Abstract
Background
Survival in malignant cutaneous melanoma has improved but increasing survival will result in an increased likelihood of the occurrence of second primary cancers (SPCs). SPCs may adversely interfere with survival. We quantified survival in patients with different types of SPCs, in comparison to known poor prognostic indicators of metastatic disease.
Methods
Data for melanoma and any SPCs were obtained from the Swedish Cancer Registry for years 2003 through 2015, including clinical TNM classification. SPCs were grouped into three ‘prognostic groups’ based on 5-year relative survival of these cancers as first primary cancer. Kaplan-Meier survival curves were generated and hazard ratios were estimated using Cox regression, adjusted for a number of variables and treating diagnosis of SPC as a time-dependent variable.
Results
The total number of first melanoma patients was 28,716 followed by 3,202 (11.1%) SPCs, 1/3 of which had a second melanoma while 2/3 had other SPCs. Among men diagnosed at age over 70 years, who survived at least 10 years, 31.4% had SPC. HRs (95% CI) for survival increased systematically from the reference rate of 1.00 (no SPC) to 1.59 (1.35–1.87) with SPC of good prognosis (78.6% of SPCs) to 3.49 (2.58–4.72) of moderate prognosis (12.0%) and to 7.93 (5.50–11.44) of poor prognosis (9.4%). In patients without SPC, the HRs increased to 2.62 (2.02–3.39) with any nodal metastases and to 5.88 (4.57–7.57) with any distant metastases compared to patients without local or distant metastases.
Conclusion
The data showed that SPCs are an increasingly common negative prognostic factor for melanoma. Future attempts to improve melanoma survival need to target SPCs.
Introduction
Survival rates among patients with malignant cutaneous melanoma (shortly melanoma) have improved in spite of increasing incidence in many countries.Citation1–Citation3 The improvements in survival have been ascribed to earlier diagnosis of thinner lesions due to alertness and screening programs, which may have also caused “overdiagnosis”.Citation1,Citation2,Citation4 It is likely that therapy has also contributed, or will contribute in the future, although breakthrough successes in immunotherapy are yet to show at the population level.Citation5,Citation6 Increasing survival will result in an increased likelihood of the occurrence of second primary cancers (SPCs). SPCs were diagnosed in 13.3% of melanoma patients in Sweden and these were often found to be the causes of death.Citation7 Second melanoma is the most common SPC in melanoma patients and the risk factors may be shared by first and second melanoma, including atypical cutaneous nevi, fair skin color, light eye color, common cutaneous nevi, propensity to sunburn and sunbathing habits, cutaneous freckles, some occupational exposures and predisposition to high-risk and low-risk melanoma-related genes.Citation5,Citation7,Citation8 Second primary melanoma is not always possible to distinguish from a skin metastasis from a previous melanoma. Family history of cancer X may also predispose melanoma patients to cancer X as SPC.Citation7
The object of the present article is to highlight the assumed adverse role of SPCs in melanoma survival. We posit that any second cancer, including melanoma, is a burden which may interfere with survival. Moreover, as most cancers show worse survival than melanoma as first primary cancers, we further posit that they would additionally worsen melanoma survival as SPCs.
Methods
Cancer data for first and any subsequent cancers were obtained from the Swedish Cancer Registry recording cancers according to the International Classification of Diseases 7th revision (ICD-7) and later revisions. The clinical TNM classification was started in 2002/2003. We considered local nodal (N) and distant (M) metastases, denoted by N+ (N1, N2, N3) or M+ (M1, M2). Because of the clinical TNM system, many patients had an undefined metastatic status at diagnosis; these were denoted Nx and Mx and there were included in the analysis but not listed in the tables. We followed newly diagnosed patients with melanoma from 1st January 2003 until 31st December 2015 for diagnosis of any of the 35 different SPCs including second melanomas. The follow-up for survival time was terminated at emigration, death, or 31st December 2015, whichever occurred earliest. Because the novel aspect of the study was to consider the contribution of SPC to melanoma mortality we considered overall mortality as the survival end point.
We grouped SPCs into three ‘prognostic groups’ based on 5-year relative survival of these cancers as first primary cancer:Citation9,Citation10 “good survival” (relative survival >60%) included cancers in the lip, larynx, anus, breast, cervix, endometrium, prostate, testis, male genitals, kidney, bladder, melanoma, skin (squamous cell, SCC), eye, thyroid gland and endocrine, and Hodgkin lymphoma, hairy cell leukemia, polycythaemia vera, myelofibrosis, chronic and unspecified lymphatic leukemia; “moderate survival” (40–60%) included cancers in the remaining upper aerodigestive tract, salivary glands, small intestine, colorectum, female genitals, bone and connective tissue, and non-Hodgkin lymphoma, acute lymphatic leukemia, chronic and unspecified myeloid leukemia; “poor survival” (<40%) included cancers in the stomach, esophagus, liver, pancreas, lung, ovary and nervous system, and myeloma, acute myeloid leukemia and acute monocytic leukemia.
In this study, SPC diagnoses were considered either by comparing second melanomas to other SPC, or by assessing survival in prognostic groups of SPC (good, moderate and poor prognoses). Kaplan-Meier survival curves were generated for patients with or without diagnoses of SPC. Hazard ratios (HRs) were estimated with Cox regression, adjusted for gender, age at diagnosis (≤50, 51–60, 61–70, 71–80, 81–90 and ≥91years old), year of diagnosis (≤2005, 2006–2010 and 2011–2015), tumor size (T1, T2, T3, T4 and others) and histology (superficial spreading, nodular, lentigo maligna and other melanomas) of the first melanoma (see Supplementary Table 1). In the analyses, the diagnosis of SPC was treated as a time-dependent variable in order to avoid the immortal time bias.Citation11 For Kaplan-Meier survival curves, individuals with occurrence of multiple events (first cancer and second cancer diagnosed in the same year, death occurred in the same year of first or second cancer diagnosis) were deleted (N=4,567). Analyses related to metastasis were performed among cases with TNM information. Statistical analyses were done with R version 3.4, SAS version 9.4 and Stata 15.1.
The study was approved (February 6, 2013) by the Ethical Committee of Lund University, without requirement for informed consent (Dnr 2012/795). Through advertisements in the major newspapers people were informed about opting out before the research database was constructed. The project database is located at Center for Primary Health Care in Malmö, Sweden.
Results
The total number of first melanoma patients was 28,716 and during the follow-up from 2003 to 2015, 3,202 (11.1%) patients developed SPCs, including 1082 second melanomas and 2120 other SPCs, i.e., 33.8% and 66.2%, respectively. The number of melanoma patients with a defined T class was 24,828. Detailed patients characteristics are shown in Supplementary Table 1.
shows the numbers and percentages of SPCs in diagnostic age groups of men and women by minimal survival time after melanoma diagnosis. It shows modest systematic increases (with a few exceptions) in proportions of second melanomas and large systematic increases in other SPCs by increasing age and minimal survival time after first melanoma diagnosis. For men diagnosed at over 70 years, second melanoma was found in 5.7%, 7.4% and 7.2% of patients who survived at least 1, 5 and 10 years, respectively. For non-melanoma SPC the respective percentages were 14.0%, 20.0% and 24.2%. Among men diagnosed at age over 70 years who survived at least 10 years only 68.6% were free from SPC (31.4% had SPC). For women diagnosed at age over 70 years, second melanoma was found in between 2% to 4% of cases but non-melanoma SPCs increased in minimal survival groups from 10.2, to 13.7 and to 21.0% of cases. Among women who survived at least 10 years, 76.4% were free from SPC. Female percentages of SPSs were always lower than male percentages, except for non-melanoma SPCs in those diagnosed below age 51 years.
Table 1 Distribution of Second Primary Cancer in Melanoma Patients Depending on Survival Time After First Melanoma Diagnosis
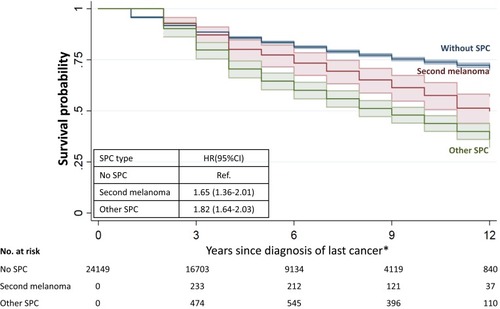
Kaplan-Meier survival data are shown in for melanoma without SPC and with second melanoma or non-melanoma SPC. At the end of the 13-year follow-up, 50% of patients were alive with second melanoma compared to 36% when the SPC was another cancer. Melanoma survival without SPC was 70%. Compared to melanoma without SPCs the HR was 1.65 for second melanoma and 1.82 for non-melanoma SPC.
Figure 1 Survival probability after first melanoma diagnosis in patients without SPC, with second melanoma and with non-melanoma SPC. *Last cancer was melanoma for patients during the time with one single melanoma, and was SPC for those from the time with SPC.
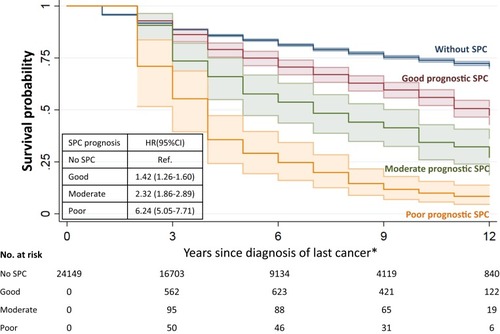
In survival data are shown for SPCs in prognostic groups, based on the known survival of these cancers as first primaries. At the end of the 13-year follow-up the survival probabilities were 46%, 27% and 9% for patients with SPC of good, moderate and poor prognosis, respectively. The respective HRs were 1.42, 2.32 and 6.24. Note that second melanoma was included in the good prognosis group.
Figure 2 Survival probability after first melanoma diagnosis in patients without SPC and with SPC of good, moderate and poor prognosis. *Last cancer was melanoma for patients during the time with one single melanoma, and was SPC for those from the time with SPC.
Multivariable Cox survival data are shown in for melanoma patients divided into prognostic classes. The difference to was that patients in had no local or distant metastases (N0/M0) at the first melanoma diagnosis. The reference category included patients without SPC and included 91.3% of the cases considered; good prognosis 6.9%, moderate prognosis 1.0% and poor prognosis 0.8% of the cases. HRs (95% CI) increased systematically from the reference rate to 1.59 (1.35–1.87) with SPC of good prognosis (78.6% of SPCs) to 3.49 (2.58–4.72) of moderate prognosis (12.0%) and to 7.93 (5.50–11.44) of poor prognosis (9.4%). The respective numbers of deaths were 1494 (reference, 81.2% of all), 207 (11.3%), 57 (3.1%) and 82 (4.5%). The HR of any SPC was 2.21 (1.93–2.54), accounting for 18.8% of all deaths.
Table 2 Survival in Melanoma Patients Without Local and Distant Metastases (N0/M0) Depending on Second Primary Cancers of Different Prognostic Groups
Multivariable Cox survival data in consider melanoma patients with metastases and SPCs. The reference category constituted patients without nodal or distant metastases (N0/M0) or SPC, the same as . In patients without SPC, the HRs increased to 2.62 (2.02–3.39) with N+ and to 5.88 (4.57–7.57) with M+ compared to the reference. When SPC was melanoma, the HR was 1.45 (1.15–1.84) in patients with N0/M0, and it increased to 4.28 (2.37–7.71) with N+ and to 11.61 (6.82–19.74) with M+. When SPC was non-melanoma, the HR for patients with N0/M0 was 2.59 (2.21–3.04) and it was 6.82 (3.21–14.48) in patients with M+. Case numbers were low in all categories of patients with SPC and metastases (N+ or M+).
Table 3 Survival in Melanoma Patients Depending on Metastases and Second Primary Cancers
In addition to HRs, it is relevant to consider the proportions of patients. This is however impeded by the large number of patients with undefined metastatic status. Among 22,267 melanoma patients with a defined T class, 195 (0.9%) patients had distant metastases (M+) but many patients were diagnosed as Mx.
Discussion
Based on the Swedish Cancer Registry we showed that SPCs are an increasingly common negative prognostic factor for melanoma and the prognostic consequences of SPCs could be predicted by their known survival data as first primary cancers. The incidence of melanoma has vastly increased in Sweden during the past half century but it is not known how this might have influenced the incidence of SPCs.Citation2 SPCs following melanoma were reported in an earlier Swedish study with a 31-year follow-up from 1958 through 1988; 7.6% of patients were diagnosed with SPC and of these only 15% were second melanomas.Citation12 In the present study of 13-year follow-up, 11.1% of patients were diagnosed with SPC and of these 1/3 were second melanomas. Improving survival may be a contributing factor for these differences of apparently increasing proportions of SPCs, but increased reporting of SPCs to the cancer registry may be the main explanation.
Around year 2005 the 5-year survival in melanoma was 89% in USA, and in Europe it was a few percent points lower.Citation1,Citation13 While the US 5-year survival for localized melanoma was 96%, it was only 41% for metastatic melanoma.Citation13 The importance of distant metastases is well established as a prognostic indicator but in the present study, we show that SPCs are another factor worsening survival in melanoma, irrespective of the metastatic state. The survival implications of SPCs have not been considered earlier with the exception of second or multiple melanomas.Citation14 We showed that among men diagnosed with melanoma at age over 70 years, who had survived at least 10 years, 7.2% were diagnosed with second melanoma, and 24.2% with non-melanoma SPC, thus adding up to 31.4%. Among women the respective percentage was 23.5.
These proportions are much higher than those for patients with metastases. Unfortunately, the Swedish clinical TNM data do not allow assessment of the proportions of patients diagnosed with metastases (because of the number of undefined cases, Nx, Mx) but a source using the Swedish Melanoma Register reported the proportions for stage 3 (i.e., N+/M0) at 2.3% and stage 4 (i.e., M+) at 0.7%.Citation14 The literature on the proportions of localized and distant metastases varies widely; e.g., in the US SEER data they are given as 9.9% and 3.4%, and in the Norwegian Cancer Registry as 5.0% and 3.2%, respectively.Citation15,Citation16
The effects of multiple melanoma diagnosis on melanoma mortality have been shown in the literature.Citation14,Citation17,Citation18 However, limited studies reported how SPC other than melanoma can influence survival in melanoma patients. We showed that the prognostic consequences of SPCs could be predicted by their known survival data as first primary cancers, in line with recent findings on benign hematological neoplasms.Citation19 The poor prognosis group, affecting around 0.9% (139/15,436) of all melanoma patients but 4.5% of their deaths, showed an HR of 7.93 and included fatal malignancies such as pancreas, lung, ovarian and nervous system cancers and myeloma. The most common SPC was melanoma, constituting 33.8% of all SPCs in this study of maximally 13 years of follow-up. The caveat is that the spectrum of SPCs changes over follow-up time. shows that the proportions of second melanomas modestly increased over the minimal survival time, while for non-melanoma SPCs the increases were large. The implication was that second melanomas were diagnosed relatively early after first melanoma as a consequence of clinical follow-up focusing on the skin. The alternative reasons are that second melanomas are in fact metastases from the first melanoma, or that the first melanoma had modified host skin susceptible to second melanoma, through immunological mechanisms for example.
The HR for second melanoma in was 1.45, in line with a Swedish study measuring melanoma-specific survival (HR 1.48).Citation14 That paper discussed other studies where no survival disadvantage was observed for patients with second melanoma. Nevertheless, a recent Norwegian study reported an HR of 2.67 for patients with second melanoma.Citation16 An important technical note concerning survival studies is that they are liable to the immortal time bias.Citation11 This can be avoided by treating SPCs as a time-dependent variable, as was done by us and by the above authors.
While early diagnosis of thin, non-ulcerated lesions promotes favorable survival in melanoma, what can be done for prevention or early diagnosis of SPCs at large? For the prevention of SPC, guidelines of general cancer prevention are valid because the causes of SPCs for patients not undergoing chemo-or radiotherapy are believed to be the same that causes of primary cancers.Citation20 For early detection, medical follow-up for second melanoma is a normal dermatological and oncological practice, which might have contributed to the present large numbers of second melanomas – close monitoring of the skin as part of follow-up would increase the likelihood of melanoma diagnosis. However, this probably also allows for earlier diagnosis and less melanoma mortality. Family history of melanoma should signal vigilance for clinical follow-up.Citation21,Citation22 Our recent study showed that a family history of cancer X is a risk factor of second cancer X in melanoma patients.Citation7 Common SPCs with increased familial risks in melanoma patients included prostate, breast, colorectal and squamous cell skin cancers, for which screening methods would be available.Citation7 It is therefore important for dermatologists and other health care professionals performing follow-up to inquire about family histories of cancers other than melanoma. Family history plays a role also for lung cancer which is probably largely explained by shared smoking habits.
Strengths and Limitations
This study is based on registered data with practically complete nationwide coverage of medically diagnosed cancers, which enables enough power of detection and provides relatively robust and accurate estimates. Specifically, in Swedish Cancer Registry diagnosis of second cancer is as rigorous as that for the first primary cancer, which mandatorily requests separate and consistent tumor notifications from clinicians and pathologists. According to an ad hoc study on the accuracy of SPC diagnosis in the Swedish Cancer Registry, 98% of them were observed to be correctly classified.Citation23 Only one study reported the effects of SPC on the survival of melanoma,Citation7 but it did not consider diagnosis of SPC as time-dependent variable, which may generate immortal time bias. Furthermore, SPCs in our study were analysed based on the survival as the first primary cancer, so it has important clinical guidance for melanoma management.
Some information related to melanoma survival is not available in our study, for example the treatment for the patients, although the primary treatment is surgery. The maximal follow-up time in the present study was 13 years (because the TNM classification was taken to use in 2003 in the Swedish Cancer Registry) which is probably too short to gauge the maximal proportions of SPCs. We predict that the full scope of survival disadvantage by SPC is yet to be recognized. There is a small but unfortunate group of patients who are struck by both of the negative predictors, metastases and SPC. However, we were unable to estimate the exact risk due to the current numbers of such patients.
Conclusions
In conclusion, we show here that SPCs are an increasingly common negative prognostic factor for melanoma. Second melanoma is the most common single SPC and early detection of suspicious lesions can save lives but requires rigorous monitoring of skin and lymph nodes. Family history is a risk factor for many SPCs, in addition to melanoma, but how well his knowledge will penetrate the clinical reality may be a challenge. It is a paradox in cancer medicine that favorable survival comes along with an increasing risk of SPCs, thus expanding the frontier in the fight against cancer deaths.
Data Access, Responsibility, and Analysis
KH, GZ had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author Contributions
Design: KH, GZ. Acquisition of data: JS, KS. Statistical analysis and interpretation: GZ, SC, KH, AF, AH. Manuscript writing: KH and all other authors. Approval of the final text: All authors. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
A.H. is shareholder in Targovax ASA. A.H. is an employee and shareholder in TILT Biotherapeutics Ltd. The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Crocetti E, Mallone S, Robsahm TE, et al. Survival of patients with skin melanoma in Europe increases further: results of the EUROCARE-5 study. Eur J Cancer. 2015;51(15):2179–2190. doi:10.1016/j.ejca.2015.07.03926421821
- Lyth J, Eriksson H, Hansson J, et al. Trends in cutaneous malignant melanoma in Sweden 1997–2011: thinner tumours and improved survival among men. Br J Dermatol. 2015;172(3):700–706. doi:10.1111/bjd.2015.172.issue-325323770
- Jemal A, Ward EM, Johnson CJ, et al. Annual report to the Nation on the status of cancer, 1975–2014, featuring survival. J Natl Cancer Inst. 2017;109:9. doi:10.1093/jnci/djx030
- Weyers W. Screening for malignant melanoma-a critical assessment in historical perspective. Dermatol Pract Concept. 2018;8(2):89–103. doi:10.5826/dpc.0802a0629785325
- Eggermont AM, Spatz A, Robert C. Cutaneous melanoma. Lancet. 2014;383(9919):816–827. doi:10.1016/S0140-6736(13)60802-824054424
- Hartman RI, Lin JY. Cutaneous Melanoma-A review in detection, staging, and management. Hematol Oncol Clin North Am. 2019;33(1):25–38. doi:10.1016/j.hoc.2018.09.00530497675
- Chattopadhyay S, Hemminki A, Försti A, Sundquist K, Sundquist J, Hemmiinki K. Familial risks and mortality in second primary cancers in melanoma. JNCI Cancer Spectr. 2019;2:pky068. doi:10.1093/jncics/pky06831360883
- Read J, Wadt KA, Hayward NK. Melanoma genetics. J Med Genet. 2016;53(1):1–14. doi:10.1136/jmedgenet-2015-10315026337759
- Talback M, Dickman PW. Predicting the survival of cancer patients recently diagnosed in Sweden and an evaluation of predictions published in 2004. Acta Oncol. 2012;51(1):17–27. doi:10.3109/0284186X.2011.62644422023089
- De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE–5-a population-based study. Lancet Oncol. 2014;15(1):23–34. doi:10.1016/S1470-2045(13)70546-124314615
- Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1(11):710–719. doi:10.1200/JCO.1983.1.11.7106668489
- Wassberg C, Thorn M, Yuen J, Ringborg U, Hakulinen T. Second primary cancers in patients with cutaneous malignant melanoma: a population-based study in Sweden. Br J Cancer. 1996;73:255–259. doi:10.1038/bjc.1996.458546916
- Pollack LA, Li J, Berkowitz Z, et al. Melanoma survival in the United States, 1992 to 2005. J Am Acad Dermatol. 2011;65(5 Suppl 1):S78–S86. doi:10.1016/j.jaad.2011.05.03022018071
- Utjes D, Lyth J, Lapins J, Eriksson H. Reduced disease-specific survival following a diagnosis of multiple primary cutaneous malignant melanomas-a nationwide, population-based study. Int J Cancer. 2017;141(11):2243–2252. doi:10.1002/ijc.v141.1128799271
- Enninga EAL, Moser JC, Weaver AL, et al. Survival of cutaneous melanoma based on sex, age, and stage in the United States, 1992–2011. Cancer Med. 2017;6(10):2203–2212. doi:10.1002/cam4.115228879661
- Robsahm TE, Helsing P, Nilssen Y, et al. High mortality due to cutaneous melanoma in Norway: a study of prognostic factors in a nationwide cancer registry. Clin Epidemiol. 2018;10:537–548. doi:10.2147/CLEP.S15124629780262
- Youlden DR, Baade PD, Soyer HP, et al. Ten-year survival after multiple invasive melanomas is worse than after a single melanoma: a population-based study. J Investig Dermatol. 2016;136(11):2270–2276. doi:10.1016/j.jid.2016.03.01427019458
- Pardo L, van der Leest R, De Vries E, Soerjomataram I, Nijsten T, Hollestein L. Comparing survival of patients with single or multiple primary melanoma in the Netherlands: 1994–2009. Br J Dermatol. 2017;176(2):531–533. doi:10.1111/bjd.2017.176.issue-227377396
- Zheng G, Chattopadhyay S, Sud A, et al. Types of second primary cancers influence survival in chronic lymphocytic and hairy cell leukemia patients. Blood Cancer J. 2019;9(4):40. doi:10.1038/s41408-019-0201-030914634
- Travis LB, Demark Wahnefried W, Allan JM, Wood ME, Ng AK. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol. 2013;10(5):289–301. doi:10.1038/nrclinonc.2013.4123529000
- Chen T, Fallah M, Forsti A, Kharazmi E, Sundquist K, Hemminki K. Risk of next melanoma in patients with familial and sporadic melanoma by number of previous melanomas. JAMA Dermatol. 2015;151(6):607–615. doi:10.1001/jamadermatol.2014.477725671687
- Chen T, Hemminki K, Kharazmi E, Ji J, Sundquist K, Fallah M. Multiple primary (even in situ) melanomas in a patient pose significant risk to family members. Eur J Cancer. 2014;50(15):2659–2667. doi:10.1016/j.ejca.2014.07.00725103454
- Frödin J-E, Ericsson J, Barlow L. Multiple primary malignant tumors in a national cancer registry. Reliability of reporting. Acta Oncol. 1997;36:465–469. doi:10.3109/028418697090013009292741
