Abstract
Background
Individuals with a family history of Parkinson’s disease (PD) appear to have a higher risk of developing PD and other neuropsychiatric diseases. However, estimates of the relative risks (RRs) of PD and the roles of genetic and environmental factors in PD susceptibility are unclear. The aim of this study was to examine familial aggregation and genetic contributions to PD and the RRs of other neuropsychiatric diseases in relatives of PD patients.
Methods
In this population-based family cohort study, the records of all individuals actively registered in the Taiwan National Health Insurance Research Database in 2015 were queried (N=24,349,599). In total, 149,187 individuals with a PD-affected parent, 3,698 with an affected offspring, 3,495 with an affected sibling, and 15 with an affected twin were identified. Diagnoses of PD were ascertained between January 1, 1999, and December 31, 2015. The prevalence and RRs of PD and other neuropsychiatric diseases in individuals with first-degree relatives with PD, as well as the contributions of heritability and environmental factors to PD susceptibility were investigated.
Results
The prevalence of PD was 0.46% in the general population and 0.52% in individuals with first-degree relatives with PD. The RR (95% CI) for PD was 2.20 (1.41–3.45) for siblings, 1.59 (1.47–1.73) for parents, 1.86 (1.63–2.11) for offspring, 63.12 (16.45–242.16) for twins, and 1.46 (1.41–1.52) for spouses. The RR (95% CI) in individuals with first-degree relatives with PD was 1.66 (1.57–1.76) for essential tremor, 1.68 (1.61–1.75) for schizophrenia, and 1.20 (1.12–1.28) for Alzheimer’s disease. The estimated contribution to the phenotypic variance of PD was 11.0% for heritability, 9.1% for shared environmental factors, and 79.9% for non-shared environmental factors.
Conclusion
First-degree relatives of PD patients are more likely to develop PD and other neuropsychiatric diseases. Environmental factors account for a high proportion of the phenotypic variance of PD.
Introduction
Parkinson’s disease (PD) is a common age-related neurodegenerative disorder that is characterized by the degeneration of dopaminergic neurons in the substantia nigra, leading to resting tremor, rigidity, bradykinesia, postural instability, and disability.Citation1 The accompanying degeneration of other neurons in PD can cause non-motor manifestations, including autonomic symptoms such as constipation, psychiatric problems such as anxiety and depression, and cognitive decline.Citation1 PD mainly affects elderly individuals and its incidence increases steadily with age; only around 5%–6% of PD cases have an onset age under 50 years (i.e., early-onset PD).Citation2,Citation3 As there are currently no available treatments that can cure or halt disease progression, the increasing prevalence of PD worldwide has imposed an overwhelming burden on aging societies.Citation4
Although most PD cases are sporadic, the etiology of PD is generally ascribed to a combination of genetic and environmental factors.Citation5,Citation6 A large-scale genome-wide association study (GWAS) of European cohorts estimated that genetic factors explain at least one-fourth of the PD liability; however, known mutations explain only 6%–7% of the phenotypic variance.Citation7 Additionally, a large GWAS of East Asian populations revealed novel Asian-specific loci and diverse associations with PD risk loci, suggesting that population differences contribute to the genetic heterogeneity in PD risk found between Europeans and East Asians.Citation8,Citation9 Environmental factors are considered to be another main cause of PD susceptibility, especially in late-onset PD.Citation1 These putative risk factors for PD include pesticides, well water, organic solvents, and metal exposure, while inverse correlations exist for smoking and caffeine consumption.Citation1
The familial aggregation of PD has been identified by case–control and twin cohort studies, with most studies reporting stronger familial aggregation for early-onset PD than in late-onset PD.Citation10 A meta-analysis including 29 familial aggregation studies of PD estimated that the overall relative risk (RR) (95% CI) for PD in affected first-degree relatives was 2.9 (2.2–3.8) and that the RR (95% CI) for early-onset PD was 4.7 (3.2–6.8), while the RR (95% CI) for late-onset PD was 2.7 (1.9–3.9).Citation11 Linkage analyses and whole exome sequencing of PD-affected families have so far revealed >20 associated loci and 11 associated genes; these studies provide convincing evidence that polymorphic variants in these genes contribute to sporadic PD.Citation12,Citation13 The causative genes in the pathogenesis of PD might be involved in abnormal protein aggregation, mitochondrial or lysosomal dysfunction, the ubiquitin–proteasome system, and/or kinase signaling pathways.Citation12 Even in the early stages of PD, neuropsychiatric abnormalities are frequently detected in patients with PD. The coaggregation of PD with other neuropsychiatric diseases, such as essential tremor, depression, anxiety, and dementia, has been reported in the literature.Citation14–Citation16
Previous studies have identified the existence of causative genes and putative environmental factors in the familial aggregation of PD, but the relative contributions of inheritance and environmental factors to PD susceptibility remain unclear. Besides, understanding the coaggregation of PD with other neuropsychiatric diseases is valuable information for individuals with a family history of PD. Therefore, we conducted this population-based cohort study using nationwide genealogy reconstructed from the National Health Insurance (NHI) database of Taiwan to estimate the extent of familial aggregation of PD by determining the RRs of PD in specific kinships and to assess the relative contributions of genetic, shared, and non-shared environmental factors to PD susceptibility. Moreover, we also estimated the RRs for other neuropsychiatric diseases in individuals with PD-affected first-degree relatives.
Materials and methods
We conducted a population-based family cohort study based on the NHI research database (NHIRD) of Taiwan. The NHI Program is a single-payer centralized disbursement system in Taiwan that was founded in 1995. By the end of 2015, the database covered over 99.6% of the residents of Taiwan.Citation17 The NHI database records patients’ demographic variables, insurance information (such as payroll, whom to cover, and start and end date of insurance), and original claims data, which contain the comprehensive medical information of the beneficiaries. All of the disease diagnosis data recorded during our study period (from 1999 to 2015) were coded using the International Classification of Diseases, Ninth Revision (ICD-9) codes. To protect the patients’ privacy, all tractable personal information was de-identified before being released for research purposes.
The present study was approved by the Institutional Review Board of Chang Gung Medical Foundation, Taiwan (approval number: 201701505B0). As the data used in this study were completely anonymized, the requirement for informed consent was waived.
Study population
We included all individuals (N=24,349,599) with a valid insurance status in the NHI in 2015 as our source population. We searched the entire study population in the NHIRD to identify all individuals who had at least two outpatient visits to a neurologist or one hospital admission with a diagnostic code of PD (ICD-9 code: 332.0) in any calendar year between 1999 and 2015 in order to derive the PD cases for this study. In Taiwan, individuals suspected of having PD are usually referred to a neurologist for a definite diagnosis of PD, which adheres to the clinical diagnostic criteria for PD from the Movement Disorder Society.Citation18 To improve diagnostic accuracy, patients with a diagnostic code of secondary parkinsonism, cerebrovascular disease, or head trauma at the time of diagnosis of PD were excluded.
Identification of first-degree relatives of PD patients
The registry of beneficiaries, a subset of the NHIRD, contains information on the family relationships between the insured person and his or her blood relatives and spouse. A unique personal identification number for each beneficiary in Taiwan enables valid internal linkages among different subsets of the NHI database, and we were thereby able to establish family genealogy for this study.Citation19 Parent–offspring and spousal relationships were identified directly from the NHIRD, while full siblings were identified as individuals with the same parents. Twins were identified as full siblings with the same date of birth (±1 day). However, twin zygosity could not be distinguished in the database. Finally, we grouped subjects into families after determining the family relationships of each individual within the family.Citation19 We excluded twins from the sibling analysis and all half-siblings from the whole analysis.
Ascertainment of other neuropsychiatric diseases
We further estimated the extent of coaggregation with other neuropsychiatric diseases in the first-degree relatives of PD patients. Patients were identified as having a specific neuropsychiatric disease in the NHIRD if they had at least one hospital admission or two specialist outpatient visits using that specific ICD-9 diagnostic code, and patients were included in our analysis only if their diagnosis was made by a specialist. The neuropsychiatric diseases and accompanying ICD-9 codes analyzed in our study were as follows: schizophrenia (295), major depression (296.2, 296.3), anxiety (300.0), phobic disorder (300.2), Tourette syndrome (307.2), Alzheimer’s disease (331), essential tremor (333.1), dystonia (333.8), spinocerebellar disease (334), amyotrophic lateral sclerosis (335.2), multiple sclerosis (340), and epilepsy (345).
Statistical analysis
The prevalence of PD was calculated between January 1, 1999, and December 31, 2015, for the general population and for individuals with PD-affected relatives. Individuals with valid insurance registration in 2015 who met the definition of PD were defined as PD for the determination of prevalence. We assessed the prevalence ratios between individuals with an affected relative and the general population as RRs.Citation20
The Breslow–Cox proportional hazard model was used to estimate the prevalence risk ratios in this cross-sectional study by applying the same follow-up time to all subjects.Citation21 In this study, the threshold liability model was used to estimate heritability and familial transmission. Heritability was defined as the relative contribution of genetic factors to phenotypic variance, and familial transmission was defined as the sum of the heritability and shared environmental proportions. The liability scale between siblings and spouses was compared under the assumption that spouses share the same environmental factors and siblings share both the same genetic and environmental factors for phenotypic variance.Citation19 We restricted the family history within first-degree relatives and assumed a mean of two siblings per family. Moreover, we further estimated the coaggregation of PD with other neuropsychiatric diseases in the first-degree relatives of patients with PD using a marginal Cox proportional hazard model with the same follow-up time for all participants. The adjusted RRs of coaggregation with other neuropsychiatric diseases were estimated by using the adjusted prevalence ratios of a specific disease between affected first-degree relatives of PD patients and the general population. Covariates such as age, sex, occupation category, income level, place of residence, and family size were included to model the familial aggregation of PD and coaggregation with other neuropsychiatric diseases. All analyses were performed using SAS software, version 9.3 (SAS Institute Inc., Cary, NC, USA). Two-sided p values ≤0.05 were considered statistically significant.
Results
PD prevalence in individuals with PD-affected relatives versus the general population
In 2015, there were 112,037 patients with a diagnosis of PD out of the 24,349,599 individuals in the general population of Taiwan, which was equivalent to a crude prevalence of 0.46%. The prevalence of PD in women (0.48%) was slightly higher than the prevalence in men (0.44%). A total of 156,048 individuals had at least one affected first-degree relative with PD: 149,187 had an affected parent, 3,698 had an affected offspring, 3,495 had an affected sibling, and 15 had an affected twin. Among the individuals with a PD-affected first-degree relative, the crude prevalence of PD was 0.52%. The comparison between the demographic characteristics of the individuals with PD-affected first-degree relatives and those of the general population are shown in . shows that the age-specific prevalence of PD in individuals with PD-affected first-degree relatives was significantly higher than that in the general population of Taiwan. Analysis of the age-specific prevalence of PD showed that the crude prevalence of PD steeply accelerated after 65 years of age in both groups (). shows the onset age-specific prevalence of PD in individuals with affected first-degree relatives of PD and that in the general population of Taiwan. The onset age-specific prevalence of PD in individuals with affected first-degree relatives of PD increased after the age of 60 years and then peaked at the age of 85 years.
Figure 1 The age-specific prevalence of PD in individuals with affected first-degree relatives of PD and in the general population of Taiwan in 2015.
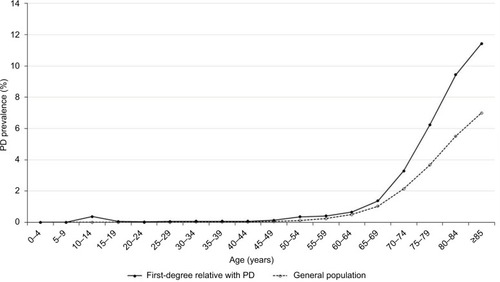
Figure 2 The onset age-specific prevalence of PD in individuals with affected first-degree relatives of PD and in the general population of Taiwan in 2015.
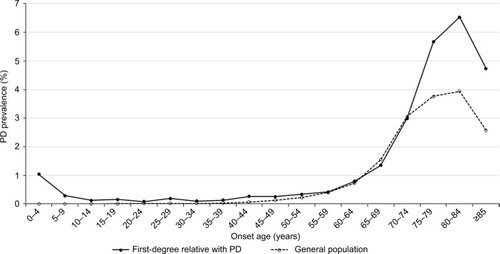
Table 1 Demographics of individuals with PD-affected first-degree relatives compared with the general population of Taiwan
Table 2 Age-specific prevalence of PD in individuals with PD-affected first-degree relatives and in the general population of Taiwan in 2015
RRs for PD in individuals with PD-affected first-degree relatives
The prevalence and adjusted RRs for PD in individuals with affected first-degree relatives of PD by the type of affected relative and sex are shown in . Overall, having a PD-affected first-degree relative was associated with an adjusted RR (95% CI) for PD of 1.69 (1.56–1.83), and there was no significant difference between males (1.73 [1.56–1.92]) and females (1.68 [1.52–1.85]). The adjusted RR (95% CI) for PD among the first-degree relatives of PD patients was the most significant (63.12 [16.45–242.16]) for twins, that is, those with the highest genetic similarity, followed by 2.20 (1.41–3.45) for siblings, 1.86 (1.63–2.11) for offspring, 1.59 (1.47–1.73) for parents, and 1.46 (1.41–1.52) for spouses without genetic similarity. Using a threshold liability model, we estimated that genetic factors (heritability), shared environmental factors, and non-shared environmental factors accounted for 11.0%, 9.1%, and 79.9% of the phenotypic variance of PD in the Taiwanese population, respectively.
Table 3 Relative risk of PD in individuals with PD-affected first-degree relatives compared with the general population of Taiwan
Coaggregation of PD with other neuropsychiatric diseases
The prevalence and adjusted RRs (95% CIs) for other neuropsychiatric diseases in individuals with PD-affected first-degree relatives compared with the general population of Taiwan are presented in . Among all analyzed neuropsychiatric diseases, the adjusted RR (95% CI) in individuals with PD-affected first-degree relatives was the highest for schizophrenia with an RR (95% CI) of 1.68 (1.61–1.75), followed by 1.66 (1.57–1.76) for essential tremor, 1.46 (1.23–1.73) for spinocerebellar disease, 1.42 (1.28–1.56) for phobic disorder, 1.35 (1.24–1.46) for dystonia, 1.29 (1.26–1.33) for major depression, 1.27 (1.24–1.30) for anxiety, 1.27 (1.03–1.56) for multiple sclerosis, 1.23 (1.07–1.42) for Tourette syndrome, 1.22 (1.17–1.27) for epilepsy, 1.20 (1.12–1.28) for Alzheimer’s disease, and 1.20 (0.94–1.54) for amyotrophic lateral sclerosis.
Table 4 Relative risk of other neuropsychiatric diseases in individuals with PD-affected first-degree relatives
Discussion
This population-based family cohort study investigated the RRs of PD in individuals with PD-affected first-degree relatives and estimated the accountability of heritability and familial transmission for PD in the general population of Taiwan. The adjusted RR of PD in PD-affected first-degree relatives was 1.69-fold higher than that in the general population, and the observed magnitude of RR was associated with genetic distance. The relative contributions of heritability and familial transmission to PD susceptibility in the Taiwanese population were estimated to be 11.0% and 20.1%, respectively, which were relatively low compared to the 79.9% contribution of non-shared environmental factors. In addition, individuals with PD-affected first-degree relatives were at a higher risk of developing other neuropsychiatric diseases, particularly essential tremor, schizophrenia, spinocerebellar disease, and phobic disorder, than the general population.
Although most PD cases were sporadic, studies on the familial aggregation of PD have identified that autosomal recessive inheritance in early-onset PD, variable genetic penetrance, and gene–environment interactions are all responsible for the heterogenetic presentation of PD.Citation11 Inheritance also plays a substantial role in the susceptibility to sporadic PD, as demonstrated by a twin study which showed that subclinical dopaminergic dysfunction was concordant with 75% of clinical PD cases among monozygotic twins, but with only 22% of cases among dizygotic twins.Citation22 Moreover, familial pedigree analyses have consistently shown higher RRs in siblings than in parents or offspring with the same genetic distance, suggesting that shared environmental exposure may increase PD susceptibility.Citation11
Previous familial aggregation studies have reported much higher RRs for PD (range: 2.7–3.4) in the first-degree relatives of PD patients than we found in our study.Citation23,Citation24 However, those studies most often employed less robust sampling strategies, such as small numbers of familial pedigrees, hospital records, or telephone interviews, thus increasing selection bias and limiting generalizability. A recent population-based study using family history questionnaires found that the first-degree relatives of patients with PD had a higher risk of developing PD (RR: 1.988, p=0.036), which was more in line with our result.Citation25 However, our estimate of heritability having only 11.0% of the accountability for PD in the Taiwanese population is relatively low compared with previous estimations made from genome-wide association analyses in European cohorts.Citation7,Citation26 This difference might be ascribed to differences in study populations and estimation methods. Further GWAS focusing on the contribution of heritability to PD in other populations is warranted.
Genetic and environmental interactions play a crucial role in PD susceptibility. Environmental exposure can cause epigenetic modifications such as DNA methylation or histone modification, thus triggering or accelerating the development of PD in genetically susceptible individuals.Citation27 An umbrella review that evaluated 75 meta-analyses on observational studies of risk factors for PD identified convincing evidences of causative associations with PD for head trauma, constipation, and depression, while protective roles were found for physical exercise and smoking.Citation28 Other reported potential linkages between PD and environmental or behavioral exposures, including well water consumption, pesticide use, organic solvents, lead or manganese exposure, and higher education, have weaker evidence-based associations with PD.Citation29–Citation31 Further investigation on the genuine causative agents of PD and clarification of its underlying mechanisms is required.
Coaggregation of PD with other neuropsychiatric abnormalities has been reported in the literature.Citation32 Genetic association studies have implied that PD might share common genetic mechanisms with other neuropsychiatric diseases; however, the variable magnitude of pathogenic overlap might contribute to diverse disease manifestations.Citation32 Among all of the neuropsychiatric diseases analyzed in our study, individuals with PD-affected first-degree relatives had the highest risk of developing schizophrenia (RR: 1.68). Visual hallucination is the most common presentation of PD psychosis in PD patients, and it is also a prominent feature of schizophrenia.Citation33 Furthermore, patients with schizophrenia exhibited the highest risk of developing subsequent PD among the patients with pre-existing psychiatric illnesses (hazard ratio: 8.9; 95% CI: 7.74–10.24).Citation34 Genetic risk profile studies have shown that schizophrenia shares spatially overlapping loci with PD and that their possible pathologic linkage might originate from dopaminergic system imbalance or dysfunction.Citation32 Essential tremor was also found to have a higher coaggregation with PD in our study (RR: 1.66). Both essential tremor and PD are characterized by pathologic tremor with progressively disabling entity, and they might also share the same Lewy body pathogenesis.Citation16 Our coaggregation analysis found higher RRs for the development of spinocerebellar diseases (RR: 1.46) and dystonia (RR: 1.35) in the first-degree relatives of PD patients. Spinocerebellar degeneration, also known as spinocerebellar ataxia (SCA), can present as parkinsonism, particularly the SCA2, SCA3, and SCA17 subtypes.Citation35 Patients who suffer from dystonic tremor and dopa-responsive dystonia might have PD-like resting tremor and cogwheel rigidity, but their results of dopamine transporter imaging are normal.Citation36,Citation37
Alzheimer’s disease and PD are both age-related neurodegenerative diseases. They are thought to share the same cardinal mechanisms of neural damage, which are mainly characterized by the abnormal aggregation of misfolded proteins (amyloid β in Alzheimer’s disease and α-synuclein in PD), oxidative stress, and mitochondrial dysfunction.Citation38,Citation39 A recent systematic review that included 16 familial coaggregation studies of PD and Alzheimer’s disease concluded that there was only modest familial coaggregation of Alzheimer’s disease in individuals with PD-affected relatives (RRs: 1.18–1.40), which is consistent with our result.Citation40 Other psychiatric diseases, such as anxiety, depression, panic disorder, and phobia, are common non-motor presentations of PD, and their coaggregation with PD has been reported in previous studies, with RRs ranging from 1.45 to 1.87.Citation41–Citation43 The modest familial coaggregation with PD implies that these psychiatric diseases might share common familial susceptibility factors with PD.Citation15
Our present study had several strengths and important implications. First, our population-based cohort study utilized the NHI database, which contains health information covering nearly the entire population of Taiwan, and its representativeness and clinical consistency are well documented. Second, we had stringent case ascertainment criteria for PD and other neuropsychiatric diseases in that required at least two outpatient visits to a specialist or one hospital admission diagnosis made by a specialist, which minimized selection and recall bias. Third, the reconstructed familial relationships could be ascertained for most permanent residents of Taiwan based on the solid linkage by unique personal identification numbers. Fourth, the threshold liability model is effective for estimating familial aggregation, and its applications have been previously validated.Citation19 Altogether, our study provided objectively quantitative data that estimated the relative contributions of heritability and environmental factors for PD susceptibility, which is valuable information for the clinical consultation of individuals with a family history of PD.
However, there were several limitations to our study. First, disease identification was based on the ICD-9 diagnosis codes for each patient registered in the NHI database. Detailed information on clinical symptoms, laboratory testing, and examinations was unobtainable in the NHIRD. Second, this cross-sectional study analyzed the entire Taiwanese population registered in the NHI database in 2015, so the incidence rate and onset age for each individual with PD were not evaluated. Third, the zygosity of twins was not recorded in the NHI database, so a classic twin study design could not be utilized to estimate the relative contribution of heritability. Fourth, our threshold liability model was imperfect, as spouses may not share the same environmental factors as those shared by siblings. Finally, our study results are restricted to the population of Taiwan; therefore, it is not clear whether our results are applicable to other populations. Further studies are required to determine the generalizability of our results.
Conclusion
This population-based cohort study showed that individuals with PD-affected first-degree relatives had a 1.69-fold higher risk of developing PD and a higher risk of developing other neuropsychiatric diseases, such as schizophrenia, essential tremor, and phobic disorder. PD should be considered an age-related multifactorial syndrome with mainly genetic and environmental components. The estimated relative contributions to PD susceptibility in the Taiwanese population were 11.0% for heritability, 9.1% for shared environmental factors, and 79.9% for non-shared environmental factors. Our findings provide information useful for counseling families of PD patients. The environmental causative agents responsible for the majority of PD susceptibility and the contribution of heritability to PD phenotypic variance in other populations warrant further investigation.
Acknowledgments
This study was based in part on data from the NHIRD provided by the Bureau of National Health Insurance, Department of Health, and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent the views of the Bureau of National Health Insurance, Department of Health or the National Health Research Institutes. The work was funded by Chang Gung Memorial Hospital (CMRPG3E0132 and CMRPG3E0133) and the Maintenance Project for the Center of Big Data Analytics and Statistics (CLRPG3D0043) from Chang Gung Memorial Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
- WirdefeldtKAdamiHOColePTrichopoulosDMandelJEpidemiology and etiology of Parkinson’s disease: a review of the evidenceEur J Epidemiol201126Suppl 1S1S5821626386
- SchragASchottJMEpidemiological, clinical, and genetic characteristics of early-onset ParkinsonismLancet Neurol20065435536316545752
- WickremaratchiMMPereraDO’LoghlenCPrevalence and age of onset of Parkinson’s disease in cardiff: a community based cross sectional study and meta-analysisJ Neurol Neurosurg Psychiatry200980780580719531689
- KowalSLDallTMChakrabartiRStormMVJainAThe current and projected economic burden of Parkinson’s disease in the United StatesMov Disord201328331131823436720
- SveinbjörnsdottirSHicksAAJonssonTFamilial aggregation of Parkinson’s disease in IcelandN Engl J Med2000343241765177011114315
- PayamiHZareparsiSJamesDNuttJFamilial aggregation of Parkinson disease: a comparative study of early-onset and late-onset diseaseArch Neurol200259584885012020270
- DoCBTungJYDorfmanEWeb-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson’s diseasePLoS Genet201176e100214121738487
- SatakeWNakabayashiYMizutaIGenome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s diseaseNat Genet200941121303130719915576
- FooJNTanLCIrwanIDGenome-wide association study of Parkinson’s disease in East AsiansHum Mol Genet201726122623228011712
- TannerCMOttmanRGoldmanSMParkinson disease in twins: an etiologic studyJAMA199928143413469929087
- ThackerELAscherioAFamilial aggregation of Parkinson’s disease: a meta-analysisMov Disord20082381174118318442112
- CortiOLesageSBriceAWhat genetics tells us about the causes and mechanisms of Parkinson’s diseasePhysiol Rev20119141161121822013209
- BenitezBADavisAAJinSCResequencing analysis of five Mendelian genes and the top genes from genome-wide association studies in Parkinson’s diseaseMol Neurodegener2016112927094865
- CaballolNMartiMJTolosaECognitive dysfunction and dementia in Parkinson diseaseMov Disord200722Suppl 17S358S36618175397
- ArabiaGGrossardtBRGedaYEIncreased risk of depressive and anxiety disorders in relatives of patients with Parkinson diseaseArch Gen Psychiatry200764121385139218056546
- LouisEDClarkLOttmanRFamilial aggregation and co-aggregation of essential tremor and Parkinson’s diseaseNeuroepidemiology2016461313626606512
- National Health Insurance Administration, Ministry of Health and Welfare, Executive Yuan2015–2016 National Health Insurance Annual Report122015 Available from: https://www.nhi.gov.tw/Resource/webdata/30285_1_National%20Health%20Insurance%20in%20Taiwan%202015-2016%20(bilingual).pdfAccessed December 15, 2017
- GoetzCGTilleyBCShaftmanSRMovement Disorder Society UPDRS Revision Task ForceMovement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing resultsMov Disord200823152129217019025984
- KuoCFGraingeMJValdesAMFamilial aggregation of systemic lupus erythematosus and coaggregation of autoimmune diseases in affected familiesJAMA Intern Med201517591518152626193127
- RischNLinkage strategies for genetically complex traits. I. Multilocus modelsAm J Hum Genet19904622222282301392
- BarrosAJHirakataVNAlternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratioBMC Med Res Methodol200332114567763
- PicciniPBurnDJCeravoloRMaraganoreDBrooksDJThe role of inheritance in sporadic Parkinson’s disease: evidence from a longitudinal study of dopaminergic function in twinsAnn Neurol199945557758210319879
- MarderKLevyGLouisEDFamilial aggregation of early- and late-onset Parkinson’s diseaseAnn Neurol200354450751314520664
- ShinoMYMcGuireVVan Den EedenSKFamilial aggregation of Parkinson’s disease in a multiethnic community-based case-control studyMov Disord201025152587259420842689
- GaareJJSkeieGOTzoulisCLarsenJPTysnesOBFamilial aggregation of Parkinson’s disease may affect progression of motor symptoms and dementiaMov Disord201732224124527862270
- KellerMFSaadMBrasJInternational Parkinson’s Disease Genomics Consortium (IPDGC); Wellcome Trust Case Control Consortium 2 (WTCCC2)Using genome-wide complex trait analysis to quantify ‘missing heritability’ in Parkinson’s diseaseHum Mol Genet201221224996500922892372
- TrinhJGuellaIFarrerMJDisease penetrance of late-onset parkinsonism: a meta-analysisJAMA Neurol201471121535153925330418
- BellouVBelbasisLTzoulakiIEvangelouEIoannidisJPEnvironmental risk factors and Parkinson’s disease: an umbrella review of meta-analysesParkinsonism Relat Disord2016231926739246
- BreckenridgeCBBerryCChangETSielkenRLJrMandelJSAssociation between Parkinson’s disease and cigarette smoking, rural living, well-water consumption, farming and pesticide use: systematic review and meta-analysisPLoS One2016114e015184127055126
- GoldmanSMQuinlanPJRossGWSolvent exposures and Parkinson disease risk in twinsAnn Neurol201271677678422083847
- ValdesEGAndelRSieurinJOccupational complexity and risk of Parkinson’s diseasePLoS One201499e10667625198429
- NallsMASaadMNoyceAJGenetic comorbidities in Parkinson’s diseaseHum Mol Genet201423383184124057672
- GibsonGMottramPGBurnDJFrequency, prevalence, incidence and risk factors associated with visual hallucinations in a sample of patients with Parkinson’s disease: a longitudinal 4-year studyInt J Geriatr Psychiatry201328662663122927195
- LinHLLinHCChenYHPsychiatric diseases predated the occurrence of Parkinson disease: a retrospective cohort studyAnn Epidemiol201424320621324462274
- van GaalenJGiuntiPvan de WarrenburgBPMovement disorders in spinocerebellar ataxiasMov Disord201126579280021370272
- BrooksDJMolecular imaging of dopamine transportersAgeing Res Rev20163011412126802555
- CiliaRRealeCCastagnaANovel DYT11 gene mutation in patients without dopaminergic deficit (SWEDD) screened for dystoniaNeurology201483131155116225150291
- JiangTSunQChenSOxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s diseaseProg Neurobiol201614711927769868
- GangulyGChakrabartiSChatterjeeUSasoLProteinopathy, oxidative stress and mitochondrial dysfunction: cross talk in Alzheimer’s disease and Parkinson’s diseaseDrug Des Devel Ther201711797810
- FeldmanALJohanssonALLambertPCFamilial coaggregation of Alzheimer’s disease and Parkinson’s disease: systematic review and meta-analysisNeuroepidemiology2014422698024296900
- PontoneGMPalanciJBienvenuOJFamilial aggregation of panic disturbances in Parkinson’s diseaseJ Neuropsychiatry Clin Neurosci201123441742422231313
- GustafssonHNordströmANordströmPDepression and subsequent risk of Parkinson disease: a nationwide cohort studyNeurology201584242422242925995056
- BroenMPNarayenNEKuijfMLDissanayakaNNLeentjensAFPrevalence of anxiety in Parkinson’s disease: a systematic review and meta-analysisMov Disord20163181125113327125963
