related death and how these were affected by successful antiviral treatment in patients with CHC and cirrhosis in Denmark. We performed an observational cohort study of nationwide data, and found that among patients with CHC and cirrhosis, HCV genotype 3 and alcohol overuse were associated with development of liver cancer, whereas diabetes mellitus was associated with development of liver complications. Curing CHC significantly reduced the rate of liver cancer and complications, as well as the rate of death. There is an urgent need to cure patients with CHC and cirrhosis, especially those with HCV genotype 3, and special attention needs to be given to patients with diabetes mellitus or alcohol overuse.
Introduction
Chronic infection with hepatitis C virus (HCV) is a disease of global importance with a large burden of morbidity and mortality.Citation1–Citation6 For 5%–20% of chronic hepatitis C (CHC) patients, the disease will progress to liver cirrhosisCitation7 associated with complications, such as liver decompensation, hepatocellular carcinoma (HCC), and increased mortality.Citation8 The commonest causes of death among patients with CHC and cirrhosis are liver-related, particularly HCC.Citation8,Citation9 Several prognostic factors, such as diabetes mellitus, male sex, alcohol abuse, and HCV genotype 3, are thought to be associated with the development of HCC, decompensation, and mortality.Citation10–Citation14
Absence of HCV RNA 24 weeks after end of treatment (EOT24) is considered a cure for CHC, and is termed sustained virologic response (SVR).Citation15 Patients with CHC and cirrhosis have previously been difficult to cure; however, achieving a cure for CHC has been shown to be associated with decreased morbidity and mortality.Citation10,Citation12 With the introduction of new, very effective antiviral drugs that cure CHC in nearly all treated patients,Citation16 large cohorts of patients with CHC and cirrhosis who fail treatment in comparison with patients with SVR will be difficult to assemble.
Moreover, our knowledge of liver-related morbidity and mortality and how SVR affects these among patients with CHC and cirrhosis on a nationwide scale is limited. Most previous studies of HCC, decompensation, and liver-related mortality in CHC patients with cirrhosis have been single- or few-center studies, and included fairly selected cohorts from tertiary centers.Citation5,Citation8,Citation10,Citation12,Citation13
The aims of this nationwide cohort study were to estimate liver-related morbidity and mortality among patients with CHC and cirrhosis in Denmark with and without antiviral treatment, and to estimate liver-related morbidity and mortality associated with SVR in a relatively large cohort of treated CHC patients with cirrhosis. Furthermore, we aimed to estimate the rates of HCC and decompensation associated with certain prognostic factors.
Materials and methods
This observational cohort study used prospectively collected data. The Danish health-care system is publicly funded and provides health-care services free of charge to the individual. Patients were identified in the Danish Database for Hepatitis B and C (DANHEP). DANHEP is a nationwide database with ongoing enrolment established January 1, 2002.Citation17 It contains demographic, clinical, liver-biopsy, laboratory, and treatment data, and transient-elastography measurements on patients seen with CHC and/or CHB in any of the specialized outpatient clinics responsible for CHC patients in Denmark after January 1, 2002. Cross-linkage between nationwide registries is possible due to the unique 10-digit personal identification numberCitation18 given to all residents in Denmark and registered in the Danish civil registration system, along with date of birth, sex, vital and migration status, and address. All hospital-admission dates and diagnoses are registered in the Danish national patient registry (NPR),Citation19 histopathologic diagnoses are registered in the national Danish pathology database (Patobank),Citation20 causes of death are registered in the Danish registry of causes of death,Citation21 and cancer diagnoses are registered in the Danish cancer registry.Citation22 Further information about the Danish registry of causes of death is provided in the Supplementary material, and the remaining registries are described in more detail elsewhere.Citation23
Patient cohort
Eligible patients were registered in DANHEP and fulfilled the following criteria: a positive HCV RNA test, cirrhosis and enrolment in DANHEP before December 31, 2013, ≥18 years of age, and a valid personal identification number and address in Denmark. All ICD and systematized nomenclature of medicine (SNOMED)Citation24 codes used in the definitions of inclusion and exclusion criteria, outcomes, and covariates are provided in the Supplementary material. To avoid reverse causation and inclusion of prevalent cases of decompensation and HCC, the first 6 months of observation were excluded. Baseline was thus defined as 6 months (183 days) after the first date of a diagnosis of cirrhosis and enrolment in DANHEP, whichever occurred last, and could not precede January 1, 2002. Cirrhosis was defined as: liver biopsy (Metavir fibrosis score of F4),Citation25 transient elastography (FibroScan; Echosens, Paris, France) median elasticity ≥17 kPaCitation26,Citation27 (10 or more valid measurements and an interquartile range ≤30% of median elasticity),Citation28 or clinical cirrhosis (cirrhosis registered in NPR, ascites, hepatic encephalopathy, esophageal varices, esophageal variceal hemorrhage, spontaneous bacterial peritonitis). Patients were excluded if they had CHB, HIV infection, autoimmune hepatitis, or hemochromatosis at baseline.
Definition of outcomes and covariates
The two primary outcomes in the separate analyses of liver-related morbidity were HCC and decompensation. We defined HCC as a diagnosis registered in Patobank, the Danish registry of causes of death, the Danish cancer registry, or the NPR. Decompensated liver cirrhosis was defined as a diagnosis registered in the NPR, the Danish registry of causes of death, and/or DANHEP of ascites, esophageal variceal hemorrhage, hepatic encephalopathy, or spontaneous bacterial peritonitis.
In mortality analyses, the primary outcome was liver-related and all-cause mortality. All-cause mortality was defined as death due to any cause with a date of death registered in the Danish civil registration system, which was updated throughout the study period. Liver-related mortality was based on the underlying cause of death registered on the death certificate and divided into liver-related and liver-unrelated. Because the Danish registry of causes of death was only updated through December 2012 at the time of data extraction, analyses of liver-related mortality were limited to before December 31, 2012.
Antiviral treatment was registered in DANHEP and defined as reception of at least one dose of antiviral therapy. Based on information registered in DANHEP, successful treatment was defined as the absence of HCV RNA (SVR) at EOT24. Only patients who were alive and had negative HCV RNA samples at ≥EOT24 were categorized as having SVR. Non-SVR was defined as a treatment with known treatment response that did not result in SVR. Reinfection after SVR was defined as at least one positive HCV RNA after a period with undetectable HCV RNA after the EOT24 date.
Comorbidity was assessed by a time-updated, cumulative Charlson comorbidity index (CCI)Citation29 score calculated based on ICD codes assigned to hospital contacts registered in the NPR (ICD codes modified by Quan et alCitation30 are provided in the Supplementary material). Originally, the CCI score was developed to predict short-term, in-hospital mortality, but has subsequently been shown to predict long-term mortality.Citation31 Liver disease, HCC, and HIV diagnoses were excluded from the CCI score. Orthotopic liver transplantation (LTx) was defined as an LTx procedure registered in the NPR. Diabetes mellitus was based on ICD codes registered in the NPR. Intravenous drug use (IDU; ever) was defined as having an IDU-related diagnosis registered in the NPR prior to the end of follow-up, self-reported IDU, or IDU as route of HCV transmission registered in DANHEP. Alcohol overuse was defined as any of the following prior to the end of follow-up: alcohol abuse-related diagnosis in NPR, a liver biopsy with a histopathologic diagnosis of alcoholic hepatitis or alcoholic cirrhosis, or self-reported daily alcohol consumption of >36 g alcohol for men and >24 g alcohol for womenCitation32 registered in DANHEP. Psychiatric disease was defined as a diagnosis of psychiatric disease registered in the NPR.
Statistical analysis
Patient characteristics were compared between patient groups with Mann–Whitney U test, χ2 test, or χ2 test with Monte Carlo simulations using 10,000 samples. All rates and rate ratios were calculated using Poisson regression, where time at risk and number of events in different strata were calculated using the stratify macro.Citation33
Patients were followed from baseline until the primary outcome, exit for other reasons, or end of the study period, whichever occurred first. The study ended on December 31, 2013 except for liver-related mortality, where the last day of observation was December 31, 2012. When estimating rates in addition to reaching the end of the study period, infection with HIV, CHB, autoimmune hepatitis, or hemochromatosis led to censoring. Patients with prevalent cases of the primary outcome or LTx at baseline were excluded from morbidity analyses. A diagnosis of HCC before decompensation when decompensation was the primary outcome did not lead to the end of follow-up and vice versa. Cumulative-incidence functions of liver-related morbidity in all patients were calculated with death, coinfection with HIV or HBV, autoimmune hepatitis, hemochromatosis, and LTx as competing risks. Incidence rate ratios (IRRs) of HCC and decompensation were calculated to estimate the rates associated with predefined prognostic factors (sex, diabetes, alcohol overuse, and HCV genotype). In all Poisson regression analyses, CCI score (0, 1, 2, 3, 4, 5, ≥6), LTx, diabetes, psychiatric disease, HCC, and decompensation were introduced as time-dependent categorical covariates, whereas sex, alcohol overuse, IDU, and genotype (1, 2, 3, 4–6, multiple genotypes) were introduced as baseline categorical covariates. Age (<40, 40–44,45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80–84, 85–89, and ≥90 years) was used as a continuous, time-dependent covariate after testing for log linearity or as a categorical variable. In cases of insufficient events, appropriate covariate strata were combined to ensure events in all strata.
In estimates of rates of HCC, decompensation and mortality when comparing patients with SVR vs non-SVR time at risk were calculated from the first EOT24 date with known treatment response or baseline, whichever occurred last, until the primary outcome or end of follow-up. In addition to the reasons mentioned previously, patients were censored at reinfection. SVR was introduced as a time-dependent covariate, and the clock-reset approach was applied. Cumulative-incidence functions in SVR, non-SVR, and untreated patients treated death, LTx, SVR, treatment, coinfection with HIV or HBV, and other liver diseases as competing events. Untreated patients were followed from baseline until initiation of event, treatment, or end of follow-up. All statistical tests were two-sided with a=0.05. Data handling and statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA), except for cumulative-incidence functions and figures, where R (version 3.2.2)Citation34 was used.
Ethics
This study was approved by the Danish Data Protection Agency (2013-41-2323) in accordance with Danish law.
Results
Data were extracted from nationwide registries on 5,779 patients with positive HCV RNA. Of these, 1,306 patients had cirrhosis, and after the exclusion of 268 patients, 1,038 patients were eventually included (inclusion and exclusion of patients can be seen in ). One patient (0.1%) was lost to follow-up with unknown address and vital status in the Danish civil registration system. Baseline characteristics of all patients, untreated and treated, are shown in . Before the end of follow-up, 537 (52%) patients had initiated antiviral treatment, of whom 519 patients reached an EOT24 date at least once. The majority of treatment courses with a known treatment outcome consisted of a backbone of IFN (with or without Ribavirin).Citation15 Only 45 (7%) patients were treated with polyethylene glycolated IFN combined with Ribavirin and a direct-acting antiviral (DAA) drug. Two patients were treated with Ribavirin monotherapy and failed to achieve SVR. Overall, 233 patients achieved SVR (45%), 276 patients achieved non-SVR (53%) at least once, and 10 patients had unknown treatment response (2%). A total of 27 patients achieved SVR24 after initially failing therapy. Three patients were reinfected and did not achieve a second SVR24. Characteristics of patients at their first EOT24 are listed in .
Table 1 Baseline characteristics of CHC patients with cirrhosis, and subgroups of untreated patients and patients who initiated treatment before the end of follow-up
Patients were followed for a median of 3.8 (range 0.01–12) and 4,909 years total. After the exclusion of patients with prevalent cases of HCC and/or LTx at baseline, among 989 patients at risk of HCC, 115 patients were diagnosed with HCC during follow-up. Similarly among 811 patients at risk of decompensation, 153 decompensated during followup. Of 1,038 patients included, 360 eventually died during follow-up. Of 299 deaths prior to December 31, 2012, 186 (62%) died of liver-related causes. The cumulative-incidence functions of liver-related morbidity are shown in . Liver-related morbidity IRs and mortality rates (MRs) for all patients and for SVR, non-SVR, and untreated patients are shown in . Adjusted liver-related morbidity IRRs are shown in for all patients. In adjusted analyses, alcohol overuse was associated with increased rates of both HCC and decompensation among patients at risk, and compared to patients with genotype 1 or 2, genotype 3 was associated with an increased rate of HCC. In contrast, male sex and diabetes were not significantly associated with HCC. Genotype was not associated with decompensation, but diabetes was associated with an IRR of 1.65 for decompensation after adjusting for confounding.
Figure 1 Cumulative incidence functions of liver-related morbidity among patients with chronic hepatitis C and cirrhosis, with death and liver transplantation as competing risks.
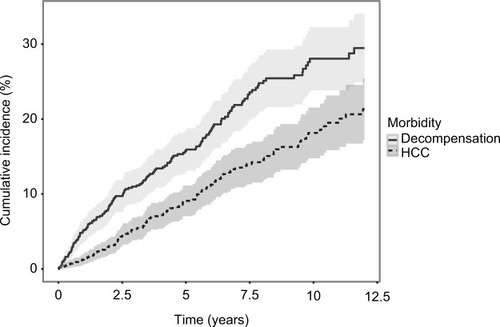
Table 2 Liver-related morbidity and mortality incidence rates for patients with chronic hepatitis C and for patients with SVR, non-SVR and for untreated patients
Table 3 Adjusted liver-related morbidity IRRs among all patients with chronic hepatitis C and cirrhosis
Median and total follow-up years were 4.8 (range 0.04–12) and 1,197 after SVR and 3.8 (range 0.04–12) and 1,444 after non-SVR. Cumulative-incidence functions of liver-related morbidity for SVR, non-SVR, and untreated patients are shown in and . Of the 226 patients who achieved SVR and were at risk of HCC, 10 developed HCC maximum 6.1 years after SVR24. In contrast, of 289 patients at risk after non-SVR, 47 were diagnosed with HCC. After non-SVR, 56 of 266 patients at risk decompensated, whereas only 11 of 211 patients decompensated after SVR. One patient with SVR and 11 with non-SVR received LTx. Adjusted liver-related morbidity IRRs and MRRs for SVR vs non-SVR and untreated patients are presented in . Cumulative incidences for 1-, 5-, and 10-year liver-related morbidity with death and LTx as competing risks are presented in . Achieving SVR was associated with a reduced rate of HCC, even after adjusting for sex, age, alcohol overuse, genotype, and diabetes. The rate of decompensation was also reduced substantially in the presence of SVR, and of the 11 patients who decompensated after SVR, seven had a history of alcohol overuse. Adjusted all-cause and liver-related MRs were reduced by 34% and 40% with SVR, respectively, when taking confounding into account.
Figure 2 Cumulative incidence functions of decompensation comparing SVR, non-SVR, and untreated patients.
Abbreviations: SVR, sustained virologic response (24 weeks after end of treatment); HBV, hepatitis B virus.
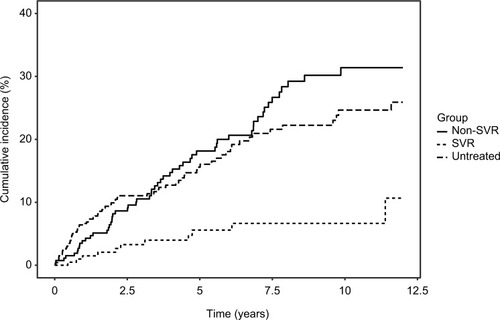
Figure 3 Cumulative incidence functions of hepatocellular carcinoma comparing patients with SVR, non-SVR, and untreated patients.
Abbreviations: SVR, sustained virologic response (24 weeks after end of treatment); HBV, hepatitis B virus.
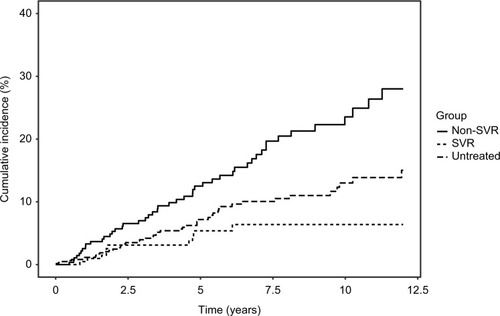
Table 4 Adjusted liver-related morbidity and MRRs for patients with SVR vs non-SVR
Discussion
This nationwide cohort study based on prospectively collected data adds to our knowledge of liver-related morbidity and mortality among CHC patients with cirrhosis in a real-life cohort. We found major differences between untreated and treated patients with regard to baseline characteristics associated with mortality. A history of alcohol overuse and genotype 3 were associated with increased rates of HCC, whereas diabetes increased the rate of decompensation, as did alcohol overuse. Achieving SVR was associated with markedly reduced rates of liver-related morbidity and mortality after adjustment for confounding. However, a history of alcohol overuse was associated with diagnoses of HCC and decompensation after SVR.
Patients who did not initiate treatment differed from treated patients with respect to several baseline characteristics, such as comorbidity, decompensated cirrhosis, HCC, and a history of alcohol overuse or IDU. These differences were reflected in the high all-cause mortality among untreated patients, even compared to patients with non-SVR24. These differences are probably primarily a result of the selection of healthier patients for antiviral treatment, in part due to contraindications to polyethylene glycol–IFN-based therapyCitation15 and other possible factors, such as continuous links to care.
We found IRs of HCC ranging from 0.85 after SVR to 3.53 after non-SVR per 100 person-years (PYs). Some studies have reported an increased rate of HCC associated with HCV genotype 3 independently of alcohol overuse, obesity, and diabetes.Citation12,Citation35 The confirmed increased rate of HCC associated with genotype 3 observed in our adjusted analyses did not take SVR into account. However, given the fact that both genotypes 2 and 3 were associated with higher chances of SVRCitation36 in the era of IFN and Ribavirin compared with genotype 1, we find it unlikely that differences in SVR rates among patients with different genotypes should explain the observed increased rate of HCC associated with genotype 3. We found no statistically significant association between sex or diabetes and HCC, as has been described elsewhere,Citation10,Citation11,Citation13,Citation37,Citation38 whereas diabetes did increase the rate of decompensation. Previously, it has been suggested that diabetes and genotype 3 are associated with fibrosis progressionCitation39–Citation41 and complications in cirrhotic patients.Citation14 The complex carcinogenic biological mechanisms involved in the development of HCC in the presence of diabetes and HCV genotype 3 are not fully understood. However, steatosis is thought to play a key role,Citation42 and diabetes and HCV show signs of a synergistic effect in the formation of HCC.Citation13,Citation43 Apart from being associated with obesity and diabetes, steatosis is also generally accepted to be associated with HCV genotype 3,Citation44 which may partly explain the increased risk of HCC. Nonetheless, the absence of information on steatosis and steatohepatitis in this study precludes any further conclusions on this matter. Alcohol overuse is a well-known risk factor,Citation38 and was also strongly associated with HCC in this study. We did not observe an association between male sex and HCC, as demonstrated elsewhere,Citation8,Citation10,Citation12,Citation13,Citation45 and we have no good explanation for this (crude rate male vs female 1.18 [95% CI 0.83–1.68] vs adjusted rate 1.21 [95% CI 0.86–1.69] per 100 PYs). While it was unclear which covariates were included in the final multivariate Cox regression models performed in previously published studies,Citation8,Citation10,Citation12,Citation13,Citation45 we find it unlikely that the lack of an association in our study is the result of confounding. It may simply be due to chance. Death would be considered a competing event with HCC, and the MR was higher among men than women (adjusted MRR 1.39 [95% CI 1.07–1.8]); however, a rate is independent of competing events. Similarly, we found no association between diabetes and HCC, which has been demonstrated consistently,Citation11–Citation13,Citation38 though mainly after SVR. In our study, diabetes was based entirely on the NPR. Misclassification in cases of diabetes not registered in the hospital registry may have occurred, which would bias the results toward the null. The positive predictive value of a diagnosis of diabetes in the NPR has been demonstrated to be good.Citation46,Citation47 However, the prevalence of diabetes of 12% at baseline in our cohort was at the lower end compared to the 12%–24% in other cohorts,Citation11–Citation13,Citation48 which could suggest that we have underestimated the true diabetes prevalence. Alternatively, since other studies have mainly been performed in single or few tertiary centers, it may also be that the association between diabetes and HCC demonstrated by some could be the result of selection bias. Because diabetes was introduced as a time-dependent variable in our regression analyses, one could argue that this could lead to reverse causation of diabetes and HCC, if diabetes was more likely to be diagnosed in patients with yet-undiagnosed HCC. However, this would lead to a stronger association between diabetes and HCC, which was not the case in our study. Furthermore, the median time from diabetes to HCC was 4.6 years and 3.9 years from diabetes to decompensation, which makes reverse causation unlikely. Interestingly, in our cohort diabetes was associated with HCC in an unadjusted analysis and when adjusting for everything but age (data not shown). Patients with diabetes were also slightly older than patients without diabetes, and the rate of HCC increased significantly with age. Therefore, at least in this study, an association between diabetes and HCC was confounded by age. The HCC IR of 0.85/100 PYs after SVR observed in our cohort is consistent with the HCC IRs of 0.55–1.39/100 PYs described by others after SVR.Citation10,Citation12,Citation38,Citation49,Citation50 The lowest HCC IR after SVR was reported in a study by van der Meer et al (0.55/100 [95% CI 0.14–0.96] PYs),Citation12 which had a long median follow-up (8.4 years), but included 46% patients with advanced fibrosis, slightly younger patients (median age 48 years), and only patients with compensated cirrhosis. The highest HCC IR estimate reported of 1.39/100 PYs was found in a large cohort consisting of 95% males with a relatively high prevalence of diabetes and alcohol abuse,Citation38 all risk factors previously described to be associated with HCC.Citation10,Citation12,Citation13,Citation38 Our results on the continuously elevated rate of HCC after non-SVR are well within the estimates reported in other cohorts of 1.44–5.85/100 PYs.Citation10,Citation12,Citation49–Citation51 We were able to confirm the reduced rate of HCC in cirrhotic CHC patients associated with SVR reported previously.Citation10,Citation12,Citation49,Citation50 These results underscore the benefits associated with a cure for CHC in cirrhotic patients. Despite small differences in the definition of liver complications applied here and elsewhere, we also found a markedly reduced rate of decompensation associated with SVR, in line with previous results.Citation10,Citation12,Citation49,Citation50 It is noteworthy that the annualized risk of HCC of 1.08% during the first 5 years after SVR found in our cohort is below the threshold of cost-effectiveness of 1.5%/year for HCC surveillance.Citation52,Citation53 However, the cutoff was estimated in the 1990s based on patients with multiple etiologies and exclusively cirrhosis Child–Pugh A. This was during in an era when CHC was difficult to cure, with presumably shorter life expectancies. At this point, the European Association for the Study of the Liver recommends continued HCC surveillance after SVR in cirrhotic patients.Citation16 Further studies are needed to determine whether surveillance for HCC is cost-effective after SVR, and if so, for how long after SVR surveillance should be recommended.
The fact that adjusted all-cause and liver-related MRRs associated with SVR did not reach statistical significance in this study may have been due to underpowering when adjusting for confounding. We are confident that SVR was truly associated with reduced mortality in this real-life cohort, as has been demonstrated previously.Citation23 With highly effective DAAs, even patients with advanced liver disease and comorbidities can undergo treatment and achieve very high SVR rates.Citation16 Therefore, it will be of interest to follow CHC patients cured with IFN-free DAA regimens to study the long-term effects of eradicating HCV in patients with advanced liver disease, comorbidity, and/or substance abuse that would have precluded them from IFN and Ribavirin-based therapy.
The unique tradition for nationwide health registries and opportunity to cross-link between registries ensured complete follow-up in this cohort study of prospectively collected data. Unfortunately, only a fraction of the total number of patients with CHC in Denmark are aware of their CHC-status and attend specialized care,Citation54 which limits the generalizability of our morbidity-rate and MR estimates, due to potential selection bias. For example, if only the sickest patients attend specialized care with symptoms of and complications due to CHC, we could have overestimated the rates of liver-related morbidity and mortality associated with CHC.
Reporting cancer diagnoses to the Danish cancer registry by physicians is mandatory, and since hospitals in Denmark are reimbursed based on admissions and procedures registered in the NPR, the number of HCC cases diagnosed in hospitals not registered in the cancer registry, Patobank, or NPR is likely to be low. A recent study from Sweden suggests that relying on a single registry could lead to substantial underestimation of primary liver cancers, possibly due to the noninvasive nature of HCC diagnosis.Citation55 In our study, we extracted information from four different registries, which confers validity to our results. However, it is possible that we underestimated the number of HCC cases due to unrecognized HCC in deceased patients in the absence of diagnostic imaging or autopsy. Only two patients were diagnosed with HCC exclusively in the registry of causes of death. With regard to decompensation, it is likely that the registration of this complication is less rigorous than HCC, since we had to rely on only two registries, and thus it is possible that we underestimated the occurrence of decompensation.
Other limitations include the fact that we did not have information about smoking status, valid HCV RNA concentrations, body-mass index, nonalcoholic fatty-liver disease, nonalcoholic steatohepatitis, CHC disease duration, Child–Pugh or MELD (model for end-stage liver disease) score, systematic information about cirrhosis regression, quantification and duration of alcohol intake, or information about current alcohol overuse or IDU. These are all important potential confounding factors.
In conclusion, we found high rates of liver-related morbidity and mortality among patients with CHC and cirrhosis, in particular among untreated patients. Genotype 3 and alcohol overuse were associated with increased rates of HCC, and diabetes and alcohol overuse increased the rate of decompensation. Successful eradication of HCV was associated with substantially reduced rates of liver-related mortality, HCC, and liver decompensation when adjusting for confounding. There continues to be an urgent need to cure patients with CHC and cirrhosis before the development of complications, particularly in those with genotype 3. Special attention should be given to patients with a history of alcohol overuse in whom an elevated rate of HCC and decompensation remain after SVR.
Author contributions
NW, PBC, SH, and HBK contributed to the concept and design of this study. SH managed and sorted data and performed statistical analyses in collaboration with statistician SL, who also contributed to the design of the study. SH wrote the manuscript in collaboration with NW. All authors contributed toward data collection and analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
SH received financial support from the Bonén Foundation. NW received financial support from the Danish Innovation Foundation, The Liver Score Project (ID 139-2012-3). None of the funding sources was involved in the design of the study, data collection or analysis, or writing of the final manuscript. We would like to acknowledge the DANHEP group and all patients in DANHEP and their families. The abstract of this paper was presented at the Liver Meeting held by the American Association for the Study of Liver Diseases (AASLD), November 11–15, 2016, Boston, MA, as an oral presentation with interim findings. The presentation’s abstract was published in the AASLD abstract book (abstract 176: http://onlinelibrary.wiley.com/doi/10.1002/hep.28796/epdf).
Supplementary materials
Data sources
Danish civil registration system, Danish Database for Hepatitis B and C (DANHEP), Danish national patient registry (NPR), national Danish pathology database (Patobank), and Danish cancer registry provided in supplementary material published previously.Citation1
The Danish register of causes of death (DAR) is a nationwide registry that contains information on nearly every death in Denmark since 1943. On each death certificate, a physician has registered one or more diagnoses considered to be the cause(s) of death, and coded with appropriate ICD-10 codes during this study period.Citation2
Definition of covariates
Lists of ICD-8, ICD-10, and SNOMED codes used in the definition of inclusion and exclusion criteria, covariates, and causes of death are supplied in –.
Figure S1 Inclusion and exclusion of patients with CHC and cirrhosis.
Abbreviations: PIN, personal identification number; HCV, hepatitis C virus; CHC, chronic hepatitis C; CHB, chronic hepatitis B; TE, transient elastography; SVR, sustained virologic response; HCC, hepatocellular carcinoma.
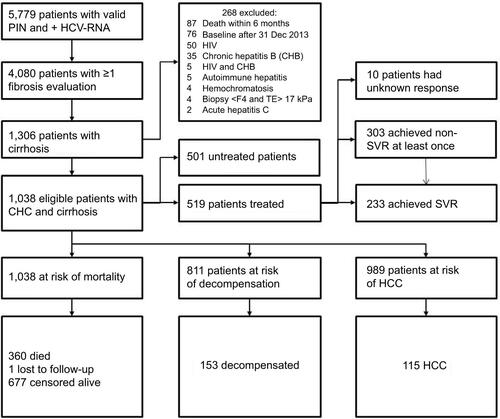
Table S1 Codes for assorted conditions
Table S2 ICD codes used in definition of Charlson comorbidity index score
Table S3 Causes of death
Table S4 Characteristics of patients with SVR and non-SVR at their first EOT24 date or baseline, whichever occurred last
Table S5 Cumulative incidences for liver-related morbidity among all patients at risk after SVR, after non-SVR, and among untreated patients, with death and LTx as competing risks
References
- HallagerSChristensenPBLadelundSMortality in patients with chronic hepatitis C and cirrhosis compared to the general population: a Danish cohort studyJ Infect Dis2017215219220127803168
- Helweg-LarsenKThe Danish register of causes of deathScand J Public Health2011397 Suppl2629
- QuanHSundararajanVHalfonPCoding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative dataMed Care200543111130113916224307
Disclosure
SH has served as an unpaid speaker at an event sponsored by MSD. PBC has received unrestricted grants from Gilead, AbbVie, Roche, and Schering and served as an advisory board member for Roche. MK has served as a speaker for AbbVie and received travel grants from BMS, AbbVie, and Bayer. BTR has served as advisory board member for BMS and received travel grants from BMS. EB has received travel grants from Norgine. TSB has served as a speaker and advisory board member for AbbVie and received travel grants from Gilead, AbbVie, and BMS. LGM has served as an advisory board member for BMS and AbbVie and served as a speaker for Medivir and BMS. JG has received grants, participated in advisory boards, or served as speaker for AbbVie, ViiV, Gilead, BMS, MSD, and Medivir. BT has received grants from Gilead and served as an advisory board member for AbbVie. HBK has received travel grants from Gilead, Medivir, MSD, and BMS. NW has received lecture honoraria from AbbVie, BMS, Gilead, Janssen, and MSD; has served as an advisory board member for AbbVie, BMS, Gilead, Medivir, and MSD; and has worked as a clinical Investigator for AbbVie, BMS, and MSD. The other authors report no conflicts of interest in this work.
References
- HanafiahKMGroegerJFlaxmanADWiersmaSTGlobal epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalenceHepatology20135741333134223172780
- El-SeragHBEpidemiology of viral hepatitis and hepatocellular carcinomaGastroenterology2012142612641273.e122537432
- PoynardTYuenMFRatziuVLaiCLViral hepatitis CLancet200336294012095210014697814
- RazaviHWakedISarrazinCThe present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigmJ Viral Hepat201421Suppl 1345924713005
- FattovichGGiustinaGDegosFMorbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patientsGastroenterology199711224634729024300
- OmlandLHKrarupHJepsenPMortality in patients with chronic and cleared hepatitis C viral infection: a nationwide cohort studyJ Hepatol2010531364220400197
- SeeffLBNatural history of chronic hepatitis CHepatology2002365 Suppl 1S35S4612407575
- SangiovanniAPratiGMFasaniPThe natural history of compensated cirrhosis due to hepatitis C virus: a 17-year cohort study of 214 patientsHepatology20064361303131016729298
- BenvegnuLNatural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complicationsGut200453574474915082595
- AlemanSRahbinNWeilandOA risk for hepatocellular carcinoma persists long-term after sustained virologic response in patients with hepatitis C-associated liver cirrhosisClin Infect Dis201357223023623616492
- HedenstiernaMNangarhariAWeilandOAlemanSDiabetes and cirrhosis are risk factors for hepatocellular carcinoma after successful treatment of chronic hepatitis CClin Infect Dis Off Publ Infect Dis Soc Am2016636723729
- van der MeerAJVeldtBJFeldJJAssociation between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosisJAMA2012308242584259323268517
- VeldtBJChenWHeathcoteEJIncreased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitusHepatology20084761856186218506898
- ElkriefLChouinardPBenderskyNDiabetes mellitus is an independent prognostic factor for major liver-related outcomes in patients with cirrhosis and chronic hepatitis CHepatology201460382383124841704
- ChristensenPBClausenMRKrarupHLaursenALSchlichtingPWeisNTreatment for hepatitis B virus (HBV) and hepatitis C virus (HCV) infection: Danish national guidelines 2011Dan Med J2012596C446522677253
- European Association for Study of LiverEASL recommendations on treatment of hepatitis C 2015J Hepatol201563119923625911336
- HansenNObelNChristensenPBPredictors of antiviral treatment initiation in hepatitis C virus-infected patients: a Danish cohort studyJ Viral Hepat200916965966519486467
- PedersenCBThe Danish Civil Registration SystemScand J Public Health2011397 Suppl222521775345
- AndersenTFMadsenMJørgensenJMellemkjoerLOlsenJHThe Danish national hospital register: a valuable source of data for modern health sciencesDan Med Bull199946326326810421985
- Patobank [website on the Internet]2017 Available from: http://www.patobank.dkAccessed November 9, 2015
- Helweg-LarsenKThe Danish register of causes of deathScand J Public Health2011397 Suppl2629
- GjerstorffMLThe Danish cancer registryScand J Public Health2011397 Suppl424521775350
- HallagerSChristensenPBLadelundSMortality in patients with chronic hepatitis C and cirrhosis compared to the general population: a Danish cohort studyJ Infect Dis2017215219220127803168
- CôtéRARobboySProgress in medical information management: systematized nomenclature of medicine (SNOMED)JAMA198024387567626986000
- BedossaPPoynardTAn algorithm for the grading of activity in chronic hepatitis C: the METAVIR cooperative study groupHepatology19962422892938690394
- DegosFPerezPRocheBDiagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study)J Hepatol20105361013102120850886
- ChristiansenKMMössnerBKHansenJFJarnbjerEFPedersenCChristensenPBLiver stiffness measurement among patients with chronic hepatitis B and C: results from a 5-year prospective studyPloS One2014911e11191225369038
- BoursierJZarskiJPde LedinghenVDetermination of reliability criteria for liver stiffness evaluation by transient elastographyHepatology20135731182119122899556
- CharlsonMEPompeiPAlesKLMacKenzieCRA new method of classifying prognostic comorbidity in longitudinal studies: development and validationJ Chronic Dis19874053733833558716
- QuanHSundararajanVHalfonPCoding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative dataMed Care20051130113916224307
- GannaAIngelssonE5 Year mortality predictors in 498,103 UK Biobank participants: a prospective population-based studyLancet2015386999353354026049253
- Danish Health AuthorityAnbefalinger om alkohol2010 Available from: https://sundhedsstyrelsen.dk/da/sundhed-og-livsstil/alkohol/anbefalingerAccessed February 25, 2016
- RostgaardKMethods for stratification of person-time and events: a prerequisite for Poisson regression and SIR estimationEpidemiol Perspect Innov20085719014582
- FoundationRThe R project for statistical computing2017 Available from: https://www.r-project.orgAccessed January 21, 2016
- KanwalFKramerJRIlyasJDuanZEl-SeragHBHCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. veterans with HCVHepatology20146019810524615981
- European Association for the Study of the LiverEASL clinical practice guidelines: management of hepatitis C virus infectionJ Hepatol201155224526421371579
- van der MeerAJFeldJJHoferHRisk of cirrhosis-related complications in patients with advanced fibrosis following hepatitis C virus eradicationJ Hepatol201766348549327780714
- El-SeragHBKanwalFRichardsonPKramerJRisk of hepatocellular carcinoma after sustained virologic response in veterans with HCV-infection: HCC after SVR2016 Available from: https://dissem.in/p/80365084/risk-of-hepatocellular-carcinoma-after-sustained-virologic-response-in-veterans-with-hcv-infection-hcc-after-svrAccessed August 31, 2017
- RatziuVMunteanuMCharlotteFBonyhayLPoynardTFibrogenic impact of high serum glucose in chronic hepatitis CJ Hepatol20033961049105514642625
- BochudPYCaiTOverbeckKGenotype 3 is associated with accelerated fibrosis progression in chronic hepatitis CJ Hepatol200951465566619665246
- McMahonBJBrudenDTownshend-BulsonLInfection with hepatitis C virus genotype 3 is an independent risk factor for end-stage liver disease, hepatocellular carcinoma, and liver-related deathClin Gastroenterol Hepatol2017153431437.e227765729
- BaffyGBruntEMCaldwellSHHepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menaceJ Hepatol20125661384139122326465
- DavilaJAMorganROShaibYMcGlynnKAEl-SeragHBDiabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control studyGut200554453353915753540
- AsselahTRubbia-BrandtLMarcellinPNegroFSteatosis in chronic hepatitis C: why does it really matter?Gut200655112313016344578
- DegosFChristidisCGanne-CarrieNHepatitis C virus related cirrhosis: time to occurrence of hepatocellular carcinoma and deathGut200047113113610861275
- ThygesenSKChristiansenCFChristensenSLashTLSørensenHTThe predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish national registry of patientsBMC Med Res Methodol2011118321619668
- NielsenGLSørensenHTPedersenABSabroeSAnalyses of data quality in registries concerning diabetes mellitus: a comparison between a population based hospital discharge and an insulin prescription registryJ Med Syst19962011108708487
- LokASSeeffLBMorganTRIncidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver diseaseGastroenterology2009136113814818848939
- BrunoSStroffoliniTColomboMSustained virological response to interferon-α is associated with improved outcome in HCV-related cirrhosis: a retrospective studyHepatology200745357958717326216
- CardosoACMoucariRFigueiredo-MendesCImpact of pegin-terferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosisJ Hepatol201052565265720346533
- DienstagJLGhanyMGMorganTRA prospective study of the rate of progression in compensated, histologically advanced chronic hepatitis CHepatology201154239640521520194
- European Association for Study of Liver, European Organisation for Research and Treatment of CancerEASL-EORTC clinical practice guidelines: management of hepatocellular carcinomaJ Hepatol201256490894322424438
- SarasinFPGiostraEHadengueACost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosisAm J Med199610144224348873514
- ChristensenPBHayGJepsenPHepatitis C prevalence in Den-mark: an estimate based on multiple national registersBMC Infect Dis201212122214291
- TörnerAStokkelandKSvenssonAThe underreporting of hepatocellular carcinoma to the cancer register and a log-linear model to estimate a more correct incidenceHepatology201765388589227533761
