Abstract
Objectives
COPD is associated with reduced physical activity, an increased risk for pulmonary infections, and impaired survival in nontransplant patients. The aim of this study was to investigate the influence of COPD in patients after heart transplantation (HTX).
Methods
We performed an observational retrospective single-center study of 259 patients receiving HTX at Heidelberg University Hospital between 2003 and 2012. Patients were stratified by the Tiffeneau index (forced expiratory volume in 1 second/forced vital capacity [FEV1/FVC]) <0.70 before HTX. The analysis included demographics, posttransplant medication, length of the initial hospital stay after HTX, early posttransplant atrial fibrillation (AF), mortality, and causes of death.
Results
In total, 63 (24.3%) patients had an FEV1/FVC <0.70. These patients showed a prolonged hospital stay after HTX (52.0 days vs 43.4 days, mean difference (MD) = 8.6 days, 95% CI: 0.2, 17.0 days), a higher rate of early posttransplant AF (19.0% vs 8.2%, MD = 10.8%, 95% CI: 0.4%, 21.2%), and an increased 30-day mortality (9.5% vs 2.6%, HR = 3.79, 95% CI: 1.16, 12.40). Kaplan– Meier analysis showed a significant inferior 5-year survival in patients with an FEV1/FVC <0.70, along with a higher percentage of death due to transplant failure and infection/sepsis. In addition, a multivariate analysis for mortality within 5 years after HTX indicated an FEV1/FVC <0.70 as a significant risk factor for impaired 5-year posttransplant survival (HR =4.77, 95% CI: 2.76, 8.22).
Conclusion
COPD in patients after HTX is associated with a prolonged hospital stay, early posttransplant AF, and impaired posttransplant survival.
Introduction
COPD is a worldwide burden and one of the leading causes of death.Citation1–Citation5 It is characterized by a limitation of airflow due to airway obstruction.Citation6 Several surveys suggest a further increase in COPD prevalence in the future with up to a quarter of all adults aged ≥40 years being affected.Citation2–Citation5
Tobacco smoking has been described as the most common cause for COPD.Citation4,Citation5 Continuous exposure to tobacco smoke over several years causes inflammation, tissue remodeling, and loss of elasticity of small airways, resulting in the manifestation of COPD after a cumulative dose of about 20 pack-years and a decline in the Tiffeneau index (forced expiratory volume in 1 second/forced vital capacity [FEV1/FVC]).Citation4,Citation5 A Tiffeneau index (FEV1/FVC) <0.70 has been used for the diagnosis of COPD with further classification into four stages by FEV1: mild (FEV1 ≥80%), moderate (FEV1 =50%–79%), severe (FEV1 =30%–49%), and very severe (FEV1 <30%).Citation7
The presence of COPD has been associated with an increased morbidity and mortality including an elevated risk for cardiovascular events.Citation1,Citation6–Citation9 As a consequence, patients with COPD often suffer from reduced physical stamina, a higher rate of hospitalization, and an increased risk for infections and malignancies and exhibit an elevated prevalence of cardiovascular diseases.Citation1,Citation6–Citation9
Given these COPD-linked comorbidities, it may be speculated that patients after heart transplantation (HTX) with preexisting COPD have worse posttransplant outcomes as these patients are more vulnerable to pulmonary infections and malignancies due to the required immunosuppressive drug regimen to prevent acute rejection episodes.Citation10,Citation11 In addition, cardiovascular events and atrial fibrillation (AF) in the early posttransplant period pose a substantial threat to patients after HTX.Citation12
However, although an FEV1 <40% has been described as a relative contraindication for HTX, no reliable data regarding the influence of COPD on posttransplant outcomes are currently available.Citation13,Citation14 Thus, due to the lack of data, this study aimed to analyze the effects of COPD in patients after HTX focusing on the duration of the initial posttransplant hospital stay, early posttransplant AF, mortality after HTX, and causes of death after HTX.
Patients and methods
Patients
This study complied with the ethical principles for medical research of the Declaration of Helsinki. Approval was given by the ethics committee of the University of Heidelberg, Heidelberg, Germany (ethical approval number: S-286/2015, date of ethical approval: June 22, 2015). All adult patients (aged ≥18 years) receiving HTX at the Heidelberg Heart Center between January 2003 and December 2012 were included except for those with repeated HTX. Data were retrieved from the medical records and analyzed in pseudonymized form. No additional written informed consent was required for this observational retrospective single-center study as only routine clinical data were used.Citation12,Citation15–Citation19
Patients were stratified based on the results of the spirometry which was performed as part of the HTX evaluation and listing process. A Tiffeneau index (FEV1/FVC) <0.70 was defined as the presence of COPD. Hence, all patients were initially divided into two groups: patients with an FEV1/FVC <0.70 and those with an FEV1/FVC ≥0.70. Then, patients with an FEV1/FVC <0.70 were further stratified into patients with an FEV1 <50% and patients with an FEV1 ≥50%.
Follow-up
The follow-up period for this study started at the time of HTX and ended 5 years after HTX. As patients after HTX require a close follow-up at a specialized center, they were continuously cared for by the medical team of the Heidelberg Heart Center. Therefore, 5-year follow-up data could be obtained in all patients requiring no censoring.
Patients’ follow-up was performed according to the usual standard of care at the Heidelberg Heart Center. After discharge, patients were followed up monthly during the first 6 months after HTX, then bimonthly from month 6 to month 12 after HTX, and three to four times per year thereafter (or more often if clinically indicated). Routine follow-up included medical history, physical examination, electrocardiography (ECG), echocardiography, endomyocardial biopsy, and blood tests including immunosuppressive drug monitoring.Citation10–Citation12,Citation15–Citation19
Posttransplant medication
After HTX, patients received an antithymocyte globulin-based immunosuppression induction therapy. At the beginning of the study period, the initial standard immunosuppressive drug regimen consisted of cyclosporine A (CsA) and mycophenolate mofetil (MMF), which was subsequently switched to tacrolimus (TAC) and MMF from 2006 onward. Within the first months after HTX, steroids (prednisolone) were tapered incrementally and discontinued finally after 6 months, if possible.Citation12,Citation15–Citation19
Statistical analysis
Statistical analysis of data was carried out with SAS (Version 9.3; SAS Institute, Cary, NC, USA). Data were displayed as count (n) with % or as mean ± SD. In addition, results were reported in terms of measures of association (mean difference [MD] or HR) and their 95% CI. Chi-squared test was applied for categorical variables and Student’s t-test was used for continuous variables. Kaplan–Meier estimator was used to display 5-year posttransplant survival.Citation12,Citation15–Citation19
Univariate analyses to test for differences between groups to reduce potential bias and confounding affecting survival included recipient data, previous open-heart surgery, the principal diagnosis for HTX, donor data, transplant sex mismatch, perioperative data, and posttransplant medication including immunosuppressive drug therapy.
In addition, the influence of COPD (FEV1/FVC <0.70) on mortality in patients after HTX was analyzed in combination with the most clinically relevant parameters linked to increased mortality in patients after HTX using a multivariate analysis (Cox regression model) for mortality within 5 years after HTX. Consequently, the following nine variables were included: FEV1/FVC <0.70, recipient age (>60.0 years), recipient body mass index (BMI >25.0 kg/m2), coronary artery disease (CAD), dyslipidemia, history of smoking, ischemic cardiomyopathy (CMP) as the principal diagnosis for HTX, nonischemic CMP as the principal diagnosis for HTX, and ischemic time (≥240 minutes). In order to ensure a stable number of events (deceased patients) per analyzed variable and to avoid biased regression coefficients, we did not include further, clinically less relevant parameters.Citation12,Citation15–Citation19
Moreover, a sensitivity analysis was performed to test the robustness of the study results. For this purpose, an analysis using a subcohort of patients with an immunosuppressive drug regimen consisting of TAC and MMF was carried out, as the initial standard immunosuppressive drug therapy was switched from 2006.
The primary outcome of this study was mortality after HTX. Secondary outcomes included the incidence of tracheostomy after HTX, time to extubation after HTX, length of the initial intensive care unit (ICU) stay, length of the initial hospital stay, and 30-day follow-up occurrence of AF.
Results
Baseline characteristics
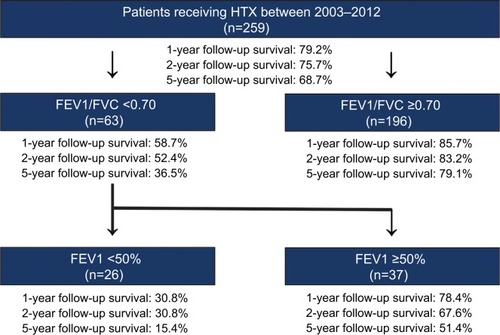
In this observational retrospective single-center study with 259 patients, 63 (24.3%) patients had an FEV1/FVC <0.70 and 196 (75.7%) patients had an FEV1/FVC ≥0.70. Patients with an FEV1/FVC <0.70 presented with a significantly higher BMI (>25.0 kg/m2, 61.9% vs 39.8%, MD: 22.1%, 95% CI: 8.3%, 35.9%), a higher rate of CAD (60.3% vs 38.3%, MD: 22.0%, 95% CI: 8.2%, 35.8%), a higher percentage of dyslipidemia (76.2% vs 58.2%, MD: 18.0%, 95% CI: 5.4%, 30.6%), and a higher rate of history of smoking (95.2% vs 43.9%, MD: 51.3%, 95% CI: 42.6%, 60.0%). Both groups showed no significant differences in recipient age, male recipient sex, arterial hypertension, diabetes mellitus, renal insufficiency, or glomerular filtration rate.
In terms of the principal diagnoses prior to HTX, patients in the FEV1/FVC <0.70 group had a significantly higher rate of ischemic CMP (50.8% vs 31.1%, MD: 19.7%, 95% CI: 5.7%, 33.7%) and a significantly lower rate of nonischemic CMP (33.3% vs 51.5%, MD: 18.2%, 95% CI: 4.6%, 31.8%). There were no significant differences between groups for valvular heart disease or cardiac amyloidosis as the principal diagnosis for HTX. Furthermore, no statistically significant differences between groups could be found related to previous open-heart surgery, donor data, transplant sex mismatch, or perioperative data. shows the baseline characteristics.
Table 1 Baseline characteristics
Initial medication after HTX
The analysis of the immunosuppressive drug regimen showed no statistically significant differences between groups concerning the use of CsA, TAC, azathioprine, or MMF. There were also no statistically significant differences related to the administration of acetylsalicylic acid, β-blockers, ivabradine, calcium channel blockers in general, dihydropyridine calcium channel blockers, non-dihydropyridine calcium channel blockers, angiotensin-converting enzyme inhibitors, or statins. provides the overview of the initial medication after HTX including immunosuppressive drug therapy.
Table 2 Initial medication after HTX
Posttransplant outcomes after HTX
In terms of the primary outcome of this study, patients in the FEV1/FVC <0.70 group showed a significantly higher 30-day follow-up mortality (9.5% vs 2.6%, HR: 3.79; 95% CI: 1.16, 12.40), 1-year follow-up mortality (41.3% vs 14.3%, HR: 3.48, 95% CI: 2.04, 5.94), 2-year follow-up mortality (47.6% vs 16.8%, HR: 3.50, 95% CI: 2.13, 5.75), and 5-year follow-up mortality (63.5% vs 20.9%, HR: 4.13, 95% CI: 2.67, 6.40). In addition, patients with an FEV1/ FVC <0.70 showed an inferior 5-year survival after HTX in the Kaplan–Meier survival analysis. Furthermore, patients with an FEV1/FVC <0.70 and an FEV1 <50% had an inferior 5-year posttransplant survival in comparison with patients with an FEV1/FVC <0.70 and an FEV1 ≥50%. and present the Kaplan–Meier estimators.
Figure 1 Survival after HTX by FEV1/FVC (Kaplan–Meier estimator).
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEV1/FCV, Tiffeneau index; HTX, heart transplantation.
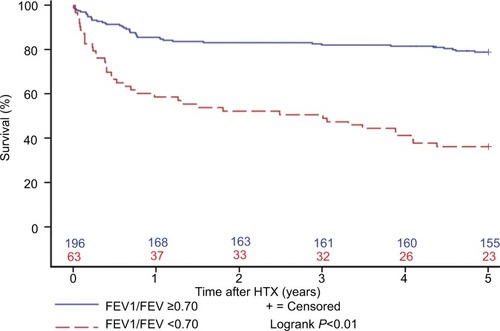
Figure 2 Survival after HTX by FEV1 in patients with an FEV1/FVC <0.70 (Kaplan–Meier estimator).
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEV1/FCV, Tiffeneau index; HTX, heart transplantation.
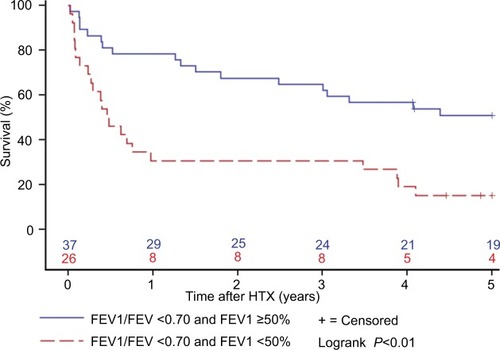
Further survival analysis revealed that patients with an FEV1/FVC >0.70 had the best 5-year survival (79.1%), followed by all patients (68.7%) and patients with an FEV1/FVC <0.70 and an FEV1 ≥50% (51.4%). Of note, patients with an FEV1/FVC <0.70 and an FEV1 <50% showed the worst survival of all groups (15.4%). shows the survival after HTX by FEV1/FVC and FEV1.
Figure 3 Survival after HTX by FEV1/FVC and FEV1.
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEV1/FCV, Tiffeneau index; HTX, heart transplantation.
With regard to the secondary outcomes of this study, patients with an FEV1/FVC <0.70 had a higher incidence of tracheostomy after HTX (25.4% vs 4.1%, MD: 21.3%, 95% CI: 10.2%, 32.4%), a longer time to extubation after HTX (13.8±23.9 days vs 4.1±4.5 days, MD: 9.7 days, 95% CI: 3.6, 15.8 days), a longer initial ICU stay (33.5±31.9 days vs 20.0±22.1 days, MD: 13.5 days, 95% CI: 4.8, 22.2 days), a longer initial hospital stay after HTX (52.0±30.8 days vs 43.4±22.6 days, MD: 8.6 days, 95% CI: 0.2, 17.0 days), and a higher rate of early posttransplant AF (19.0% vs 8.2%, MD: 10.8%, 95% CI: 0.4%, 21.2%) compared with patients with an FEV1/FVC ≥0.70. provides the posttransplant outcomes after HTX.
Table 3 Posttransplant outcomes
Causes of death after HTX
A total of 81 patients (31.3%) deceased within 5 years after HTX. In the FEV1/FVC <0.70 group, 40 patients (63.5%) passed away, while 41 patients (20.9%) deceased in the other group. In terms of causes of death, significantly more patients died from transplant failure (17.5% vs 5.1%, MD: 12.4%, 95% CI: 2.6%, 22.2%), infection/sepsis in general (31.7% vs 12.8%, MD: 18.9%, 95% CI: 6.5%, 31.3%), pulmonary infection (23.8% vs 9.7%, MD: 14.1%, 95% CI: 2.8%, 25.4%), and thromboembolic event/bleeding (9.5% vs 1.0%, MD: 8.5%, 95% CI: 1.1%, 15.9%) in the FEV1/FVC <0.70 group, whereas there was no significant difference between groups concerning acute rejection, abdominal infection, or malignancy. displays the causes of death.
Table 4 Causes of death within 5 years after HTX
Multivariate analysis for survival after HTX
A multivariate analysis for mortality within 5 years after HTX was conducted with the following nine clinically relevant variables: FEV1/FVC <0.70 (HR: 4.77, 95% CI: 2.76, 8.22), recipient age >60.0 years (HR: 1.62, 95% CI: 0.95, 2.73), recipient BMI >25.0 kg/m2 (HR: 1.16, 95% CI: 0.73, 1.85), CAD (HR: 1.36, 95% CI: 0.59, 3.11), dyslipidemia (HR: 0.93, 95% CI: 0.49, 1.77), history of smoking (HR: 0.69, 95% CI: 0.38, 1.28), ischemic CMP as the principal diagnosis for HTX (HR: 0.50, 95% CI: 0.21, 1.18), nonischemic CMP as the principal diagnosis for HTX (HR: 0.36, 95% CI: 0.20, 0.66), and ischemic time ≥240 minutes (HR: 0.72, 95% CI: 0.46, 1.12). shows the multivariate analysis for mortality within 5 years after HTX.
Table 5 Multivariate analysis for mortality within 5 years after HTX
Sensitivity analysis
A sensitivity analysis to test the robustness of the study results was performed with a subcohort of patients with an immunosuppressive drug regimen consisting of TAC and MMF (190 of 259 [73.4%] patients). Here, similar results regarding the primary outcome (mortality after HTX) and the secondary outcomes (incidence of tracheostomy after HTX, time to extubation after HTX, length of the initial ICU stay, length of the initial hospital stay, and 30-day follow-up occurrence of AF) were observed confirming the robustness of the study results.
Discussion
COPD in patients after HTX
As the prognostic effects of COPD in patients after HTX have been poorly studied, this retrospective observational single-center study with 259 patients investigated the influence of COPD in patients after HTX. Patients with an FEV1/ FVC <0.70 had an increased 30-day and 5-year mortality along with a higher rate of death due to transplant failure, infection/sepsis in general, pulmonary infection, and thromboembolic event/bleeding. Furthermore, patients with an FEV1/FVC <0.70 had a significantly higher incidence of tracheostomy after HTX, a longer time to extubation after HTX, a longer initial ICU stay, a longer initial hospital stay after HTX, and a higher rate of early posttransplant AF compared with patients with an FEV1/FVC ≥0.70.
COPD is a chronic inflammatory pulmonary disease characterized by airway obstruction and recurring episodes of acute exacerbation.Citation20 However, there is a growing evidence that this inflammatory pulmonary disease is not confined to the lungs and is instead part of a complex systemic inflammatory process affecting the entire circulatory system including the heart and extrapulmonary vessels.Citation20–Citation22 Therefore, it is not surprising that patients with COPD also often concurrently suffer from cardiovascular diseases such as CAD, peripheral artery disease (PAD), and cardiac arrhythmias.Citation1,Citation6–Citation9 Although CAD, PAD, and cardiac arrhythmias share common risk factors with COPD such as tobacco smoking and advanced age, these cardiovascular diseases show a higher prevalence in patients with COPD than in the general population, independently of common risk factors.Citation20
Given these characteristics, new cardiac allografts in patients with COPD encounter a systemic setting with an increased vulnerability for infections, development of malignancies, and cardiovascular events. Hence, the purpose of this study was to investigate the effects of COPD in patients after HTX focusing on the duration of the initial posttransplant hospital stay, early posttransplant AF, mortality after HTX, and causes of death after HTX.
Posttransplant AF and length of the initial hospital stay
Cardiac arrhythmias, including AF, are common in nontransplant patients with COPD.Citation20,Citation23–Citation26 In the Copenhagen City Heart Study with 13,430 subjects, a reduced FEV1 was an independent predictor for the occurrence of AF irrespective of age, gender, smoking, blood pressure, and BMI.Citation27 Furthermore, Psaty et alCitation28 discovered an independent, inverse association between FEV1 and AF.
Several risk factors have been linked to cardiac arrhythmias in patients with COPD including altered cardiopulmonary physiology, pulmonary hypertension, hypoxemia, hypercarbia, acidosis, oxidative stress, inflammation, and smoking.Citation20,Citation24,Citation27,Citation29,Citation30 Oxidative stress and inflammation have been associated with AF as hypoxemia and acidosis can cause an increase of pulmonary vascular resistance and atrial remodeling.Citation29,Citation31 Alterations in the pulmonary veins due to pulmonary hypertension or changes in gas composition can provoke AF, and atypical foci of AF (located in the right atrium) are more frequent in patients with COPD.Citation27,Citation32,Citation33 Moreover, medication for COPD including β-agonists and glucocorticoids may contribute to the development of AF.Citation20,Citation34–Citation36
Nontransplant patients with COPD show an increased risk for hospitalization and prolonged hospital stays due to COPD-associated comorbidities.Citation37,Citation38 This is in line with our findings, as we detected a higher incidence of tracheostomy, a prolonged time to extubation, a longer initial ICU stay as well as a longer initial hospital stay after HTX in patients with COPD.
In summary, we uncovered a significantly higher rate of early posttransplant AF and a longer initial hospital stay after HTX in patients with an FEV1/FVC <0.70. Because AF is associated with an increased risk for morbidity including thromboembolic complications such as stroke and patients after HTX with posttransplant AF show an impaired survival compared with patients without AF, close monitoring of patients with COPD via ECG and Holter monitoring seems advisable.Citation12,Citation39–Citation41
Posttransplant survival and causes of death
The presence of COPD has been linked to an increased risk for morbidity and mortality including cardiovascular events in nontransplant patients.Citation1,Citation6–Citation9 To our knowledge, this is the first study to analyze the influence of COPD on posttransplant survival in patients after HTX. We found a statistically significant inferior short-, mid-, and long-term posttransplant survival in patients with COPD.
As survival in patients after HTX may be affected by several risk factors,Citation12,Citation15–Citation19 patients with and without COPD were analyzed to account for differences in baseline characteristics and posttransplant medication including immunosuppressive drug therapy. Here, patients in the COPD group showed a higher percentage of CAD, ischemic CMP as the principal diagnosis for HTX, dyslipidemia, history of smoking, and an elevated BMI. These results are not surprising as COPD has been associated with tobacco smoking and the presence of cardiovascular diseases.Citation1–Citation9 Consequently, patients in the no-COPD group had a lower percentage of ischemic CMP and a higher percentage of nonischemic CMP as principal diagnosis prior to HTX. There were no significant differences between both groups with regard to the remaining baseline characteristics or the posttransplant medication, including immunosuppressive drug therapy.
As part of our study, we performed a multivariate analysis for mortality within 5 years after HTX including the presence of COPD (FEV1/FVC <0.70) and eight clinically relevant variables. Here, COPD was found to be a relevant risk factor for mortality within 5 years after HTX with an HR of 4.8 indicating that patients with COPD have an almost fivefold increased risk of death within 5 years after HTX.
In terms of causes of death, patients with COPD had a significantly higher likelihood of death due to transplant failure (17.5% vs 5.1%), infection/sepsis in general (31.7% vs 12.8%), pulmonary infection (23.8% vs 9.7%), and thromboembolic event/bleeding (9.5% vs 1.0%).
Interestingly, we could not detect a significant difference between groups in the occurrence of malignancies within 5 years after HTX although COPD has been linked to pulmonary malignancies and patients after HTX are at a higher risk for malignancies due to the immunosuppressive drug therapy.Citation4,Citation5,Citation19,Citation42,Citation43 A possible explanation for this might be the carcinogenic effect of the needed immunosuppressive medication in patients after HTX which could mask the influence of COPD on the development of malignancies. Another reason for this finding might be that the follow-up period of 5 years in this study was not long enough to detect the effects of COPD on malignancies as the development of cancer may take >5 years.Citation19 However, when looking at the types of post-transplant malignancies, there was a trend toward a higher risk for lung cancer in patients with COPD as two of three patients in the COPD group died of lung cancer (the third patient died of bladder cancer), while no patient in the no-COPD group died of lung cancer (one patient died of prostate cancer and another one of esophageal adenocarcinoma).
In summary, our results show an impaired posttransplant survival in patients with COPD. However, as survival after HTX may be affected by several risk factors, it is uncertain whether these findings are representative of patients after HTX in general. In addition, the impact of an optimized anti-obstructive therapy and the results of regularly performed spirometries on survival in patients after HTX require further evaluation in future studies.
Study limitations
Our results were derived from an observational retrospective single-center study with 259 patients. Outcomes should therefore be treated with caution as the retrospective, nonrandomized study design carries certain limitations and may therefore be subject to unmeasured confounders. However, as our data were derived from a single-center study, patients received a standardized center-specific pre-, peri-, and posttransplant course of treatment and follow-up reducing the likelihood of potential selection bias and confounders.Citation10–Citation12,Citation15–Citation19
This study included data from patients receiving HTX at the Heidelberg Heart Center between January 2003 and December 2012 with a subsequent 5-year follow-up period until December 2017. Given this long study period, a possible era effect due to changes in medical care cannot be ruled out. As the initial standard immunosuppressive drug regimen was subsequently switched from CsA and MMF to TAC and MMF from 2006 onward, we performed a sensitivity analysis including only patients with TAC and MMF to test the robustness of the study results. Here, similar results were observed. Another change of medical treatment during the study period was the clinical introduction of ivabradine in 2006 which is used for heart rate reduction in patients after HTX. Nevertheless, there was no relevant difference between patients with and without COPD in the administration of ivabradine.Citation15
A particular characteristic of this study site is the relatively large number of patients with cardiac amyloidosis as the principal diagnosis for HTX. This is because Heidelberg Heart Center is closely linked with the Heidelberg Amyloidosis Center which is the largest center for amyloidosis in Germany.
Importantly, the results of this study should be considered as hypothesis-generating, especially in terms of survival as multiple factors may influence survival such as recipient age and recipient comorbidities. Finally, the retrospective design of this study cannot prove or disprove a causal relationship between COPD and an increased mortality in patients after HTX but merely demonstrates an association between the two. Therefore, to confirm our results, further large prospective multicenter trials are desirable to investigate the influence of COPD in patients after HTX.
Conclusion
Patients with an FEV1/FVC <0.70 before HTX had a significantly prolonged initial hospital stay after HTX, a higher rate of early posttransplant AF, an increased posttransplant short- and long-term mortality, as well as a higher percentage of death due to transplant failure and infection/sepsis. Moreover, Kaplan–Meier estimator and a multivariate analysis for mortality within 5 years after HTX showed a significantly inferior survival in these patients.
In summary, COPD in patients after HTX is associated with a prolonged hospital stay, early posttransplant AF, and impaired posttransplant survival.
Acknowledgments
We acknowledge financial support by the Deutsche Forsc-hungsgemeinschaft (DFG – German Research Foundation) and the University of Heidelberg within the funding program “Open Access Publishing”. We thank Viola Deneke and Berthold Klein for their assistance and advice.”
Disclosure
Rasmus Rivinius is funded by research grants from the Faculty of Medicine, University of Heidelberg (Physician Scientist Program Scholarship). The other authors report no conflicts of interest in this work.
References
- ManninoDMGagnonRCPettyTLLydickEObstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994Arch Intern Med2000160111683168910847262
- ManninoDMBuistASGlobal burden of COPD: risk factors, prevalence, and future trendsLancet2007370958976577317765526
- BuistASMcBurnieMAVollmerWMInternational variation in the prevalence of COPD (the BOLD Study): a population-based prevalence studyLancet2007370958974175017765523
- DecramerMJanssensWMiravitllesMChronic obstructive pulmonary diseaseLancet201237998231341135122314182
- RabeKFWatzHChronic obstructive pulmonary diseaseLancet2017389100821931194028513453
- BarnesPJCelliBRSystemic manifestations and comorbidities of COPDEur Respir J20093351165118519407051
- BevacquaBKPre-operative pulmonary evaluation in the patient with suspected respiratory diseaseIndian J Anaesth201559954254926556912
- SinDDWuLManSFThe relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literatureChest200512761952195915947307
- AnthonisenNRConnettJEKileyJPEffects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health StudyJAMA199427219149715057966841
- HelmschrottMRiviniusRRuhparwarAAdvantageous effects of immunosuppression with tacrolimus in comparison with cyclosporine A regarding renal function in patients after heart transplantationDrug Des Devel Ther2015912171224
- HelmschrottMRiviniusRBrucknerTKatusHADoeschAORenal function in heart transplant patients after switch to combined mammalian target of rapamycin inhibitor and calcineurin inhibitor therapyDrug Des Devel Ther20171116731680
- RiviniusRHelmschrottMRuhparwarAThe influence of surgical technique on early posttransplant atrial fibrillation – comparison of biatrial, bicaval, and total orthotopic heart transplantationTher Clin Risk Manag20171328729728331331
- ManciniDLietzKSelection of cardiac transplantation candidates in 2010Circulation2010122217318320625142
- MehraMRCanterCEHannanMMThe 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year updateJ Heart Lung Transplant201635112326776864
- RiviniusRHelmschrottMRuhparwarAControl of cardiac chronotropic function in patients after heart transplantation: effects of ivabradine and metoprolol succinate on resting heart rate in the denervated heartClin Res Cardiol2018107213814729098378
- RiviniusRHelmschrottMRuhparwarAChronic digitalis therapy in patients before heart transplantation is an independent risk factor for increased posttransplant mortalityTher Clin Risk Manag2017131399140729075124
- RiviniusRHelmschrottMRuhparwarAComparison of post-transplant outcomes in patients with no, acute, or chronic amiodarone use before heart transplantationDrug Des Devel Ther20171118271837
- RiviniusRHelmschrottMRuhparwarALong-term use of amiodarone before heart transplantation significantly reduces early post-transplant atrial fibrillation and is not associated with increased mortality after heart transplantationDrug Des Devel Ther201610677686
- RiviniusRHelmschrottMRuhparwarAAnalysis of malignancies in patients after heart transplantation with subsequent immunosuppressive therapyDrug Des Devel Ther2015993102
- BhattSPDransfieldMTChronic obstructive pulmonary disease and cardiovascular diseaseTransl Res2013162423725123727296
- SinDDManSFWhy are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary diseaseCirculation2003107111514151912654609
- ManninoDMFordESReddSCObstructive and restrictive lung disease and markers of inflammation: data from the Third National Health and Nutrition ExaminationAm J Med2003114975876212829203
- BhattSPNandaSKintzerJSArrhythmias as trigger for acute exacerbations of chronic obstructive pulmonary diseaseRespir Med201210681134113822595809
- SarubbiBEspositoVDucceschiVEffect of blood gas derangement on QTc dispersion in severe chronic obstructive pulmonary disease: evidence of an electropathy?Int J Cardiol19975832872929076557
- ShihHTWebbCRConwayWAPetersonETilleyBGoldsteinSFrequency and significance of cardiac arrhythmias in chronic obstructive lung diseaseChest198894144482454781
- TükekTYildizPAkkayaVFactors associated with the development of atrial fibrillation in COPD patients: the role of P-wave dispersionAnn Noninvasive Electrocardiol20027322222712167183
- BuchPFribergJScharlingHLangePPrescottEReduced lung function and risk of atrial fibrillation in the Copenhagen City Heart StudyEur Respir J20032161012101612797497
- PsatyBMManolioTAKullerLHIncidence of and risk factors for atrial fibrillation in older adultsCirculation1997967245524619337224
- KorantzopoulosPKolettisTMGalarisDGoudevenosJAThe role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillationInt J Cardiol2007115213514316764958
- OgiHNakanoYNiidaSIs structural remodeling of fibrillated atria the consequence of tissue hypoxia?Circ J20107491815182120631454
- GasparovaIKubatkaPOpatrilovaRPerspectives and challenges of antioxidant therapy for atrial fibrillationNaunyn Schmiedebergs Arch Pharmacol20173901114
- RohSYChoiJILeeJYCatheter ablation of atrial fibrillation in patients with chronic lung diseaseCirc Arrhythm Electrophysiol20114681582221946388
- OlssonSBAtrial fibrillation–Where do we stand today?J Intern Med20012501192811454138
- SalpeterSROrmistonTMSalpeterEECardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysisChest200412562309232115189956
- ChristiansenCFChristensenSMehnertFCummingsSRChapurlatRDSørensenHTGlucocorticoid use and risk of atrial fibrillation or flutter: a population-based, case-control studyArch Intern Med2009169181677168319822824
- van der HooftCSHeeringaJBrusselleGGCorticosteroids and the risk of atrial fibrillationArch Intern Med200616691016102016682576
- CurkendallSMDeLuiseCJonesJKCardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patientsAnn Epidemiol2006161637016039877
- CurkendallSMLanesSde LuiseCChronic obstructive pulmonary disease severity and cardiovascular outcomesEur J Epidemiol2006211180381317106760
- HohnloserSHPajitnevDPogueJIncidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W SubstudyJ Am Coll Cardiol200750222156216118036454
- DasariTWPavlovic-SurjancevBPatelNIncidence, risk factors, and clinical outcomes of atrial fibrillation and atrial flutter after heart transplantationAm J Cardiol2010106573774120723655
- PavriBBO’NunainSSNewellJBRuskinJNWilliamGPrevalence and prognostic significance of atrial arrhythmias after orthotopic cardiac transplantationJ Am Coll Cardiol1995257167316807759722
- HuntSATaking heart–cardiac transplantation past, present, and futureN Engl J Med2006355323123516855261
- HauptmanPJMehraMRIt is time to stop ignoring malignancy in heart transplantation: a call to armsJ Heart Lung Transplant20052481111111316102448
