Abstract
Objective
Reports on the epidemiology of vestibular schwannoma (VS) indicate an increase in diagnosed cases, often based on selected materials over a limited period of time. This report presents prospective 40-year epidemiological data from an unselected national cohort of all patients diagnosed with a VS in Denmark since 1976.
Study-design
Data on gender, age, tumor localization and size registered during the period 1976–2015 were retrieved.
Results
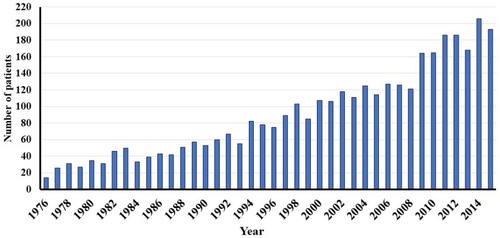
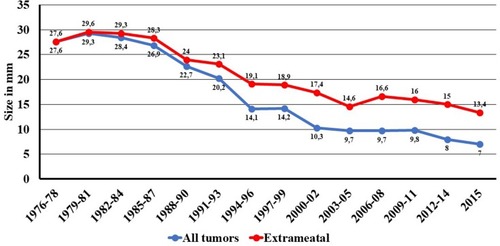
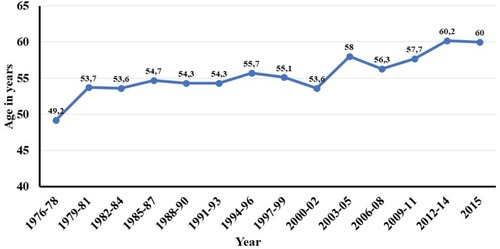
3637 new cases of VS were diagnosed during the 40-year period. The annual number of diagnosed VS increased from 14 in 1976 to 193 in 2015. Mean extrameatal tumor size decreased from 26mm in 1976 to 13.4mm in 2015. Large and giant tumors were more frequent during the first decades, whereas predominantly smaller tumors were diagnosed during the recent years. Median age at diagnosis increased gradually from 49.2 years in 1976 to 60 years in 2015.
Conclusion
Over the past 40 years, the incidence rate of vestibular schwannomas has increased steadily from 3 VS/million/year to 34 VS/million/year, primarily due to easier access to improved diagnostics and the finding of more tumors in older people. Concurrently, the diagnostic tumor size has decreased from 26mm to 7mm, and the age at diagnosis has increased from 49 to 60 years.
Introduction
All vestibular schwannomas diagnosed in Denmark have been managed at our tertiary referral center and registered prospectively in a database since 1976. Prior to this, data occur primarily in Danish neurosurgical publications.Citation1 Following the initiation of the database and the introduction of the translabyrinthine approach,Citation2 the recorded incidence rate of VS has been published successively, firstly by Tos and Thomsen and more lately by Stangerup et al.Citation1,Citation3–Citation7 In 2004, an increasing incidence rate of VS was reported, based on 1446 patients diagnosed during the period from 1976 to 2001Citation1 and in 2010 reported further increasing to a peak of 22.8 VS/million in 2004.Citation6 In 1976, the annual incidence rate was 3.1 VS/million, increasing to 19.4 VS/million in 2008, based on 2283 patients.Citation6
Several other studies have also published data showing an increasing incidence rate of VS. Generally, incidences between 10 and 20 VS/million/year are reported, including data from America (Nestor et alCitation8), Canada (Frohlich and SutherlandCitation9), United Kingdom (Evans et alCitation10) and locally in Denmark (Mirz et alCitation11). Lately, American population-based registry studies have found similar incidences. Thus, Carlson et alCitation12 reported an incidence rate of 11 VS/million/year based on 8330 patients diagnosed during the period 2004–2011, and data on 23,729 patients registered in the Central Brain Tumor Registry of the United States during the period 2004–2010 showed an incidence rate of 10.9 million/year.Citation13 An earlier publication from the same registry reported an incidence rate of 6 VS/million/year.Citation14 From the Netherlands Cancer Registry, a publication from 2016 reported on 3663 patients diagnosed with VS during the period 2001 to 2012, showing an increase in incidence rate from 10.3 to 15.5 VS/million/year.Citation15 From Taiwan, a publication reported an increase in incidence rate from 26.6/million in 2001 to 37.2/million in 2012.Citation16 Lastly, in 2018, a publication from the USA (Minnesota) reported an increase in incidence rate from 15 VS/million/year in 1966 to 42 VS/million/year in 2016.Citation17
The aim of this study is to report prospective 40-year epidemiological data from the Danish VS database, which contains the unselected, unbiased national cohort of all Danish patients diagnosed with a VS since 1976. Data on incidence rate, gender, age at diagnosis, tumor localization (purely intrameatal or extrameatal) and size registered during the period 1976–2015 are presented.
Methods
Since 1976, data from all patients in Denmark with a sporadic/unilateral VS have been referred to our national treatment center, at which the patient data have been entered prospectively into the national VS database. The data are thus unselected and unbiased. Among other data, the tumor size, the tumor localization, the initial treatment strategy, gender and age of the patient at diagnosis were registered. The tumors were categorized as either intrameatal or extrameatal (intra- and extrameatal). The size of the extrameatal tumors was determined as the largest extrameatal diameter by linear measurements. This classification follows the Tokyo recommendations on reporting size of VS.Citation18
This study reports the data on the annual incidence rate, the tumor localization and size at diagnosis, the age at diagnosis, and the relation between age and tumor size at diagnosis during the 40-year period from January 1, 1976 to January 1, 2016. A total of 3637 patients with sporadic/unilateral VS were registered and included in the database during this period, thus excluding patients diagnosed with Neurofibromatosis 2. The population in Denmark was 5.1 (5.065) million in 1976, increasing to 5.7 (5.707) million as of January 1, 2016.
Results
Gender And Tumor Localization At Diagnosis
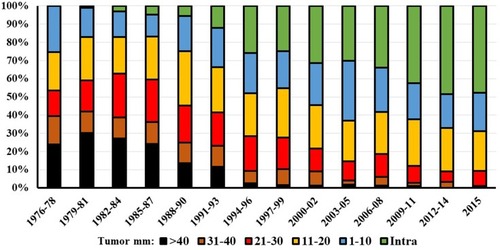
Of the 3637 diagnosed patients, 1804 were female and 1833 male. 1095 of the tumors had an intrameatal localization at diagnosis and 2509 were extrameatal. Localization data is missing for 33 patients ().
Table 1 Patients diagnosed with a VS in Denmark during the 40-year period 1976–2015
Annual Incidence Rate
Since 1976, the annual number of diagnosed VS has increased almost linearly from 14 tumors in 1976 to 193 in 2015 (), corresponding to an increase in incidence rate from 2.8 VS/million/year in 1976 to 33.8 VS/million/year in 2015.
Diagnostic Tumor Size
Diagnostic tumor size has decreased markedly, from a mean size of 27.6 mm in the period 1976 to 1978 to 7 mm in 2015 for all tumors (). The mean extrameatal tumor size was 26 mm in 1976 decreasing to 13.4 mm in 2015. During the first three years, no intrameatal tumors were found, whereas such tumors constituted nearly half of the diagnosed tumors at the end of the 40-year period (). The large and giant tumors constituted 40% of all tumors during the first years, only a few percent toward the end of the period ().
Patient Age At Diagnosis
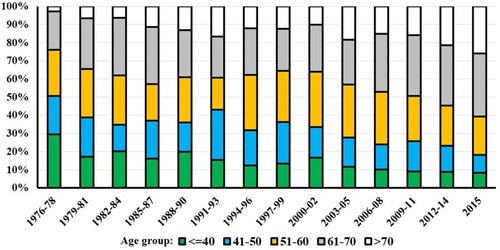
The median age at diagnosis has increased slowly, from 49.2 years in 1976 to 60 years in 2015 (). The age distribution at the time of diagnosis changed during the period, as seen in . Initially, 51% of the VS patients were 50 years or younger, decreasing to 18% at the end of the period (). In contrast, only 2.8% of the patients were older than 70 years in the beginning of the period, increasing to 26% at the end of the period.
Tumor Size And Age At Diagnosis
shows that the tumor size at diagnosis was larger in younger patients and smaller in older patients. Thus, patients below the age of 40 years had a mean tumor size of 19.7mm extrameatal, whereas patients above the age of 60 years had a mean tumor size of 11.1mm extrameatal.
Table 2 Mean tumor size (mm extrameatal ±SD) related to age at diagnosis in patients diagnosed with a VS during the 40-year period 1976 – 2015
Discussion
This prospective study on 40-year epidemiological data from the unselected, unbiased national cohort of all Danish patients diagnosed with a VS since 1976 shows that the incidence rate of diagnosed tumors has been increasing steadily over the years, from 2.8 VS/million/year in 1976 to 33.8 VS/million/year in 2015. Improved diagnostics and increased availability of improved diagnostics are probably the main reasons for this increase, although an increased tumor occurrence cannot entirely be ruled out. The first MRI scanner in Denmark was functional in 1989 and by 2015, the number had increased to approximately 100. Increased awareness among patients and physicians is likely a contributing factor, as is an increasing number of older people, who in addition may be less and less likely to accept even a small loss of hearing, thus seeking a physician. More and more incidental findings due to an increased number of MRI scans, performed for reasons other than hearing loss, is also likely to be part of the explanation. On this basis, it is reasonable to assume that the incidence rate will increase continuously over the coming years.
Studies with higher incidence rates, ie 37,Citation16 42Citation17 or 54Citation19 VS/million/year, have been published, from eg USA. A number of biases may be contributing, including easier access to health care in more affluent parts of the world or less strict criteria of indication to perform MRI, based on eg tinnitus, a smaller degree of hearing loss, or any other symptom or finding that may lead to MRI of the head and thus an incidental tumor finding. The two reports from America both have populations with easier access to the healthcare system (ie Mayo Clinic, Rochester) or belong to a very affluent part of the population (ie Beverly Hills, California). Denmark has a state-funded healthcare system financed through general taxation and every citizen can consult an ENT specialist (and have an MRI if indicated) free of charge and without a referral. Despite this, even in a very equal and homogeneous population such as the Danish, a study on socio-demographic factors demonstrated lower incidence rates in remote parts of the country which is relatively small, but the same diagnostic age and tumor size across the country.Citation20 Thus, socio-economic and socio-demographic factors can explain some of the differences in reported incidence rates but are unlikely to directly explain the degree of occurrence of higher incidences reported by other centers.
Over the four decades, diagnostic tumor size has decreased steadily, from 28 mm extrameatal in the late seventies to 7 mm extrameatal in 2015, which is in agreement with an increased incidence rate associated with improved diagnostic tools (contemporary imaging can detect very small tumors, which were undetectable before the introduction of MRI). It is also in agreement with the assumption that patients seek medical consultation based on fewer symptoms. Thus, tumors are found at an earlier stage of development, when the tumor size is smaller.
Tumor aggressiveness appears to be inversely related to age, as in general, large tumors are found in young patients, small tumors in older patients. This is corroborated, but remain unexplained, by studies on the natural history of tumor growth, as small intrameatal tumors (usually found in older patients) are less likely to grow compared to larger, extrameatal tumors (which are usually found in younger patients).Citation7 Studies on tumor gene expression have demonstrated the association between growth and expression of certain genes and activation of specific molecular pathways but have not accounted for age-related tumor aggressiveness.Citation21
Age at tumor diagnosis is steadily increasing, from 49.2 years of age to currently 60 years of age. Demography in Denmark, and in general, is shifting progressively towards older people, which may also be a major reason for the increasing age at diagnosis. In addition, older people are more likely to seek a physician and have an MRI performed due to higher morbidity in general, but certainly also due to the occurrence of age-related hearing loss. Finally, in continuation of the discussion on improved diagnostics, the small tumors within the older patient group were simply not found previously, before the introduction of MRI.
The etiopathogenesis of sporadic/unilateral VS is unaccounted for and several studies have investigated risk factors and associations with VS. No obvious risk factors have been found regarding age and gender. Viral infection has been indicated,Citation21–Citation23 which is in line with the fact that these tumors have been occurring for centuries. In continuation of this, the theory of inflammation as a cause of VS has given rise to studies and reported association to chickenpox,Citation24 hay feverCitation25 and conflicting studies of the protective effects of aspirin/NSAID.Citation26–Citation29 Furthermore, a few studies have reported tobacco use (not snuff tobacco) being inversely related to VS occurrence, although this is poorly understood.Citation25,Citation30–Citation33 None of the studies are prospective, randomized or blinded but mostly case–control studies. Of importance, no association has been found between occupation, traffic/loud noise nor the use of mobile phones and tumor occurrence.Citation34–Citation37 Lastly, exposure to radiation is known to cause neoplasms including intracranial.Citation38–Citation41
Conclusion
Over the past 40 years, the incidence rate of vestibular schwannomas has increased steadily from approximately 3 VS/million/year to 34 VS/million/year, primarily due to continuously easier access to improved diagnostics and the finding of more tumors in older people. Concurrently, the diagnostic tumor size has decreased from 26 mm to 7 mm, and the age at diagnosis has increased from approximately 49 to 60 years of age.
Data Sharing Statement
The data is accessible in the national vestibular schwannoma database and available for the author group.
Acknowledgments
A profound gratitude and sincere thanks are due to the late Prof. J. Thomsen and the late Prof. M. Tos for initiating the database and to all those contributing to its maintenance over the years.
Disclosure
The authors report no conflicts of interest in this work.
References
- Stangerup S-E, Tos M, Caye-Thomasen P, Tos T, Klokker M, Thomsen J. Increasing annual incidence of vestibular schwannoma and age at diagnosis. J Laryngol Otol. 2004;118(8):622–627. doi:10.1258/002221504191798915453938
- Thomsen J, Tos M, Harmsen A, Riishede J, Thornval G. Surgery of acoustic neuromas. Preliminary experience with a translabyrinthine approach. Acta Neurol Scand. 1977;56(4):277–290.920109
- Tos M, Thomsen J. Epidemiology of acoustic neuromas. J Laryngol Otol. 1984;98(7):685–692. doi:10.1017/s00222151001472926747450
- Tos M, Thomsen J, Charabi S. Incidence of acoustic neuromas. Ear Nose Throat J. 1992;71(9):391–393.1425377
- Tos M, Charabi S, Thomsen J. Incidence of vestibular schwannomas. Laryngoscope. 1999;109(5):736–740. doi:10.1097/00005537-199905000-0001110334223
- Stangerup S-E, Tos M, Thomsen J, Caye-Thomasen P. True incidence of vestibular schwannoma? Neurosurgery. 2010;67(5):1335–1340. discussion 1340. doi:10.1227/NEU.0b013e3181f2266020871439
- Stangerup S-E, Caye-Thomasen P. Epidemiology and natural history of vestibular schwannomas. Otolaryngol Clin North Am. 2012;45(2):257–268, vii. doi:10.1016/j.otc.2011.12.00822483814
- Nestor JJ, Korol HW, Nutik SL, Smith R. The incidence of acoustic neuromas. Arch Otolaryngol Head Neck Surg. 1988;114(6):680. doi:10.1001/archotol.1988.01860180094042
- Frohlich AM, Sutherland GR. Epidemiology and clinical features of vestibular schwannoma in Manitoba, Canada. Can J Neurol Sci. 1993;20(2):126–130. doi:10.1017/s03171671000476858334574
- Evans DGR, Moran A, King A, Saeed S, Gurusinghe N, Ramsden R. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol. 2005;26(1):93–97.15699726
- Mirz F, Pedersen CB, Fiirgaard B, Lundorf E. Incidence and growth pattern of vestibular schwannomas in a Danish county, 1977–98. Acta Otolaryngol Suppl. 2000;543:30–33. doi:10.1080/00016480045388310908969
- Carlson ML, Habermann EB, Wagie AE, et al. The changing landscape of vestibular schwannoma management in the United Statesâ—a shift toward conservatism. Otolaryngol Head Neck Surg. 2015;153(3):440–446. doi:10.1177/019459981559010526129740
- Kshettry VR, Hsieh JK, Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Incidence of vestibular schwannomas in the United States. J Neurooncol. 2015;124(2):223–228. doi:10.1007/s11060-015-1827-926024654
- Propp JM, McCarthy BJ, Davis FG, Preston-Martin S. Descriptive epidemiology of vestibular schwannomas. Neuro-Oncology. 2006;8(1):1–11. doi:10.1215/S152285170400109716443943
- Kleijwegt M, Ho V, Visser O, Godefroy W, van der Mey A. Real incidence of vestibular schwannoma? Estimations from a national registry. Otol Neurotol. 2016;37(9):1411–1417. doi:10.1097/MAO.000000000000116927525713
- Koo M, Lai J-T, Yang EY-L, Liu T-C, Hwang J-H. Incidence of vestibular schwannoma in taiwan from 2001 to 2012: a population-based national health insurance study. Ann Otol Rhinol Laryngol. 2018;127(10):694–697. doi:10.1177/000348941878838530032646
- Marinelli JP, Lohse CM, Carlson ML. Incidence of vestibular schwannoma over the past half-century: a population-based study of Olmsted County, Minnesota. Otolaryngol Head Neck Surg. 2018;159(4):717–723. doi:10.1177/019459981877062929712512
- Kanzaki J, Tos M, Sanna M, Moffat DA, Monsell EM, Berliner KI. New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. Otol Neurotol. 2003;24(4):642–648. discussion 648–649.12851559
- Schwartz M, Fisher L. Incidence and clinical characteristics of acoustic neuroma in beverly hills. Skull Base. 2006;16(S 1):A040. doi:10.1055/s-2006-958307
- Stepanidis K, Kessel M, Caye-Thomasen P, Stangerup S-E. Socio-demographic distribution of vestibular schwannomas in Denmark. Acta Otolaryngol. 2014;134(6):551–556. doi:10.3109/00016489.2014.89029324655069
- Sass HCR, Borup R, Alanin M, Nielsen FC, Cayé-Thomasen P. Gene expression, signal transduction pathways and functional networks associated with growth of sporadic vestibular schwannomas. J Neurooncol. 2017;131(2):283–292. doi:10.1007/s11060-016-2292-927752882
- Burkhart CG. Herpes and acoustic neuromas: is there a cause and effect to observe? Med Hypotheses. 2010;74(6):1013–1014. doi:10.1016/j.mehy.2010.01.01020153934
- Bhimrao SK, Maguire J, Garnis C, et al. Lack of association between human herpesvirus and vestibular schwannoma: analysis of 121 cases. Otolaryngol Head Neck Surg. 2015;152(3):513–517. doi:10.1177/019459981456351725560404
- Corona AP, Ferrite S, da Silva Lopes M, Rêgo MAV. Risk factors associated with vestibular nerve schwannomas. Otol Neurotol. 2012;33(3):459–465. doi:10.1097/MAO.0b013e3182487fee22377646
- Berkowitz O, Iyer AK, Kano H, Talbott EO, Lunsford LD. Epidemiology and environmental risk factors associated with vestibular schwannoma. World Neurosurg. 2015;84(6):1674–1680. doi:10.1016/j.wneu.2015.07.00726171891
- Dilwali S, Kao S-Y, Fujita T, Landegger LD, Stankovic KM. Nonsteroidal anti-inflammatory medications are cytostatic against human vestibular schwannomas. Transl Res. 2015;166(1):1–11. doi:10.1016/j.trsl.2014.12.00725616959
- Kandathil CK, Cunnane ME, McKenna MJ, Curtin HD, Stankovic KM. Correlation between aspirin intake and reduced growth of human vestibular schwannoma: volumetric analysis. Otol Neurotol. 2016;37(9):1428–1434. doi:10.1097/MAO.000000000000118027631829
- Hunter JB, O’Connell BP, Wanna GB, et al. Vestibular schwannoma growth with aspirin and other nonsteroidal anti-inflammatory drugs. Otol Neurotol. 2017;38(8):1158–1164. doi:10.1097/MAO.000000000000150628692590
- Marinelli JP, Lees KA, Tombers NM, Lohse CM, Carlson ML. Impact of aspirin and other NSAID use on volumetric and linear growth in vestibular schwannoma. Otolaryngol Head Neck Surg. 2019;160(6): 194599819827812. doi:10.1177/0194599819827812
- Schoemaker MJ, Swerdlow AJ, Auvinen A, et al. Medical history, cigarette smoking and risk of acoustic neuroma: an international case-control study. Int J Cancer. 2007;120(1):103–110. doi:10.1002/ijc.2227217019705
- Benson VS, Green J, Pirie K, Beral V. Cigarette smoking and risk of acoustic neuromas and pituitary tumours in the million women study. Br J Cancer. 2010;102(11):1654–1656. doi:10.1038/sj.bjc.660569520461083
- Palmisano S, Schwartzbaum J, Prochazka M, et al. Role of tobacco use in the etiology of acoustic neuroma. Am J Epidemiol. 2012;175(12):1243–1251. doi:10.1093/aje/kwr46522517809
- Chen M, Fan Z, Zheng X, Cao F, Wang L. Risk factors of acoustic neuroma: systematic review and meta-analysis. Yonsei Med J. 2016;57(3):776–783. doi:10.3349/ymj.2016.57.3.77626996581
- Prochazka M, Feychting M, Ahlbom A, et al. Occupational exposures and risk of acoustic neuroma. Occup Environ Med. 2010;67(11):766–771. doi:10.1136/oem.2009.04788620581419
- Schuz J, Steding-Jessen M, Hansen S, et al. Long-term mobile phone use and the risk of vestibular schwannoma: a Danish nationwide cohort study. Am J Epidemiol. 2011;174(4):416–422. doi:10.1093/aje/kwr11221712479
- Fisher JL, Pettersson D, Palmisano S, et al. Loud noise exposure and acoustic neuroma. Am J Epidemiol. 2014;180(1):58–67. doi:10.1093/aje/kwu08124786799
- Roswall N, Stangerup S-E, Cayé-Thomasen P, et al. Residential traffic noise exposure and vestibular schwannomaâ-a Danish case-control study. Acta Oncol. 2017;56(10):1310–1316. doi:10.1080/0284186X.2017.133792528609173
- Ron E, Modan B, Boice JD, et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988;319(16):1033–1039. doi:10.1056/NEJM1988102031916013173432
- Preston DL, Ron E, Yonehara S, et al. Tumors of the nervous system and pituitary gland associated with atomic bomb radiation exposure. J Natl Cancer Inst. 2002;94(20):1555–1563. doi:10.1093/jnci/94.20.155512381708
- Evans DGR, Birch JM, Ramsden RT, Sharif S, Baser ME. Malignant transformation and new primary tumours after therapeutic radiation for benign disease: substantial risks in certain tumour prone syndromes. J Med Genet. 2006;43(4):289–294. doi:10.1136/jmg.2005.03631916155191
- Pollock BE, Link MJ, Stafford SL, Parney IF, Garces YI, Foote RL. The risk of radiation-induced tumors or malignant transformation after single-fraction intracranial radiosurgery: results based on a 25-year experience. Int J Radiat Oncol Biol Phys. 2017;97(5):919–923. doi:10.1016/j.ijrobp.2017.01.00428333013