Abstract
Background
The tonsils are immunological gatekeepers against pathogens. Immunological response to tonsillitis may vary clinically from no enlargement of the tonsils to nearly obstructive conditions. In this investigation, we studied the familial aggregation of tonsillectomy, as an indicator of the extent to which tonsillar immune responses to infections might be genetically controlled.
Methods
Data on kinship relations and vital status from the Danish Civil Registration System were used to establish a cohort of Danes with relatives born since 1977. Tonsillectomies in all hospitals and clinics from 1977 to 2013 were identified in national registers together with the indication for tonsillectomy. Rate ratios (RRs) for tonsillectomy >1 year after tonsillectomy in specific types of relatives (first to fourth degree) were estimated in Poisson regression models with adjustment for calendar period, sex, age, and total number of specified relatives.
Results
A cohort of 2.4 million persons was followed for 44,100,697 million person-years (mean 18.4 years/person), and included 148,190 tonsillectomies. RRs of tonsillectomy were consistently higher when the relatedness and the number of tonsillectomized relatives were higher. RRs were similar in boys and girls, but were larger in early childhood. Additional analyses suggested that this relatively higher RR at younger ages was due to a larger influence of shared environment at younger ages, whereas the genetic influence was similar at all ages. Results were similar for tonsillectomies performed strictly due to tonsillitis.
Conclusions
Genetic factors appear to predispose to severe tonsillitis underlying tonsillectomies, regardless of age and sex. Further studies are needed to understand how genes regulate the tonsils’ immune response against infections.
Introduction
The tonsils are the immunological gatekeepers against pathogens entering through the mouth and nose, as their specialized crypt structure effectively captures antigens.Citation1 Subsequent immune responses may result in tonsillitis and tonsil enlargement of varying severity, ranging from temporary narrowing of the pharynx with accompanying tenderness to dysphagia, dyspnea, and often severe bouts of tonsillitis. Tonsillectomy is considered to reduce obstructive symptoms, the frequency of tonsillitis episodes, and the number of infections beginning in the tonsils and spreading elsewhere in the respiratory tract.Citation2–Citation4
In Denmark, 6%–9% of persons <20 years of age underwent tonsillectomy in the period between 1980 and 2001.Citation5 However, tonsillectomy rates vary greatly during childhood,Citation6–Citation9 with peaks in early childhood (age 4–5 years) and adolescence (age 16–17 years).Citation5,Citation8,Citation9 Admissions for chronic tonsillar disease (with or without accompanying tonsillectomy) demonstrate similar patterns.Citation5 The reason for these peaks remains unclear, but both environmental and genetic factors may play a role. Immune responses to infections may be attributed to a degree of genetic control, for example, as indicated by the role of the CCR5 gene in controlling time to acquired immunodeficiency syndrome and the role of the mannose-binding lectin gene in controlling effective responses to infant infections.Citation10,Citation11 In the case of tonsillitis episodes, twin studies indicate a considerable genetic component underlying recurrent tonsillitis and tonsillectomy.Citation12,Citation13 For example, 1 twin study reported age at tonsillectomy to be more similar among monozygotic than dizygotic twins.Citation13
We studied the potential influence of genetic control of the tonsillar immune response by using Denmark’s national population-based registers and the Danish Family Relations Database to describe the familial aggregation of nearly 150,000 tonsillectomies by age and sex. After considering the role of environmental factors,Citation5,Citation13 such as familial preferences for tonsillectomy, we modeled the familial aggregation to isolate a possible genetic component using principles of basic heredity patterns.
Methods
Data sources
Civil registration system
Using the unique personal identification number assigned to each Danish resident, the Civil Registration System has since 1968 registered demographic and vital status information of all persons residing in Denmark.Citation14,Citation15 The personal identification number permits follow-up of all persons living in Denmark and accurate linking of individual-level information from Denmark’s nationwide population-based registers.
National hospital discharge register
The hospital discharge register contains discharge diagnoses of all patients admitted to Danish hospitals since 1977, hospital outpatient diagnoses from 1995 onward, and procedure codes for all surgical procedures performed in Danish hospitals since 1977.Citation16 Between 1977 and 1995, surgical procedures were coded using the Danish National Board of Health’s Classification of Surgical Procedures and Treatments (OBK, first to third eds.). Since 1996, surgical procedures have been coded using the Nordic Medico-Statistical Committee Classification of Surgical Procedures (NCSP). Between 1977 and 1993, discharge diagnoses were coded using the International Classification of Diseases (ICD), version 8, with version 10 being used since 1994.
National health service register
The health service register has records of all primary health care services delivered to persons in Denmark since 1990, including the provider’s medical specialty, the type of service, and the calendar week in which the service was provided.Citation17,Citation18 The register is virtually complete because registration is required for providers to be reimbursed by the state, and the state reimburses for most services (universal healthcare). In recent decades, many planned inpatient tonsillectomies have become outpatient procedures, being performed either in hospital settings or in otolaryngology clinics.Citation19
Danish family relations database
The database is based on kinship information in the Civil Registration System and permits the identification of relatives for persons with family in Denmark.Citation20–Citation24 Parents, siblings and half-siblings can be identified for nearly all persons born from 1950 and onwards (presently 2013), whereas grandparents, aunts/uncles and cousins are identifiable for almost 90% of persons born from 1985 and onwards (presently 2013).
Ethics
The study was based on existing data in national registries and was approved by the Danish Protection Agency (j.nr. 2015-57-0102). Because subjects were not contacted as part of the study, written informed consent was not required.
Study population
Using the Civil Registration System, we established a cohort of persons born in Denmark who were alive in 1977, or born later, and for whom any of the relatives were born in Denmark since 1977. In this way, cohort members had nearly complete registration of tonsillectomies and also constituted about half of the Danish population. Thus, the sample size of the cohort was determined by the size of the Danish population and there was no pre-study calculation of sample size. Cohort members were followed from January 1, 1977, or date of birth, whichever came last, until the first of the following events: 1) tonsillectomy (outcome of interest); 2) death; 3) emigration; 4) classified “missing” in the Civil Registration System; or 5) October 31, 2013 (end of follow-up). Also see Methods section in the Supplementary materials.
Identification of tonsillectomies
Persons registered in the National Hospital Discharge Register or the National Health Services Register during the follow-up period with any of the following codes were considered to have undergone tonsillectomy: OBK code 2614 (tonsillectomy); NCSP codes EMB10 (tonsillectomy) and EMB20 (adenotonsillectomy); and service code 3015 (tonsillectomy) reimbursed to otolaryngologists (medical specialty number 21). Information on indications was available for tonsillectomies performed in hospitals: tonsillitis was identified using ICD codes for acute, chronic, recurrent, or streptococcal tonsillitis (ICD8 codes 0340.0, 4630.x, 5000.0, 5000.2; ICD10 codes J03.x, J35.0). Overall, the most common diagnosis codes underlying the tonsillectomy indication were tonsillitis (60%), tonsil hypertrophy (19%), peritonsillar abscess (11%), and vegetative adenoids (4%); the remaining included more than 50 other diagnoses (6%).
Identification of relatives and determination of relatives’ history of tonsillectomy
For each cohort member, we used the Danish Family Relations Database to identify any relatives of the same generation (twins, siblings, half-siblings, cousins, half-cousins) or the previous generation (parents, uncles/aunts, half-uncles/half-aunts) registered in the Civil Registration System. Once all relatives were identified, we determined which, if any, underwent tonsillectomy before their respective cohort member underwent tonsillectomy (exposure of interest). Biological relatedness was defined by degrees and coefficients and was as follows:Citation25 same sex twins were assumed to comprise 50% monozygotes (first-degree relatives, coefficient 1.000) and 50% dizygotes (first-degree relatives, coefficient 0.500), yielding a combined coefficient of 0.500×1.000+0.500×0.500=0.750; different sex twins comprised 100% dizygotes (first-degree relatives, coefficient 0.500); siblings and parents (first-degree relatives, coefficient 0.500); half-siblings and uncles/aunts (second-degree relatives, coefficient 0.250); cousins and half-uncles/aunts (third-degree relatives, coefficient 0.125); half-cousins (fourth-degree relatives, coefficient 0.0625).
Statistical analyses
Familial aggregation of tonsillectomy was evaluated by comparing the rates of tonsillectomy in individuals with and without previously tonsillectomized relatives. More specifically, incidence rate ratios (RRs) were estimated comparing the rate of tonsillectomy for individuals with a given number (1, 2, or ≥3) of relatives of a certain type who had previously undergone tonsillectomy with the rate for individuals with at least the same number of relatives of the given type, none of whom had undergone the procedure. For example, when evaluating the risk of tonsillectomy associated with 2 tonsillectomized siblings, the RR compared the rate of tonsillectomy in individuals with 2 previously tonsillectomized siblings with that of individuals with at least 2 siblings, all of whom still retained their tonsils. We considered only previous tonsillectomies in relatives to ensure that when a pair of individuals were both cohort members, the pair contributed only once to our estimates.
RRs were estimated for each type of relative separately using log-linear Poisson regression models in SAS, version 9.2 (SAS Institute, Inc., Cary, NC, USA), with adjustment for calendar period (1-year categories) and number of relatives of a specified type, as well as for the interaction between sex and attained age (1-year categories).
In initial analyses, we evaluated the effect of the time elapsed between a relative’s tonsillectomy and tonsillectomy in a cohort member; we found higher rates of tonsillectomy associated with very recent tonsillectomy in a relative (<1 year previously) (Table S1), whereas rates associated with longer intervals were lower. Since shared sources of infections are most likely overrepresented shortly after “exposure”, we only present results for tonsillectomies occurring ≥1 year after a relative’s tonsillectomy.
In analyses estimating the age-interval-specific RRs, we excluded tonsillectomies at age 20+ years of both cohort persons and their relatives, because of small numbers. Furthermore, we presented the rate of tonsillectomy in a given age interval in individuals with and without a relative who were tonsillectomized in the same age interval, because the RR for comparisons with different age intervals were slightly lower (Table S2).
To evaluate the degree to which observed familial aggregation was due to shared genes vs shared environment, the RRs for family history were modeled as the product of a genetic component and a shared environmental component. In this context, we differentiated between RRs estimated for family history (“observed RRs”), and RRs estimated in the model (“estimated RRs”). For the technical details, see Methods section in the Supplementary materials.
Results
The study population included 2,399,717 individuals with at least one identifiable relative, and a total of 44,100,697 person-years of follow-up (mean: 18.4 years/person). Tonsillectomy was performed in 148,190 individuals from 1977 to 2013, with 133,552 (90.1%) performed in hospitals and 14,638 (9.9%) in otolaryngology clinics. Adenotonsillectomies constituted 29.8% of tonsillectomies performed in hospitals since 1996.
shows the RR for tonsillectomy by type and number of tonsillectomized relatives. The rate of tonsillectomy following tonsillectomy in relatives was up to 11 times higher (range 1.11–10.87) than the rate in persons whose relatives had not undergone the procedure. RR magnitudes increased with increasing degree of relatedness: having tonsillectomized siblings or parents yielded the greatest RRs, while the smallest increases in tonsillectomy rate were seen among persons with tonsillectomized cousins, half-cousins and half-uncles/aunts (). RR magnitudes also increased with increasing numbers of tonsillectomized relatives of a given degree of relatedness (). For example, having 2 tonsillectomized siblings or parents was associated with a 6-fold increase in the rate of tonsillectomy, and having 3 tonsillectomized siblings was associated with an almost 11-fold increase in the tonsillectomy rate. These findings were not materially different when stratified by total number of relatives (Table S3). We observed higher RRs of tonsillectomy when having tonsillectomized relatives on the maternal side than when having tonsillectomized relatives on the paternal side, although these patterns appeared limited to close maternal relatives (maternal half-siblings, ), and were not evident in more distant relatives (e.g., maternal half-cousins, Table S4).
Table 1 Rate ratio for tonsillectomy by type and number of tonsillectomized relatives, Denmark, 1977–2013
shows observed RRs – illustrated by colored data points – for tonsillectomy at age <10 years and 10–19 years, respectively, according to coefficient of relatedness to tonsillectomized relatives. The RRs for each data point are shown in Table S5. Generally, RRs were higher for children <10 years of age () than for older children (). For example, RRs for tonsillectomy following tonsillectomy in 1 sibling were 5.03 at <10 years of age and 2.42 at 10–19 years. Furthermore, for children <10 years of age (), associations were generally stronger for tonsillectomy in relatives with the same mother (full siblings and maternal half-siblings, red data points) than for relatives with different mothers (blue data points) but the same level of genetic relatedness. Such a difference was not observed for children aged 10–19 years ().
Figure 1 RRs for tonsillectomy at age <10 years (A) and 10–19 years (B) according to relatedness to tonsillectomized relatives, Denmark, 1977–2013.
Abbreviation: RR, rate ratio.
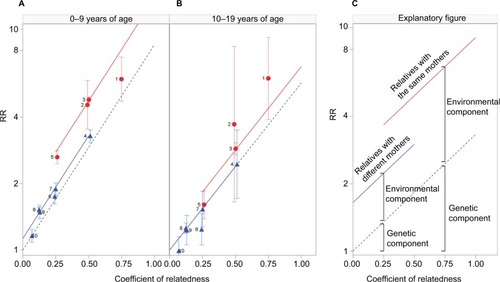
also include results from models of the genetic and environmental components of the familial aggregation of tonsillectomy in each of the 2 age groups. illustrates the key aspects of : the dashed line represents the genetic component, which increases with increasing degree of relatedness, while the red and blue lines represent the environmental component for relatives with the same mother and different mothers, respectively. Overall, the genetic component was estimated to be of similar size in the 2 age groups (as seen by the similar slopes of the dashed lines for the 2 age groups, also see the estimates in Methods section in the Supplementary materials), whereas a contribution from a shared environmental component was primarily observed in the youngest group (as seen by a larger intercept in the younger age group, also see the estimates in Methods section in the Supplementary materials). In fact, in the older group, the contribution from shared household (same mother) decreases considerably, while the non-shared household effect (different mothers) disappears.
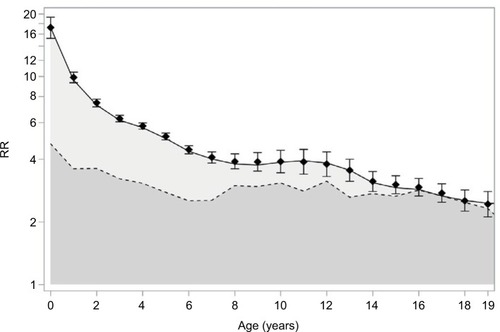
shows observed and estimated RRs for tonsillectomy in children with a previously tonsillectomized sibling, by age, along with the estimated contributions from genetics (dark gray area) and the shared environment (light gray area). These results supported the notion from that the particularly strong familial aggregation of tonsillectomy observed in younger children was due to a larger contribution of shared environment in this age group, whereas the genetic component of the association was similar for both age groups (for further background on this result, see Methods section in the Supplementary materials). Similar results for children with a tonsillectomized cousin are shown in Figure S1.
Figure 2 Age-specific RR for tonsillectomy following tonsillectomy in a sibling, Denmark, 1977–2013; an analysis among individuals with 1 sibling (i.e., RRs for pairs of siblings).
Abbreviation: RR, rate ratio.
The age-specific pattern shown in was observed in both males and females (Figure S2A and B). Furthermore, the RR for tonsillectomy associated with 1 sibling with tonsillectomy did not differ substantially by sex of the tonsillectomized sibling, for both males and females and among children <10 years and 10–19 years of age (Table S6).
When we repeated the analyses restricting to tonsillectomies indicated strictly due to tonsillitis, the results were similar to those shown in (Table S7).
Discussion
In a cohort of 2.4 million persons, we observed strong and consistent familial aggregation of tonsillectomy. A family history of tonsillectomy was associated with as much as an 11-fold increased risk of tonsillectomy, depending on the number and degree of relatedness of tonsillectomized relatives. The familial aggregation was strongest for children <10 years of age. The observed hereditary pattern of associations was compatible with a strong genetic component both in children <10 years of age and in those aged 10–19 years. However, there was also a substantial shared environmental component among the younger children that was not evident among children aged 10–19 years.
Our findings, based on almost 150,000 tonsillectomies, add population-based clinical evidence that there is a genetic component to chronic and/or recurrent acute tonsillitis leading to tonsillectomy, and describe age- and sex-specific aspects of this component that have never before been reported. Previously, a Norwegian study of 9479 twins aged 18–25 years provided evidence of a genetic predisposition for self-reported recurrent tonsillitis independent of sex,Citation12 and an Australian study of 7620 adult twins hinted at a genetic predisposition for self-reported tonsillectomy.Citation13 On the basis of the same case definition as in the present study, we recently conducted a genome-wide association study (GWAS) of tonsillectomy (~3000 cases) and identified and replicated an association between a genetic variant in the gene HORMAD2 and tonsillectomy.Citation26 Another recent GWAS based on self-reported tonsillectomy identified 36 genetic variants associated with tonsillectomy (60 cases),Citation27 but did not include replication of these associations in samples with clinically documented tonsillitis and tonsillectomy. Further studies are needed to characterize the functional mechanisms behind the associations.
Observed associations with tonsillectomy in relatives were strongest for children <10 years of age. Modeling of the genetic and environmental contributions to these associations suggested that this was due to a stronger contribution of shared environment in this age group than in older children. In contrast, the genetic contribution to the familial aggregation of tonsillectomy appeared to be constant throughout childhood.
Although our genetic model made certain assumptions, estimation of the genetic and environmental components of the familial aggregation of tonsillectomy was based directly on the observed RRs, which agreed well with the expected RRs estimated by the model (). Interpretation of the shared environment findings should also occur in the context of the specific model used. First, shared environment includes not only physical proximity and shared behaviors but also a shared threshold for intervention by tonsillectomy, given that families often share a general practitioner, views on the advisability of tonsillectomy vs waiting, and/or a local hospital or clinic (at which the surgery is performed), for example, having a particular professional culture (sometimes referred to as a “surgical signature”).Citation28,Citation29 Second, since shared environment will likely be more extensive for persons living together, the shared environment component in the model was allowed to differ for pairs of relatives with and without the same mother (an indicator of shared household). Third, since the first year of risk time after tonsillectomy in a relative was excluded from the analysis, the aspect of shared environment directly related to tonsillectomy per se (i.e., a shared contagion) was, to a large degree removed from the estimation.
Age-specific differences in the environmental contribution to the familial aggregation of tonsillectomy might help to explain the age- and sex-specific differences in tonsillectomy rates that have been noted in most reports from the last 40–50 years.Citation5,Citation8,Citation9,Citation30 The general observation has been that tonsillectomy rates peak in early childhood (predominantly in males) and again in adolescence (predominantly in females).Citation5,Citation8,Citation9 It has been speculated that parental preferences to tonsillectomy or different rates of chronic and/or recurrent acute tonsillitis in males and females might explain these differences.Citation5,Citation9 Our analysis provides a possible explanation for these differences, which have previously been difficult to study. We observed no strong contribution from shared familial environment among 10–19 year olds for either males or females; the genetic contribution in this age group corresponded to that for younger children. This finding supports the suggestion that in addition to a genetic contribution, the tonsillectomy peak in this age group could have non-genetic sources that are not predominantly shared among family members (e.g., sources associated with school or friends). In contrast, our findings in younger children support the hypothesis that the peak in tonsillectomy at 4–5 years of age has a strong non-genetic source predominantly shared among family members (e.g., siblings may share a threshold for tonsillectomy intervention by using the same general practitioner and local hospital).
The study was based on mandatory national (non-retrospective) registration of vital status, kinship links and disease in Denmark over 36 years. This unbiased inclusion of about half of the entire Danish population made selection bias negligible and allowed us to accrue a very large number of tonsillectomized individuals, permitting analyses stratified not only by relatedness but also by number of relatives of a given type.
As for other surgical operations in Denmark,Citation31 registration of tonsillectomies is close to complete. The incidence was 144 per 100,000 person-years, ranging up to 1000 for the 2 age-specific peaks.Citation5 In comparison, smaller studies range from 20 to 1000.Citation6,Citation8,Citation29,Citation30,Citation32,Citation33 A potential concern when we used tonsillectomy to address genetic control of the tonsillar immune response to infection, was that tonsillectomy is increasingly performed for a less infection-related cause than tonsillitis, notably tonsilar and adenoid hyperthrophy causing obstructive sleep apnea.Citation8,Citation34,Citation35 However, we adjusted RRs for calendar year and in the additional analysis, we found similar RRs when excluding tonsillectomies caused by tonsil hypertrophy or performed as adenotonsillectomies.
Conclusion
Our findings suggest that genetic factors predispose to severe tonsillitis underlying tonsillectomy, regardless of age and sex. Further studies are needed to understand how genes regulate the tonsils’ immune response to infections, and for example, whether genetic considerations could benefit the treatment of severe tonsillitis.
Acknowledgments
This work was supported by Oak Foundation Fellowships (grant number OCAY-12-319 to Drs Bager and Feenstra).
Disclosure
The authors report no conflicts of interest in this work.
References
- PerryMEThe specialised structure of crypt epithelium in the human palatine tonsil and its functional significanceJ Anat1994185Pt 11111277559106
- BurtonMJGlasziouPPChongLYVenekampRPTonsillectomy or adenotonsillectomy versus non-surgical treatment for chronic/recurrent acute tonsillitisCochrane Database Syst Rev201411CD001802
- MitkaMGuideline cites appropriateness criteria for performing tonsillectomy in childrenJAMA2011305766166221325178
- GeorgalasCCTolleyNSNarulaPATonsillitisBMJ Clin Evid201420140503
- VestergaardHWohlfahrtJWestergaardTPipperCRasmussenNMelbyeMIncidence of tonsillectomy in Denmark, 1980 to 2001Pediatr Infect Dis J200726121117112118043448
- Van Den AkkerEHHoesAWBurtonMJSchilderAGLarge international differences in (adeno)tonsillectomy ratesClin Otolaryngol Allied Sci200429216116415113303
- KoshyEMurrayJBottleASignificantly increasing hospital admissions for acute throat infections among children in England: is this related to tonsillectomy rates?Arch Dis Child201297121064106823079872
- EricksonBKLarsonDRSauverJLStMeverdenRAOrvidasLJChanges in incidence and indications of tonsillectomy and adenotonsillectomy, 1970–2005Otolaryngol Head Neck Surg2009140689490119467411
- FreemanJLJekelJFFreemanDHJrChanges in age and sex specific tonsillectomy rates: United States, 1970–1977Am J Public Health19827254884917065339
- StephensonJStudies probe new anti-HIV strategy, long-term success of prevention methodsJAMA2011305141397139921486966
- KochAMelbyeMSorensenPAcute respiratory tract infections and mannose-binding lectin insufficiency during early childhoodJAMA2001285101316132111255386
- KvestadEKvaernerKJRoysambETambsKHarrisJRMagnusPHeritability of recurrent tonsillitisArchiv Otolaryngol Head Neck Surg20051315383387
- MartinNGKehrenUBattistuttaDMathewsJDIatrogenic influences on the heritability of childhood tonsillectomy: cohort differences in twin concordanceActa Genet Med Gemellol (Roma)19914021651721759552
- PedersenCBThe Danish Civil Registration SystemScand J Public Health2011397 Suppl222521775345
- PedersenCBGotzscheHMollerJOMortensenPBThe Danish Civil Registration System. A cohort of eight million personsDan Med Bull200653444144917150149
- AndersenTFMadsenMJorgensenJMellemkjoerLOlsenJHThe Danish National Hospital Register. A valuable source of data for modern health sciencesDan Med Bull199946326326810421985
- AndersenJSOlivariusNFKrasnikAThe Danish National Health Service RegisterScand J Public Health2011397 Suppl343721775348
- OlivariusNFHollnagelHKrasnikAPedersenPAThorsenHThe Danish National Health Service Register. A tool for primary health care researchDan Med Bull19974444494539377908
- OvesenTKamarauskasAHlidarsdottirTDahlMRMainzJGood long-term results after tonsillectomy in ear, nose and throat practicesDan Med J2013605A463723673266
- OyenNRantheMFCarstensenLFamilial aggregation of lone atrial fibrillation in young personsJ Am Coll Cardiol2012601091792122726627
- SchnackTHPoulsenGMyrupCWohlfahrtJMelbyeMFamilial coaggregation of cryptorchidism, hypospadias, and testicular germ cell cancer: a nationwide cohort studyJ Natl Cancer Inst2010102318719220026812
- BoydHAPoulsenGWohlfahrtJMurrayJCFeenstraBMelbyeMMaternal contributions to preterm deliveryAm J Epidemiol2009170111358136419854807
- RostgaardKWohlfahrtJHjalgrimHA genetic basis for infectious mononucleosis: evidence from a family study of hospitalized cases in DenmarkClin Infect Dis201458121684168924696238
- RostgaardKNielsenTRWohlfahrtJSibship structure and risk of infectious mononucleosis: a population-based cohort studyInt J Epidemiol20144351607161425436250
- WrightSCoefficients of inbreeding and relationshipAm Nat192256645330338
- FeenstraBBagerPLiuXGenome-wide association study identifies variants in HORMAD2 associated with tonsillectomyJ Med Genet201754535836427941131
- PickrellJKBerisaTLiuJZSegurelLTungJYHindsDADetection and interpretation of shared genetic influences on 42 human traitsNat Genet201648770971727182965
- WeinsteinJNBronnerKKMorganTSWennbergJETrends and geographic variations in major surgery for degenerative diseases of the hip, knee, and spineHealth Aff (Millwood)2004Suppl VariationVAR81VAR8915471768
- National Health Service PHEThe NHS atlas of variation in Healthcare. 2015 compendium, Map 88: rate of elective admission to hospital for tonsillectomy in children aged 0–17 years per population by Clinical Commission Group (211 areas)National Health Service, Public Health of England2015228229 Available from: https://fingertips.phe.org.uk/profile/atlas-of-variationAccessed May 31, 2017
- McPhersonKWennbergJEHovindOBCliffordPSmall-area variations in the use of common surgical procedures: an international comparison of New England, England, and NorwayN Engl J Med198230721131013147133068
- SchmidtMSchmidtSASandegaardJLEhrensteinVPedersenLSorensenHTThe Danish National Patient Registry: a review of content, data quality, and research potentialClin Epidemiol2015744949026604824
- BhattacharyyaNLinHWChanges and consistencies in the epidemiology of pediatric adenotonsillar surgery, 1996–2006Otolaryngol Head Neck Surg2010143568068420974339
- PearsonRJSmedbyBBerfenstamRLoganRFBurgessAMJrPetersonOLHospital caseloads in Liverpool, New England, and Uppsala. An international comparisonLancet1968275675595664175411
- MarcusCLMooreRHRosenCLA randomized trial of adenotonsillectomy for childhood sleep apneaN Engl J Med2013368252366237623692173
- KalampoukaEMoudakiAMalakasiotiGPanaghiotopoulou-GartaganiPChrousosGKaditisAGFamily history of adenotonsillectomy as a risk factor for tonsillar hypertrophy and snoring in childhoodPediatr Pulmonol201449436637123775948
