Abstract
Purpose
The purpose of this study was to investigate the effects of breast density on breast cancer risk among women screened via a nationwide mammographic screening program.
Patients and methods
We conducted a nested case–control study for a randomly selected population of 1,561 breast cancer patients and 6,002 matched controls from the National Cancer Screening Program. Breast density was measured and recorded by two independent radiologists using the Breast Imaging Reporting and Data System (BI-RADS). Associations between BI-RADS density and breast cancer risk were evaluated according to screening results, time elapsed since receiving non-recall results, age, and menopausal status after adjusting for possible covariates.
Results
Breast cancer risk for women with extremely dense breasts was five times higher (adjusted odds ratio [aOR] =5.0; 95% confidence interval [CI]) =3.7–6.7) than that for women with an almost entirely fatty breast, although the risk differed between recalled women (aOR =3.3, 95% CI =2.3–3.6) and women with non-recalled results (aOR =12.1, 95% CI =6.3–23.3, P-heterogeneity =0.001). aORs for BI-RADS categories of breast density were similar when subjects who developed cancer after showing non-recall findings during initial screening were grouped according to time until cancer diagnosis thereafter (<1 and ≥1 year). The prevalence of dense breasts was higher in younger women, and the association between a denser breast and breast cancer was stronger in younger women (heterogeneously dense breast: aOR =7.0, 95% CI =2.4–20.3, women in their 40s) than older women (aOR =2.5, 95% CI =1.1–6.0, women in their 70s or more). In addition, while the positive association remained, irrespective of menopausal status, the effect of a dense breast on breast cancer risk was stronger in premenopausal women.
Conclusion
This study confirmed an increased risk of breast cancer with greater breast density in Korean women which was consistent regardless of BI-RADS assessment category, time interval after initially non-recall results, and menopausal status.
Introduction
On a mammogram, breast density reflects the glandular tissue composition of the breast, and several meta-analyses support considering breast density as a risk factor of breast cancer.Citation1–Citation3 Although breast density is an established risk factor for breast cancer, the extent of masking effects of breast density on tumors of the breast, which increases the likelihood of false-negative results, and the resultant effect thereof on the strength of the association are yet to be determined.Citation4,Citation5 In addition, considering that breast density tends to decrease with increasing age while breast cancer rates tend to increase, the association between breast density and breast cancer is somewhat paradoxical. As an interpretation of this paradox, researchers have suggested a cumulative effect for breast density and increasing age, as both seem to be related with an increased incidence of breast cancer.Citation1 Meanwhile, however, in Asian countries, breast densities and the proportion of younger women with breast cancer are higher than in Western countries.Citation6–Citation8
Studies of the effect of breast density on breast cancer risk have mostly been conducted for Western populations,Citation2,Citation9 for whom mammographic screening is routinely recommended as part of both organized and opportunistic screening programs. Across Asia, however, mammographic screening is not commonly conducted,Citation10 and uptake rates for mammographic screening are low.Citation11 Moreover, only a few countries including Korea, Japan, and Taiwan offer mammographic screening as part of organized screening programs for individuals aged ≥40 years.Citation12,Citation13 While a few studies in Asia have aimed to assess breast density and breast cancer risk, their study populations included only individuals who underwent opportunistic screening, were of small sample sizes, and had limited representativeness.Citation14–Citation16 Thus, the effect of breast density on breast cancer risk needs to be further evaluated for Asian women.
In Korea, the National Cancer Screening Program (NCSP) provides screening services for stomach, liver, colorectal, breast, and cervical cancer. Breast cancer screening was implemented in 2002, and biennial breast cancer screening by mammography taken from craniocaudal (CC) and mediolateral oblique (MLO) views is recommended for women aged ≥40 years.Citation17
Accordingly, this study was designed to investigate the effect of mammographic breast density using the American College of Radiology Breast Imaging Reporting and Data System (ACR BI-RADS),Citation18 a standardized breast density measurement technique, on subsequent breast cancer risk in women, by assessing data from a nationwide breast cancer screening program in Korea. In addition, to identify the independent effect of breast density on breast cancer risk and the effect apart from masking bias, associations were estimated by applying the following assessment categories: time interval after initial non-recall screening results, age, breast cancer type and stage, and menopausal status.
Patients and methods
Study population
In Korea, in 2015, around 1,800,000 women aged ≥40 years received mammographic breast cancer screening through NCSP, with the participation rate of 46%. Between 2007 and 2009, about 1,000,000–1,427,000 women received mammographic breast cancer screening with the participation rate of 30.2% in 2007, 34.9% in 2008, and 40.0% in 2009. To conduct a population-based, nested case–control study, representing all participants of the NCSP with follow-up data, a total of 86 breast cancer screening units were randomly selected after stratification by regional district and the proportion of women aged ≥40 years in each region, considering the sampling strategy utilized in our previous study.Citation19 We identified all the women who underwent breast cancer screening in these units between 2007 and 2009, and who were diagnosed with ductal carcinoma in situ (DCIS) or invasive breast cancer until December 2011, through a database linkage with the Korea Central Cancer Registry, from which we also obtained information on the date of diagnosis, disease code according to the International Statistical Classification of Disease and Related Health Problems, 10th version, and stage according to Surveillance, Epidemiology, and End Results (SEER). The data utilized in this study were obtained, and were available, upon request to the National Health Insurance Service and Korea Central Cancer Registry after reviewing our study proposal, which was approved by the Institutional Review Board of the National Cancer Center, IRB number NCC2016-0162.
Thus, we found 2,522 incident breast cancer cases, including both DCIS and invasive breast cancers. For each case, four controls who underwent screening but did not develop breast cancer were randomly selected and matched for age (within 2 years), screening unit, and date of screening. All the selected 10,088 controls were confirmed as not having developed breast cancer until the end of 2011 through linkage with the Korea Central Cancer Registry.
The mammography films of these selected women were obtained from the screening units in the form of analog films or Digital Imaging and Communications in Medicine files. Among the selected women, 3,304 women (26.2%) whose social security number was missing or whose CC and MLO images of both breasts were not stored, were excluded. After we obtained the mammograms, we additionally excluded 73 individuals (0.6%) whose initial cancer diagnosis was recorded before the screening date and subjects whose film status was not re-readable. Ten women (0.1%) with breast implants were excluded as well. About 1,660 people (13.2%) were excluded due to unmatched pairs. Finally, mammograms for 1,561 incident breast cancer cases and 6,002 controls were included in this study.
Mammography reading
Mammography imaging results have been reported by the NCSP using BI-RADS since 2007. To reduce variability in BI-RADS classification between radiologists in 86 screening units, six breast radiologists who were trained in BI-RADS classification of breast density and showed strong agreement for measurement of breast density were paired into three groups to re-assess breast density. The radiologists were blinded to case–control status and initial classification and were asked to determine the participants’ BI-RADS assessment category and BI-RADS breast density classification. Each BI-RADS assessment category was classified as follows: recalled results as category 0 (inconclusive) or categories 4 and 5 (suspicious for malignancy); non-recalled results as category 1 (negative finding) or 2 (benign findings). Density classifications were characterized as almost entirely fat, scattered fibroglandular densities, heterogeneously dense, and extremely dense. If two radiologists’ reviews were not consistent, they held discussions to reach an agreement thereon.
Covariates
Information on other breast cancer risk factors was obtained from self-reported questionnaires administered at the date of screening. The questionnaire surveyed age at menarche, number of live births, breast feeding duration, menopausal status, age at menopause, hormone replacement therapy use, oral contraceptive use, height, weight, and past benign breast disease. Body mass index, calculated according to height and weight, was also included as a covariate.
Statistical analysis
The characteristics of the incident breast cancer subjects and matched controls were compared using a t-test for age (continuous variable) and the chi-square test for other categorical variables. All P-values were obtained from two-tailed tests.
The effects of breast density according to BI-RADS classification on breast cancer risk were analyzed using conditional logistic regression for matched analysis (whole analysis) or logistic regression for unmatched analysis (subgroup analysis according to age group, screening results, assessment category, time interval after non-recall screening, and menopausal status), adjusting for the covariates mentioned above. Missing variables were treated as dummies. Cochran–Armitage test was applied to test for trends in associations between increased breast density and breast cancer risk. We estimated adjusted odds ratio (aOR) and 95% confidence intervals (CIs) and stratified them according to screening results category: recall (BI-RADS categories 0, 4, and 5) and non-recall (BI-RADS categories 1 and 2). To avoid masking effects, recalled cases were divided by each BI-RADS assessment category, and non-recalled cases were divided into cases detected <1 year after initially receiving non-recall screening results and cases detected ≥1 year after initially receiving non-recall screening results. Cancer cases were divided by DCIS and invasive cancer, and invasive cancer cases were further divided by SEER stage and time after receiving non-recall screening results. In addition, the associations between breast density and breast cancer risk were evaluated according to 10-year group intervals and menopausal status (premenopause and postmenopause).
Heterogeneity in detection methods or BI-RADS assessment categories was assessed using Cochran’s Q test.Citation20 All analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, NC, USA) and Stata/SE (ver. 12.0; StataCorp LP, College Station, TX, USA) software packages.
Results
shows the distribution of breast cancer risk factors and breast densities according to BI-RADS for incident breast cancer cases and matched controls. Among the incident breast cancer cases, the proportions of heterogeneously or extremely dense breasts were higher (P<0.001). The mean follow-up periods for breast cancer cases and controls were 178 days (range: 0–977 days) and 1,278 days (range: 740–1,817 days, data not shown), respectively.
Table 1 Baseline characteristics of breast density and selected risk factors of breast cancer of 1,561 pairs of breast cancer cases and matched controls
shows the aORs of breast cancer risk, according to BI-RADS breast density and age. Compared to women with an almost entirely fatty breast, the risk of breast cancer for those who had an extremely dense breast was five times higher (OR =5.0; 95% CI =3.7–6.7). The effect of increased breast density on breast cancer risk was greater in younger age groups, and the strength of the association between denser breasts and breast cancer risk decreased as age increased. The aOR for heterogeneously dense breasts, compared to entirely fatty breasts, was 7.0 (95% CI =2.4–20.3) for women in their 40s and decreased to 2.5 (95% CI =1.1–6.0) for women in their 70s or older. Similarly, the aOR for extremely dense breasts was 9.4 (95% CI =3.2–27.4) for women in their 40s and decreased to 5.1 (95% CI =2.1–12.2) for women in their 60s. No women in their 70s had extremely dense breasts.
Table 2 Breast density as risk factor of breast cancer according to the age groupTable Footnotea
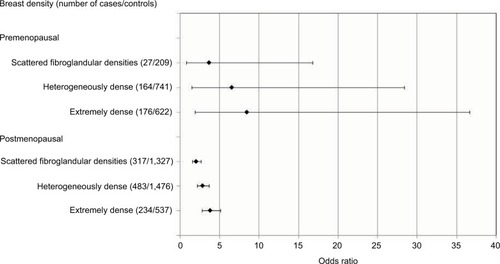
When the effect of breast density was estimated according to menopausal status (), breast cancer risk tended to increase as BI-RADS breast density increased in both premenopausal and postmenopausal women. The aORs of breast cancer for an extremely dense breast were 8.5 (95% CI =1.9–36.7) in premenopausal women and 3.8 (95% CI =2.8–5.1) in postmenopausal women.
Figure 1 Breast density as risk factor of breast cancer according to menopausal status. Odds ratios are adjusted for age, age at menarche, number of live births, breast feeding, menopausal status/age at menopause, hormone replacement therapy use, oral contraceptive use, body mass index, past benign breast disease and calculated by logistic regression. In total, 139 cases and 329 controls whose menopausal status were missing were excluded from analysis.
For the recalled cases and controls, the aOR of breast cancer for women with an extremely dense breast was 3.3 (95% CI =2.3–3.6), and in women with non-recall screening results and controls, the aOR was 12.1 (95% CI =6.3–23.3, ). For both recalled and non-recalled results, there was an increased trend of cancer risk with elevated BI-RADS density classification (P-trend <0.001). The risk of breast cancer in an extremely dense breast in comparison with an almost entirely fatty breast was significantly higher for non-recalled cases, compared with recalled cases (P-heterogeneity =0.001). Among the recalled cases, the effect of breast density on breast cancer differed according to BI-RADS assessment categories. Although breast cancer risk tended to increase as BI-RADS breast density increased among women with heterogeneously dense and extremely dense breasts, compared to those with an almost fatty breast, the risk of breast cancer was significantly higher for category 0 subjects than for category 4 or 5 subjects (P-heterogeneity =0.040 and 0.003, respectively). In patients with non-recall findings on mammography, breast cancer risk also tended to increase as BI-RADS density increased, irrespective of time interval after an initial non-recall screening (<1 year and ≥1 year); however, no heterogeneity in breast cancer risk was observed according to time interval. The effect of greater breast density on breast cancer risk remained obvious in cases detected ≥1 year after initially receiving non-recall results. A more detailed analysis according to SEER stage is presented in .
Table 3 Breast density as risk factor of breast cancer according to screening results, assessment category, and time interval after non-recall screeningTable Footnotea
Discussion
As a study of population-based screening, the present study is the first to investigate the effect of breast density on breast cancer risk in a large number of Asians. Herein, women with extremely dense breasts exhibited a five times higher risk of breast cancer than women with an entirely fatty breast. The independent effect of breast density on breast cancer risk remained prominent irrespective of masking effects. In addition, while there was a positive association between breast density and breast cancer risk, irrespective of age or menopausal status, the effect of a dense breast on breast cancer risk was stronger in women of younger age and in premenopausal women.
Previous studies conducted in Asia consistently showed that breast density was a significant risk factor of breast cancer,Citation14–Citation16,Citation21 irrespective of subtypesCitation22 and the strength of association of highest density category compared with lowest category ranged between 2.6 and 5.5.Citation14,Citation15 A study conducted in Korea also has suggested that increases of 1 standard deviation in absolute dense area and percentage dense area increase the risk of breast cancer by about 1.20-fold.Citation16 The previous Asian results were comparable with previous meta-analysis studies in which most of the included studies were from Western population.Citation2,Citation3
However, in contrast to previous meta-analyses, which showed that the risk of breast cancer in women of older age increases with greater breast densityCitation2 or that there is no association,Citation23 our study discovered a stronger effect of breast density on breast cancer in younger women, which was in line with a previous study in Korea.Citation16 In Western countries, the probability of breast cancer development increases with increasing age;Citation24 however, in Korea, breast cancer risk is highest in women aged 45–49 years.Citation25 This age-specific breast cancer rate may have influenced the reduced effect of breast density on breast cancer risk with increasing age. Similarly, we noted a higher risk of breast cancer in premenopausal women with dense breasts than postmenopausal women. Nevertheless, while the magnitude of the OR for breast cancer was much greater in premenopausal women, P for heterogeneity therein did not reflect a statistically significant difference, as has been previously reported.Citation16 Meanwhile, in Korea, the prevalence of dense breasts more rapidly decreased as age increased (from >90% in women aged <40 years to around 20% in women aged 70–79 years), compared to Western populations (from around 60% in women aged <40 years to around 20% in women aged 70–79 years).Citation26 This could explain the inconsistencies between our results and previous studies that have shown associations between dense breasts and breast cancer risk only in postmenopausal women.Citation14,Citation27
A marked increase in breast cancer risk among women with extremely dense breasts who receive non-recall screening results, compared with recalled cases, might be partly attributable to masking bias, which refers to the masking of cancers, by a highly dense breast on mammograms, that manifest in later years.Citation4,Citation28 Several studies have estimated the risk of a dense breast on breast cancer up to 12Citation2,Citation28,Citation29 or 24 months after non-recall screening resultCitation30 could be overestimated due to masking effect. In this study, due to the short follow-up time in breast cancer cases (maximum period from screening and cancer detection was 2.7 years), we could not assess the long-term breast cancer risk in relation to breast density. Notwithstanding, even in BI-RADS category 4 or 5, the risk of breast cancer increased as density increased, suggesting that the risk associated with breast density would be independent. Nevertheless, the strength of this association might be affected by masking effects, which caused more missed cases in females with dense breasts. We also identified a greater increase in the risk of breast cancer in women with a dense breast diagnosed within 1 year after a non-recalled screening result, compared to women diagnosed after 1 year of receiving non-recalled results. While this also suggests the presence of masking effects, the risk after 12 months was greater than that reported in previous studies.Citation2,Citation29 To better address the effect of masking bias on cancer risk, the invasive breast cancer cases were divided by stage (). For advanced cases, a portion of missing cases due to masking effect would have remained for those diagnosed ≥1 year after initially receiving non-recalled results. However, this would be minimized in early stage breast cancer cases (localized breast cancer). In our study, ORs for breast cancer were high in localized breast cancer cases diagnosed at ≥1 year after initially receiving non-recalled results. Thus, we suggest that, despite a certain degree of masking bias, which was more prominent in early stages, the independent risk of breast density on breast cancer apart from masking effects is prominent.
Methods of breast density measurement include qualitative and quantitative approaches, among which percentage density shows the strongest association with cancer risk.Citation2,Citation3 Measuring breast density with quantitative methods intuitively leads to classifying density results into <5%, 5%–24%, 25%–49%, 50%–74%, and ≥75%Citation2 to achieve clinical meaning and to facilitate interpretation of results. However, considering that film mammography has been recommended as a standard screening method for the past few decades, classifying breast density according to BI-RADS classification might be more practical for risk identification in screening settings. Also, good correlation between automated quantitative volumetric assessment and BI-RADS visual density categories has been reported.Citation31,Citation32 Our results using BI-RADS classification were comparable to results in both meta-analyses using BI-RADS classification and those using percent densities (<5% compared to ≥75%).Citation2
Several limitations to this study warrant consideration. First, the follow-up period was short (maximum 2.7 years), and thus, it was impossible to identify interval breast cancers or the long-term effects of breast density on cancer risk. In addition, our criterion for the follow-up time of non-recalled results (1 year) might not be enough to exclude overlooked cancers at the time of screening, resulting in overestimation of the breast density effect.Citation9 However, when we divided the results by 1.5 and 2 years after receiving non-recalled results, the results were not much different (data not shown). About 26% of the participants whose social security number was missing or whose image of both breasts were not stored were excluded. In such instances, comparing baseline characteristics between excluded people and included people is a commonly applied method to identify the presence of selection bias.Citation33 However, we could not compare them because of a lack of information. Third, information on possible confounding variables was self-reported, and thus would introduce information bias. However, the bias would be non-differential and give us outcomes that are more conservative. Fourth, some of the major confounders that affect both breast cancer and mammographic density, such as age at first birth, type of hormone replacement therapy, or oral contraceptive use, could not be considered in this study because they were not included in the questionnaires. In addition, some of the covariates, such as body mass index, hormone replacement therapy, or oral contraceptive use, had a high proportion of missing data. To keep the number of the study population high in the multivariate analysis, we treated these covariates as missing variables, although this might cause biased results, considering the differences in the proportions of missing data between cases and controls. Fifth, due to the small number of younger women with an almost entirely fatty breast (reference group) and too few older women with an extremely dense breast, the confidence intervals of the subgroup analysis were wide, despite the observed statistical significance.
Conclusion
This study confirmed an increased risk of breast cancer in association with breast density measured by BI-RADS classification in an East Asian population. The increased risk was consistent irrespective of the screening results (recalled cases and non-recalled cases), BI-RADS assessment category, and follow-up period after non-recalled results, suggesting an independent effect of breast density on subsequent cancer development. However, a larger number of overlooked cancers may have been present in women with dense breasts, and a greater breast density was observed in younger women.
Acknowledgments
This study was funded by the National Cancer Center, Korea (number 1610402) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2016R1C1B1013621).
Supplementary material
Table S1 Breast density as risk factor of breast cancer according to results of screening, time interval after negative screening, type of breast cancer, and Surveillance, Epidemiology, and End Results stageTable Footnotea
Disclosure
The authors report no conflicts of interest in this work.
References
- BoydNFMartinLJYaffeMJMinkinSMammographic density and breast cancer risk: current understanding and future prospectsBreast Cancer Res20111322322114898
- McCormackVAdos Santos SilvaIBreast density and parenchymal patterns as markers of breast cancer risk: a meta-analysisCancer Epidemiol Biomarkers Prev2006151159116916775176
- PetterssonAGraffREUrsinGMammographic density phenotypes and risk of breast cancer: a meta-analysisJ Natl Cancer Inst20141065piidju078
- ColinCPrinceVValettePJCan mammographic assessments lead to consider density as a risk factor for breast cancer?Eur J Radiol20138240441120133095
- van der WaalDRippingTMVerbeekALBroedersMJBreast cancer screening effect across breast density strata: a case-control studyInt J Cancer20161401414927632020
- FanLZhengYYuK-DBreast cancer in a transitional society over 18 years: trends and present status in Shanghai, ChinaBreast Cancer Res Treat200911740941619153831
- HanJ-gJiangY-dZhangC-hClinicopathologic characteristics and prognosis of young patients with breast cancerBreast20112037037221392995
- LiJZhangB-NFanJ-HA nation-wide multicenter 10-year (1999-2008) retrospective clinical epidemiological study of female breast cancer in ChinaBMC Cancer20111136421859480
- VachonCMVan GilsCHSellersTAMammographic density, breast cancer risk and risk predictionBreast Cancer Res2007921718190724
- Bhoo-PathyNYipCHHartmanMBreast cancer research in Asia: adopt or adapt Western knowledge?Eur J Cancer20134970370923040889
- SaikaKSobueTTime trends in breast cancer screening rates in the OECD countriesJpn J Clin Oncol20114159159221447696
- LeeEHParkBKimN-SThe Korean guideline for breast cancer screeningJ Korean Med Assoc201558408419
- HamashimaCHamashimaCCHattoriMThe Japanese guidelines for breast cancer screeningJpn J Clin Oncol20164648249227207993
- WongCLimGGaoFMammographic density and its interaction with other breast cancer risk factors in an Asian populationBr J Cancer201110487187421245860
- JakesRWDuffySWNgFCGaoFNgEHMammographic parenchy-mal patterns and risk of breast cancer at and after a prevalence screen in Singaporean womenInt J Epidemiol200029111910750598
- KimB-KChoiY-HNguyenTLMammographic density and risk of breast cancer in Korean womenEurJ Cancer Prev201524542242925370685
- SuhMChoiKSParkBTrends in Cancer Screening Rates among Korean Men and Women: Results of the Korean National Cancer Screening Survey, 2004-2013Cancer Res Treat20164811025943324
- American College of RadiologyACR BI-RADS® Breast Imaging Reporting and Data System, Breast Imaging Atlas, Mammography4th edPreston, VAAmerican College of Radiology2003
- JunJKKimMJChoiKSSuhMJungKWDevelopment of a sampling strategy and sample size calculation to estimate the distribution of mammographic breast density in Korean womenAsian Pac J Cancer Prev2012134661466423167398
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ200332755712958120
- NguyenTLChoiYHAungYKBreast Cancer Risk Associations with digital mammographic density by pixel brightness threshold and mammographic systemRadiology2017 Epub1016
- ShinJLeeJEKoHYAssociation between mammographic density and tumor marker-defined breast cancer subtypes: a case-control studyEur J Cancer Prev2017 Epub927
- RazzaghiHTroesterMAGierachGLMammographic density and breast cancer risk in White and African American WomenBreast Cancer Res Treat201213557158022864770
- BoydNFRommensJMVogtKMammographic breast density as an intermediate phenotype for breast cancerLancet Oncol2005679880816198986
- JungKWWonYJOhCMCancer statistics in Korea: incidence, mortality, survival, and prevalence in 2014Cancer Res Treat20174929230528279062
- YounIChoiSKookSHChoiYJMammographic breast density evaluation in Korean women using fully automated volumetric assessmentJ Korean Med Sci20163145746226955249
- NagataCMatsubaraTFujitaHMammographic density and the risk of breast cancer in Japanese womenBr J Cancer2005922102210615956963
- van GilsCHOttenJDVerbeekALHendriksJHMammographic breast density and risk of breast cancer: masking bias or causality?Eur J Epidemiol1998143153209690746
- BoydNFGuoHMartinLJMammographic density and the risk and detection of breast cancerN Engl J Med200735622723617229950
- YaghjyanLColditzGARosnerBTamimiRMMammographic breast density and subsequent risk of breast cancer in postmenopausal women according to the time since the mammogramCancer Epidemiol Biomarkers Prev2013221110111723603205
- GweonHMYoukJHKimJASonEJRadiologist assessment of breast density by BI-RADS categories versus fully automated volumetric assessmentAJR Am J Roentgenol201320169269723971465
- SeoJMKoESHanBKKoEYShinJHHahnSYAutomated volumetric breast density estimation: a comparison with visual assessmentClin Radiol20136869069523434202
- GordisLEpidemiology5th edPhiladelphia, PAElsevier Saunders2013
- MelnikowJFentonJJWhitlockEPSupplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task ForceAnn Intern Med2016164426827826757021
