Abstract
Background
The incidence and prevalence of multiple endocrine neoplasia 2A (MEN2A) have only been reported once in a nationwide setting. However, it is unclear whether the figures are representative of other populations, as the major component of the syndrome, hereditary medullary thyroid carcinoma (MTC), has been reported as rare in the same country. We conducted a nationwide retrospective cohort study of MEN2A in Denmark from 1901 to 2014, aiming to describe the incidence and prevalence.
Methods
This study included 250 unique MEN2A patients born or resident in Denmark before December 31, 2014. Patients were identified through the Danish REarranged during Transfection (RET) cohort, linkage of MEN2A pedigrees, the Danish MTC cohort, a nationwide collaboration of MEN2 centers, cross-checking of other relevant cohorts, and a systematic literature search.
Results
The incidence from 1971 to 2000 was 28 (95% CI: 21–37) per million live births per year. Incidence for the specific mutations or for the overall MEN2A group did not change significantly from 1901 to 2014 (P>0.05). Point prevalence at January 1, 2015, was 24 per million (95% CI: 20–28).
Conclusion
The incidence and prevalence of MEN2A in Denmark seem higher than those reported in other countries. This is likely explained by the Danish C611Y founder effect. Also, our data indicate no significant change in MEN2A incidence during the last century.
Introduction
Multiple endocrine neoplasia 2 (MEN2) is an autosomal-dominant inherited cancer syndrome subdivided into MEN2A and MEN2B. MEN2A associates medullary thyroid carcinoma (MTC), pheochromocytoma, hyperparathyroidism, cutaneous lichen amyloidosis, and Hirschsprung’s disease. MEN2B associates MTC, pheochromocytoma, and ganglioneuromatosis of the aerodigestive tract, and facial, ophthalmologic, and skeletal abnormalities. Both syndromes are caused by germline mutations of the REarranged during Transfection (RET) protooncogene.Citation1
The incidence and prevalence of MEN2B have recently been well described.Citation2–Citation4 Meanwhile, the epidemiology of MEN2A remains poorly defined. Only two studies, a German and a Norwegian, have calculated the incidence and prevalence of MEN2A.Citation2,Citation5 The German study was based on data from two major referral centers and estimated that at least half of all RET carriers born between 1991 and 2000 had been captured.Citation2 The Norwegian study was population-based and covered all RET mutation carriers born in Norway between 1965 and 2015.Citation5 However, it is unclear whether the Norwegian figures are representative of other populations, as the major component of the syndrome, hereditary MTC, has been reported as rare in another Norwegian study.Citation6
Consequently, we conducted a nationwide study of MEN2A in Denmark from 1901 to 2014, aiming to describe the incidence and prevalence.
Methods
Study design and setting
This retrospective cohort study included 250 unique MEN2A patients born or resident in Denmark before December 31, 2014.
Data sources
The Danish RET cohort formed the basis for identifying MEN2A patients. This nationwide cohort contains all patients (n=1,583) RET tested in Denmark between September 1994 and December 2014.Citation7
Pedigree linkage within the RET cohort was used to identify MEN2A patients, who had not been RET tested. Thus, we sought to find linkage between the MEN2A patients from the RET cohort carrying identical mutations. For this purpose, we excluded MEN2A patients with familial origin outside Denmark, molecular proven de novo mutations, and unique mutations within the RET cohort (). Pedigrees were constructed with a minimum of four generations by dint of the Civil Registration System (www.cpr.dk) and the Danish National Archives (www.sa.dk/en/) before comparing with one another. Where linkage was found, this was validated by haplotype.Citation8
Table 1 MEN2A families with RET germline mutationsTable Footnotea detected in Denmark and includedTable Footnoteb in this study
The Danish MTC cohort was searched for MEN2A patients.Citation9 This cohort comprises 476 patients diagnosed with histological (n=474) or cytological (n=2) MTC in Denmark between January 1960 and December 2014 and was constructed through three nationwide registries: the Danish Thyroid Cancer Database, the Danish Cancer Registry, and the Danish Pathology Register.Citation10–Citation12
A nationwide collaboration of all Danish MEN2 treatment centers (Copenhagen University Hospital, Aarhus University Hospital, Odense University Hospital, and Aalborg University Hospital) was established for MEN2A identification. Upon inquiry, each center contributed with all MEN2A patients locally registered until December 31, 2014.
Based on the abovementioned sources, we drew pedigrees from the MEN2A patients and detected the first-degree relatives, who had not been RET tested.
A cross-check between the first-degree relatives of the already identified MEN2A patients and the Danish MTC cohort and other relevant cohorts was performed. The other relevant cohorts were identified through two nationwide registries: the Danish Pathology Register and the Danish National Patient Registry.Citation12,Citation13 Cohorts from the Danish Pathology Register included patients histologically or cytologically diagnosed between September 1968 and December 2014 and comprise a pheochromocytoma cohort, hyperparathyroidism cohort, Hirschsprung cohort, and MEN2A cohort. Cohorts from the Danish National Patient Registry included patients coded between 1976 and 2014 and comprise a pheochromocytoma cohort, hyperparathyroidism cohort, Hirschsprung cohort, multiple endocrine neoplasia cohort, and lichen amyloidosis cohort. All cohorts have been described in detail elsewhere.Citation9 Cohorts with identical names were combined before the cross-check, yielding pheochromocytoma, hyperparathyroidism, Hirschsprung, and multiple endocrine neoplasia cohort of 1,298, 15,802, 1,516, and 426 unique patients, respectively. The lichen amyloidosis cohort included six patients.
A cross-check of the MTC cohort, pheochromocytoma cohort, hyperparathyroidism cohort, Hirschsprung cohort, and lichen amyloidosis cohort against one another was conducted by use of the unique personal identification number issued to each individual in Denmark upon birth or immigration.Citation14 The multiple endocrine neoplasia cohort was cross-checked against all other cohorts except the hyperparathyroidism cohort, as this would result in multiple false-positive cases due to the association of multiple endocrine neoplasia 1 and hyperparathyroidism.
A systematic literature search was performed on December 7, 2017, in the following databases: Cochrane, Embase, PubMed, Scopus, and Web of Science. The search term used was “medullary thyroid carcinoma Denmark OR medullary thyroid carcinoma Danish OR multiple endocrine neoplasia 2 Denmark OR multiple endocrine neoplasia 2 Danish.” No filters were used. MEN2A patients were traced by data on the patient (sex, age, MEN2A features, RET mutation) and pedigree level provided by the respective citations. These data were compared with the data gathered throughout the previous six steps.
MEN2A criteria
If genetic tested, a MEN2 patient was defined as 1) an individual with a pathogenic RET germline sequence change and a MEN2A phenotype in the ARUP MEN2 database on May 1, 2018.Citation15
If not genetic tested, a MEN2A patient was defined as 2) an individual with a MEN2A feature (histologically verified MTC/pheochromocytoma/Hirschsprung’s disease or clinically diagnosed cutaneous lichen) and relatedness to an individual fulfilling 1), or 3) an individual without a proven MEN2A feature, but providing linkage between two individuals fulfilling 1), or 4) an individual without a proven MEN2A feature, but providing linkage between a patient with a MEN2A feature and an individual fulfilling 1), or 5) an individual with >1 MEN2A feature.
Study participants
shows an overview of inclusion of MEN2A patients according to data sources.
The Danish RET cohort initially contained 36 MEN2 families. For the purpose of this study, all MEN2B families (n=6), three of which have been described elsewhere, were excluded.Citation16–Citation19 This left 30 MEN2A families with 155 RET mutation carriers. Several of these have been reported previously.Citation20–Citation24 One family with three carriers has subsequently been excluded from the cohort, as the pathogenicity of the RET I852M variant has been questioned and reclassified in the ARUP MEN2 database.Citation15,Citation25 In a C634R family, one patient initially had the RET test interpreted as negative. A recent revaluation, however, identified the mutation. This resulted in 153 MEN2A patients from 29 families fulfilling criterion 1) ().
Pedigree linkage between the MEN2A patients from the RET cohort carrying identical mutations identified 54 MEN2A patients. All 54 patients were new. Six and forty-eight fulfilled criteria 2) and 3), respectively. This reduced the number of C611Y families from twelve to three and the number of C618Y families from two to one. Thus, after this step we had identified 207 MEN2A patients from 19 families.
The Danish MTC cohort initially comprises 102 MTC patients with MEN2A. Linking their pedigrees to the MEN2A patients from the RET cohort provided 22 additional MEN2A patients satisfying criterion 4). Accordingly, 124 MEN2A patients were uncovered from the MTC cohort, but only 34 were new patients. Of these, 22, 11, and one fulfilled criteria 4), 2) and 1), respectively. The latter patient was a C634R carrier genetic tested subsequent to the end date of the RET cohort and unrelated to the other MEN2A families (). This resulted in 241 MEN2A patients from 20 families.
The nationwide collaboration of all Danish MEN2 centers provided 136 MEN2A patients, all related to families already known. Eight of these patients had not been identified in the previous steps. Four met criterion 1) and four met criterion 2). This yielded 249 MEN2A families from 20 families. In relation to these, we detected 289 first-degree relatives, who had not been RET tested.
A cross-check between the first-degree relatives and relevant cohorts supplied only one MEN2A patient. This patient had not been identified in prior steps and fulfilled criterion 2). Consequently, 250 MEN2A patients from 20 families had been detected.
A cross-check of relevant cohort against one another discovered no additional MEN2A patients.
The systematic literature search found 240 citations. Removal of eleven duplicates and two triplets yielded 225 unique citations, of which 65 had Danish affiliations. Full text was retrieved for all 65 citations. Nineteen reported of MEN2A patients in Denmark.Citation7,Citation8,Citation21–Citation24,Citation26–Citation38 From these citations, we found 34 MEN2A patients. All had been uncovered through previously used sources.
Figure 1 Flow chart showing identification of MEN2A patients. Dotted boxes indicate methods used and additional MEN2A patients for inclusion.
Abbreviations: MEN2A, multiple endocrine neoplasia 2A; RET, Rearranged during Transfection.
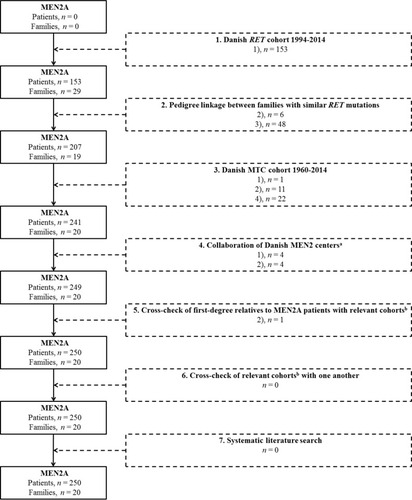
In total, we ascertained 250 MEN2A patients from 20 families. Criteria 1), 2), 3), and 4) were met by 158, 22, 48, and 22 patients, respectively.
All 250 patients were included for prevalence calculations, while the 190 patients born in Denmark between January 1, 1901, and December 31, 2014, were included for incidence calculations.
The investigation was approved by the Danish Health Authority (3-3013-395/3) and the Danish Data Protection Agency (18/17801). The Regional Committees on Health Research Ethics for Southern Denmark found that further review was not liable to notification (S-20132000-69).
Incidence
Incidence was calculated as the number of MEN2A patients born in Denmark in each decade divided by the number of live births in Denmark for the respective decade. To estimate the incidence of MEN2A, we used the period from 1971 to 2000, equivalent to that previously used to estimate the incidence of MEN2B in Denmark.Citation3
Danish population data were retrieved from Statistics Denmark (www.statbank.dk).
Prevalence
Point prevalence for each year from 1901 to 2015 was calculated as the number MEN2A patients alive at January 1 divided by the number of inhabitants alive at the same date.
Statistical analysis
Time trends in incidence were evaluated by Poisson regression. P-values<0.05 were considered significant. All analyses were done using Stata® 15.0 (StataCorp LP, College Station, TX, USA).
Results
Among the 250 MEN2A patients included, 118 were female and 132 were male yielding a female–male ratio of 0.89. The RET mutation carriers were distributed as follows: 169 (68%) C611Y carriers, 16 (6%) C618Y carriers, 16 (6%) C611W carriers, 12 (5%) C620R carriers, 10 (4%) D631Y carriers, 9 (4%) C634R carriers, 6 (2%) V804M carriers, 5 (2%) L790F carriers, 4 (2%) C618F carriers, 2 (1%) C634Y carrier, and 1 (0%) C634Y+Y791F carrier.
Most index cases had inherited their RET mutation. Only in one case was the mutation (C620R) molecularly proven to be de novo, as both parents were tested negative for the mutation. Also, haplotype analysis failed to relate the index case to the two other Danish C620R families.Citation8
Incidence
depicts the incidence of MEN2A by decade and mutation. No significant change in incidence from 1901 to 2014 was seen for the specific mutations or for the overall group of MEN2A (P>0.05) (). The incidence from 1971 to 2000 was 28 (95% CI: 21–37) per million live births per year.
Table 2 Incidence of MEN2A in Denmark according to decade and mutation
Prevalence
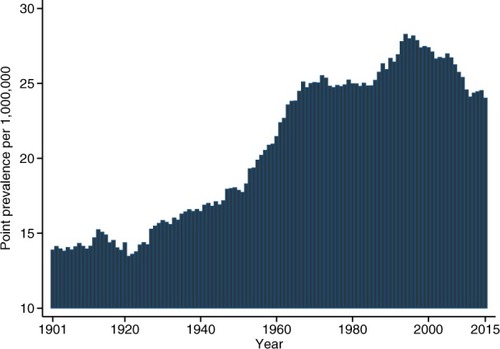
shows the point prevalence of MEN2A per million according to January 1 in each year from 1901 to 2015. The point prevalence at January 1, 2015, was 24 per million (95% CI: 20–28) (5,659,715 inhabitants in Denmark and 136 MEN2A patients alive).
Discussion
In this nationwide study of 250 unique MEN2A patients born or resident in Denmark before December 31, 2014, we report an incidence of 28 per million live births per year and a point prevalence of 24 per million.
Limitations
To estimate the true number of MEN2A patients in a country, the entire population should ideally undergo RET testing at birth. To the best of our knowledge, this is not the case anywhere in the world. Instead, we conducted a comprehensive search in the Danish RET cohort, linkage of MEN2A pedigrees, the Danish MTC cohort, a nationwide collaboration of MEN2 centers, cross-checking of other relevant cohorts, and a systematic literature search. This virtually depleted all possibilities to identify MEN2A patients in Denmark and enabled us to study the incidence and prevalence for the entire 20th century.
The registries used have different coverage in terms of calendar time, potentially having an impact on the incidence and prevalence estimates. Except for the Danish Cancer Registry, none of the utilized registries existed during the first half of the century. Similarly, fewer MEN2A patients born in the first part than in the second part of the century survived until the introduction of RET testing in Denmark (September 1994). Thus, the incidence and prevalence may be underestimated in the first half of the century, although pedigree linkage identified several MEN2A patients born in this period.
In our cohort, the MEN2A diagnosis was verified by RET testing in 63% (158/250). In 34% (86/250), genetic in vivo testing was never possible, as patients had died or emigrated before RET testing became available in Denmark or before the first MEN2A diagnosis was made in their respective families. However, if only considering patients included for calculations of incidence 1901–2014, incidence 1971–2014, and point prevalence at January 1, 2015, the figures for MEN2A diagnosis verified by RET testing increased to 80%, 100%, and 97%, respectively.
Despite our meticulous search and high proportion of RET-tested MEN2A patients for the reported incidence and prevalence calculations, we cannot rule out that some patients may not have been captured. In fact, as of this writing, a Danish L790F family is undergoing genetic workup after the presentation of MTC (T1bN0M0) in a 70-year-old index case. This exemplifies that carriers of RET mutations classified in the American Thyroid Association’s moderate categoryCitation1 and carriers unaware of their MEN2A family history may not have been captured yet. Therefore, our figures of incidence and prevalence should be regarded as minimum estimates.
Incidence
In the present study, we found an incidence of 28.4 per million live births per year from 1971 to 2000.
A German study reported the incidence of MEN2A from 1951 to 2000, with the highest incidence found from 1991 to 2000.Citation2 If excluding carriers of the benign Y791F variant,Citation15,Citation39 the incidence in this period can be calculated as 8.1 (95% CI: 6.2–10) per million live births per year. During the same period, our incidence was 31 (95% CI: 19–48) per million live births per year. Another study found the incidence of MEN2A in Norway from 1965 to 2015 to be 1 per 66,438 live births per year corresponding to 15 (95% CI: 11–20) per million live births per year.Citation5 In the same period, the incidence in Denmark was 25 (95% CI: 20–31) per million live births per year corresponding to 1 per 40,102 live births per year.
The Danish incidence was higher compared with both the German and the Norwegian incidence. The difference in the German incidence could likely be explained by ascertainment as the population in our study included all of Denmark, while the German study estimated a coverage of at least half of all RET carriers born in the period of 1991–2000.Citation2 Even though the Norwegian study is nationwide,Citation5 one might speculate that the difference in the Danish incidence is also due to ascertainment, as inclusion in the Norwegian study was based solely on a systematic search within all four Norwegian departments of medical genetics, while our inclusion was based on a systematic search in the Danish RET cohort, linkage of MEN2A pedigrees, the Danish MTC cohort, a nationwide collaboration of MEN2 centers, cross-checking of other relevant cohorts, and a systematic literature search. However, if we include only patients identified from the RET cohort, being the best comparison with the Norwegian method, the Danish MEN2A incidence in the given period at 24 (95% CI: 19–30) per live birth per million still appears higher than the Norwegian. A more likely explanation to the difference in incidence may be the C611Y founder effect in Denmark, accounting for an unusually high incidence of C611Y carriers.Citation8 This is supported by the fact that 74% (60/81) of MEN2A patients born in Denmark between January 1, 1965, and January 1, 2015, were carriers of the C611Y mutation. Unfortunately, the Norwegian study did not specify the carrier status of MEN2A patients included in the incidence calculations, but because only five carriers of codon 611 mutations were included in the entire study, the percentage of C611Y carriers in incidence calculation could maximally be 11% (5/44). This suggests that the large proportion of C611Y carriers in the Danish MEN2A population is the main reason for the disparity seen in incidence between the two Scandinavian countries.
Prevalence
Based on 5,659,715 inhabitants and 136 MEN2A patients living in Denmark at January 1, 2015, we found a point prevalence of 24 per million. Similarly, 5,165,000 inhabitants and 65 MEN2A patients were living in Norway at March 1, 2015, yielding a point prevalence of 13 per million.Citation5 Thus, the point prevalence in Denmark seems somewhat higher compared with that of Norway.
Again, if only including patients (n=131) identified from the RET cohort for the Danish prevalence calculations, the prevalence still appears higher than that in Norway. Also, for prevalence calculations, the proportion of C611Y carriers in Denmark (99/136) was substantially higher than the corresponding proportion in Norway (5/65) under the assumption that all codon 611 mutation carriers in Norway were in fact C611Y carriers and alive at prevalence day. Thus, the most likely explanation for the difference in MEN2A prevalence recorded between the two countries seems to be the Danish C611Y founder effect.Citation8 Similarly, other populations with supposed or proven RET founder mutations may also experience a rather high MEN2A prevalence.Citation40–Citation42
In our study, the prevalence was steadily increasing until the beginning of the 1960s, most likely caused by buildup of the cohort with continuing recruitment exceeding the rate of exit, a phenomenon known from other rare conditions as well.Citation43 The prevalence will be stable when recruitment equals exit (death or emigration). We do not have any obvious explanation why the prevalence is decreasing after 2000.
Conclusion
The incidence and prevalence of MEN2A in Denmark seems to be higher than those reported in other countries. This is likely explained by the Danish C611Y founder effect. Also, our data indicate no significant change in MEN2A incidence during the last century.
Acknowledgments
The authors are deeply grateful to Torben Falck Ørntoft (Aarhus) for the possibility to create and use the Danish nationwide RET cohort. We are also very grateful for the help in data collection provided by Anne Lene Riis (Horsens). This work was supported by the University of Southern Denmark, the Region of Southern Denmark, Odense University Hospital, Copenhagen University Hospital, the Danish Cancer Society, the Danish Cancer Research Foundation, and the A. P. Moller Foundation. The research salary of Ulla Feldt-Rasmussen is sponsored by an unrestricted research grant from the Novo Nordic Foundation.
Disclosure
The authors report no conflict of interest in this work.
References
- WellsSAAsaSLDralleHRevised American Thyroid Association guidelines for the management of medullary thyroid carcinomaThyroid201525656761025810047
- MachensALorenzKSekullaCMolecular epidemiology of multiple endocrine neoplasia 2: implications for RET screening in the new milleniumEur J Endocrinol2013168330731423211574
- MathiesenJSKroustrupJPVestergaardPIncidence and prevalence of multiple endocrine neoplasia 2B in Denmark: a nationwide studyEndocr Relat Cancer2017247L39L4228438782
- ZnaczkoADonnellyDEMorrisonPJEpidemiology, clinical features, and genetics of multiple endocrine neoplasia type 2B in a complete populationOncologist201419121284128625355845
- OpsahlEMBrauckhoffMSchlichtingEA nationwide study of multiple endocrine neoplasia type 2a in Norway: predictive and prognostic factors for the clinical course of medullary thyroid carcinomaThyroid20162691225123827400880
- HøieJJørgensenOGStenwigAELangmarkFMedullary thyroid cancer in Norway. A 30-year experienceActa Chir Scand19881545–63393433420998
- MathiesenJSKroustrupJPVestergaardPDistribution of RET mutations in multiple endocrine neoplasia 2 in Denmark 1994-2014: a nationwide studyThyroid201727221522327809725
- MathiesenJSKroustrupJPVestergaardPFounder effect of the RETC611Y mutation in multiple endocrine neoplasia 2A in Denmark: a nationwide studyThyroid201727121505151029020875
- MathiesenJSKroustrupJPVestergaardPIncidence and prevalence of sporadic and hereditary MTC in Denmark 1960–2014: a nationwide studyEndocr Connect20187682983929760189
- GjerstorffMLThe Danish Cancer RegistryScand J Public Health2011397 Suppl424521775350
- LonderoSCMathiesenJSKrogdahlACompleteness and validity in a national clinical thyroid cancer database: DATHYRCACancer Epidemiol201438563363725132423
- BjerregaardBLarsenOBThe Danish Pathology RegisterScand J Public Health2011397 Suppl727421775357
- LyngeESandegaardJLReboljMThe Danish National Patient RegisterScand J Public Health2011397 Suppl303321775347
- Nguyen-NielsenMSvenssonEVogelIEhrensteinVSundeLExisting data sources for clinical epidemiology: Danish registries for studies of medical genetic diseasesClin Epidemiol2013524926223966801
- MargrafRLCrockettDKKrautscheidPMMultiple endocrine neoplasia type 2 RET protooncogene database: repository of MEN2-associated RET sequence variation and reference for genotype/phenotype correlationsHum Mutat200930454855619177457
- MathiesenJSHabraMABassettJHDRisk profile of the RET A883F germline mutation: an international collaborative studyJ Clin Endocrinol Metab201710262069207428323957
- MathiesenJSStochholmKPoulsenPLVestergaardEMChristiansenPVestergaardPAggressive medullary thyroid carcinoma in a ten-year-old patient with multiple endocrine neoplasia 2B due to the A883F mutationThyroid201525113914025244518
- MathiesenJSDossingHBenderLGodballeCMedullary thyroid carcinoma in a 10-month-old child with multiple endocrine neoplasia 2BUgeskr Laeger20141765apii V07130456
- Søndergaard PedersenJHSchaffalitzky de MuckadellOChoroidal metastases in multiple endocrine neoplasia type 2BActa Ophthalmol Scand200785112012117244227
- VestergaardPKroustrupJPRønneHEngCLaurbergPNeuromas in multiple endocrine neoplasia type 2A with a RET codon 611 mutationInt J Disabil Hum Dev1999113337
- HansenHSTorringHGodballeCJägerACNielsenFCIs thyroidectomy necessary in RET mutations carriers of the familial medullary thyroid carcinoma syndrome?Cancer200089486386710951350
- EmmertsenKScreening for hereditary medullary cancer in DenmarkHenry Ford Hosp Med J19843242382436152458
- GodballeCJørgensenGGerdesAMKrogdahlASTybjaerg-HansenANielsenFCMedullary thyroid cancer: RET testing of an archival materialEur Arch Otorhinolaryngol2010267461361719823860
- KjaerAPetersenCLPrimary diagnosis of multiple pheochromocytomas in the brother of a MEN-2 patient by simultaneous MIBG scintigraphy and low-dose computed tomographyClin Nucl Med2002271286887012607865
- MathiesenJSvan Overeem HansenTRasmussenÅKNovel somatic RET mutation questioning the causality of the RET I852M germline sequence variant in multiple endocrine neoplasia 2AThyroid20172781103110428578594
- BrandrupFOMedullary carcinoma of the thyroid gland. A review and report of 4 casesUgeskr Laeger197013294304375446571
- EmmertsenKMedullary thyroid carcinoma and calcitoninDan Med Bull1985321128
- EmmertsenKElbrøndONielsenHEFamilial medullary thyroid carcinoma in multiple endocrine neoplasia (MEN) IIa: diagnosis and problems in treatmentEur J Cancer Clin Oncol19821876456506127216
- EmmertsenKMelsenFMosekildeLAltered vitamin D metabolism and bone remodelling in patients with medullary thyroid carcinoma and hypercalcitoninemiaMetab Bone Dis Relat Res19824117237121251
- SchifterSWilliamsEDCraigRKHansenHHCalcitonin gene-related peptide and calcitonin in medullary thyroid carcinomaClin Endocrinol1986256703710
- SchifterSCalcitonin gene-related peptide and calcitonin as tumour markers in MEN 2 family screeningClin Endocrinol1989303263270
- SchifterSCalcitonin and PDN-21 as tumour markers in MEN-2 family screening for medullary thyroid carcinomaEur J Cancer1992282–33413451350454
- TveteråsKPaulsenSMGreisenOJohansenJThyroid cancer in the county of North Jutland. Diagnosis, treatment and prognosisUgeskr Laeger199315520155715618316989
- HansenHSMedullary thyroid cancer–screeningUgeskr Laeger199415626389338968059474
- SchifterSJohnsenAHCalcitonin gene-related peptide in medullary thyroid carcinomas: characterization of molecular forms including the amidated C-terminusPeptides19941558979057984511
- VestergaardPMultiple endocrine neoplasia type 2a and 2bUgeskr Laeger199615855905938607216
- KroustrupJPLaurbergPMadsenPHRapid MEN 2A gene carrier identification using primer-specific PCR amplificationScand J Clin Lab Invest199959864364710691056
- VestergaardPJönssonALChristiansenPFrohnertJCan measurement of basal calcitonin replace the pentagastrin test?Ugeskr Laeger2008170413238324218940156
- ToledoRAHatakanaRLourençoDMComprehensive assessment of the disputed RET Y791F variant shows no association with medullary thyroid carcinoma susceptibilityEndocr Relat Cancer2015221657625425582
- FanisPSkordisNFrangosSMultiple endocrine neoplasia 2 in Cyprus: evidence for a founder effectJ Endocrinol Invest201841101149115729396759
- CunhaLLLindseySCFrançaMIEvidence for the founder effect of RET533 as the common Greek and Brazilian ancestor spreading multiple endocrine neoplasia 2AEur J Endocrinol2017176551551928137737
- PinnaGOrgianaGRiolaARET proto-oncogene in Sardinia: V804M is the most frequent mutation and may be associated with FMTC/MEN-2A phenotypeThyroid200717210110417316110
- GrothKAHoveHKyhlKPrevalence, incidence, and age at diagnosis in Marfan SyndromeOrphanet J Rare Dis20151015326631233