Abstract
Routine biomarker results from hospital laboratory information systems, covering hospitals and general practitioners, in Denmark are available to researchers through access to the regional Clinical Laboratory Information System Research Database at Aarhus University and the nationwide Register of Laboratory Results for Research. This review describes these two data sources. The laboratory databases have different geographical and temporal coverage. They both include individual-level biomarker results that are electronically transferred from laboratory information systems. The biomarker results can be linked to all other Danish registries at the individual level, using the unique identifier, the CPR number. The databases include variables such as the CPR number, date and time (hour and minute) of sampling, NPU code, and name of the biomarker, identification code for the laboratory and the requisitioner, the test result with the corresponding unit, and the lower and upper reference limits. Access to the two databases differs since they are hosted by two different institutions. Data cannot be transferred outside Denmark, and direct access is provided only to Danish institutions. It is concluded that access to data on routine biomarkers expands the detailed biological and clinical information available on patients in the Danish healthcare system. The full potential is enabled through linkage to other Danish healthcare registries.
Data Resource Basics
Danish Healthcare Registries
Denmark has a large network of population-based healthcare registries.Citation1 Several supplementary issues of international medical journals have been devoted to describe these registries to facilitate their use in research.Citation2,Citation3 The registries contain data on the Danish population of approximately 5.8 millions inhabitants and include data on healthcare contacts, hospital diagnoses, redeemed prescriptions, childbirths, causes of death, and a wide variety of other information. The Civil Registration System and the healthcare system in Denmark have been described in detail elsewhere.Citation1,Citation4 In short, all Danish residents are assigned a unique 10-digit personal identification number, the CPR number, upon birth or immigration. The CPR number serves as the key identifier in the nationwide registration of personal data, including contacts with the healthcare system. The CPR number also allows Statistics Denmark (www.dst.dk/en) to produce updated population statistics at the individual level on a variety of different topics, including, but not limited to demographics, income, education, and healthcare use.
The Danish healthcare system provides free healthcare to all residents at general practices and hospitals through a tax-paid system. This allows for long-term and virtually complete follow-up at the individual level across the different sources of healthcare data.
This review provides an overview of the two Danish healthcare research databases that collect routine individual-level biomarker data from general practitioners and hospital encounters: the regional Clinical Laboratory Information System Research (LABKA) Database at Aarhus UniversityCitation5 and the nationwide Register of Laboratory Results for Research (RLRR).Citation6 These specific databases enable researchers to obtain very detailed and comprehensive biological information for research purposes.
Hospital laboratories are an integrated part of the public hospital system. Private medical laboratories play a minor role for routine biomarker analyses in Denmark, as they only provide very limited number of the test results and mainly for low throughput and highly specialized biomarkers, eg lactate dehydrogenase isoenzymes and metabolic intermediates.
Routine Clinical Biomarkers in the Danish Healthcare System
Biomarkers are used in everyday clinical workstream in general practice and in the hospital setting for diagnosis, screening, monitoring, assessment of prognosis, and evaluation of treatment effects and safety. Upon physician request, biological samples (blood, urine, cerebrospinal fluid, and other body fluids) undergo biomarker analysis in public hospital laboratories, and thus biomarker data are recorded in the registries as a by-product of healthcare provision. The biomarker results are registered in the electronic laboratory information systems using the International System of Nomenclature, Properties, and Units (the NPU system).Citation7 Only very few biomarker results are recorded by non-electronic methods including some of the highly specialized biomarkers. The NPU system, an international standardised terminology for reporting on laboratory results, was established in 1960 by the International Union of Pure and Applied Chemistry (IUPAC) and the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). The NPU system is equivalent to the Logical Observation Identifiers Names and Codes (LOINC) used in other parts of the world.Citation8 The organization of the NPU system complies with recommendations from these two leading international laboratory organizations. In Denmark, the NPU system was implemented in 2001, but some biomarkers do not comply with the NPU system and are coded using national or local codes. The results for these biomarkers are also included in the laboratory information systems.
The Danish hospital laboratories use the laboratory information system for managing data on requisitions, specimen handling, transportation, and biomarker results. The LABKA database and the RLRR described in this review mainly cover routine biomarker results including some results for therapeutic drug monitoring and genetic tests. Clinical microbiology and pathology are not included in the two research databases, but are covered in other databases.Citation9–Citation11
Specifications for the LABKA database and the RLRR are described in detail below and in . The two databases show some degree of overlap, since some geographical regions of Denmark are represented in both databases.
Table 1 Basic Characteristics of the Two Laboratory Databases in Denmark
The LABKA databaseCitation5 is named after a specific laboratory information system software called LABKA. A previous publication has described the features of the first version of the laboratory information system called LABKA as a real-time computer system for handling and transfer of data to and from the hospital laboratory.Citation11 The LABKA database covers two (Northern and Central Danish Regions) of the five geographical regions in Denmark. These two regions currently have a combined population of 1.9 million inhabitants.Citation12 Data are considered complete for the geographical regions since 2005, but data from the late 1990s are available for some smaller geographical areas of the Northern and Central Danish Regions. The LABKA database holds biomarker results on more than 2.4 million individual patients dispersed over more than 6,000 different biomarker codes, coding for more than 1,700 different biomarkers. As an example, more than 8 million measurements of haemoglobin were performed on more than 1 million patients between 1999 and 2007. Further details are found in an earlier publication.Citation5 It is hosted by the Department of Clinical Epidemiology, Aarhus University Hospital, Denmark.
The RLRR contains nationwide biomarker data from the Danish population of 5.8 million inhabitants.Citation13 The RLRR has varying temporal coverage as data collection started at different time points for the five Danish regions (). The database became available to researchers in 2018 and is hosted by the Danish Health Data Authority.Citation6
Data Collection
Content
The main variables contained in the two data sources are broadly comparable. They include the CPR number, date and time (hour and minute) of sampling, the NPU (or Danish modified) code or local code, identification codes of the laboratory and the requisitioner, the biomarker result, unit (eg mmol/L or ng/L), and lower and upper reference limits. The LABKA database also records the name of the biomarker and most text-written non-numeric results. The RLRR currently includes only the following text-written results: “positive”, “negative”, “ABO blood type”, and “rhesus blood type”. Hence, other text results are missing from the RLRR. The overall proportion of missing data has not been assessed, but the electronic data transfer should ensure a limited proportion of missing data.
Requisition of biomarkers through an electronic clinical user interface is essentially the same in hospitals, general practices, and private specialist clinics. Each requisition is transferred from the clinical user interface to the laboratory information system. Most general practitioners handle sampling (eg venepuncture and urine or stool collection) and then send the samples by postal mail, courier services, or other transportation systems. At hospitals, laboratory technicians perform most venepunctures, and physicians or nursing staff collect other samples (eg urine, cerebrospinal fluid).
Point-of-care testing (POCT) equipment enables rapid bedside biomarker analysis results. POCT analyses are distinguished from analyses performed at hospital laboratories by separate NPU codes. Recording of POCT biomarker results in the databases requires online access to the laboratory information system, which is inherent in most POCT equipment used in hospitals. However, results of POCT tests performed by general practitioners or specialist clinics are not recorded in the laboratory information system.
The two laboratory databases are updated with different time intervals. The LABKA database is updated irregularly, at intervals of one to two years. The RLRR database is updated on a monthly basis from a national laboratory databank. This national databank transfers laboratory results both to a secured website for access by health professionalsCitation14 and to a personal electronic medical file with unique personal access for individual Danish residents.Citation15
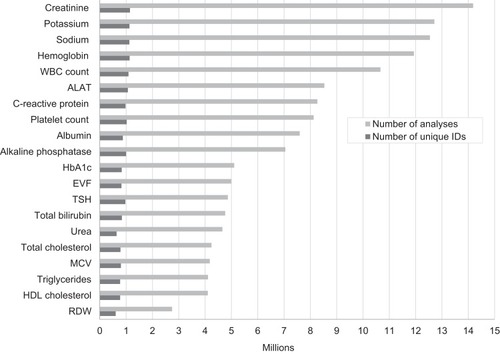
The pattern of test results follows clinical practice. provides an overview of the 20 most frequent biomarker analyses performed in the Central Denmark region from 1 January 2008 to 31 December 2018, including the total number of tests and the number of unique patients with at least one result for a given biomarker. As expected, the most commonly recorded results included basic haematological and infection tests, lipid and HbA1c levels, and measurements of liver, kidney and thyroid function. The data we present from the laboratory information system of Central Denmark is likely representative of Denmark as a whole, as Danish registries show a high degree of homogeneity for health care utilization across the five Danish regions.Citation16
Figure 1 Number of test results and unique personal identification (CPR) numbers of the 20 most frequent laboratory tests performed in the Central Denmark region, 2008–2018.
Data Quality
The quality of biomarker results in the Laboratory Information Systems Databases is generally high. Most hospital laboratories in Denmark are accredited in accordance with the standards for medical laboratory testing specified by the International Organization for Standardization (ISO 15189).Citation17 As a result, quality management systems in Danish laboratories comply with international standards, including methodological and clinically relevant quality criteria such as detection limits, variation of analyses over time and across hospital laboratories, internal and external quality assurance, electronic transfer of test results to medical files and databases, procedures for handling errors, and systematic assessment of these quality standards. The accreditation status of an individual clinical laboratory can be found at the website of the Danish Accreditation Fund.Citation18
Data Resource Use
Use of routine biomarker data in epidemiological research has tremendous potential, especially with linkage to other Danish healthcare registries. By using the CPR number, biomarker data can be linked with healthcare data at the individual level, such as hospital diagnosis, redeemed prescriptions and causes of death. We refer to other publications for descriptions of available resources of Danish healthcare data.Citation1–Citation4 Biomarker data provide detailed information about pathophysiological processes in individual patients and add significant value to the comprehensive network of Danish healthcare registries. In addition, the laboratory databases also allow identification of specific patients groups that are not readily captured by other data sources in DenmarkCitation1 eg patients diagnosed and treated in general practice. Below, we provide some study examples, where biomarker data have been used. Since RLRR data only recently became available, the studies were based on data from the LABKA database.
Validation of registry data is fundamental to all types of epidemiological research. Biomarker data are a key resource for validating registry discharge diagnoses defined by biomarkers, such as anaemia, electrolyte disorders, lipid disorders, or diabetes.Citation19 One such study examined the sensitivity of hyponatraemia diagnoses in the Danish National Patient Registry (DNPR).Citation20 Only 2% of patients with a record of low sodium levels in the LABKA database had a hyponatraemia discharge diagnosis in the DNPR. The study thus revealed the incompleteness of hyponatraemia diagnoses in the DNPR. Laboratory data also can be used to evaluate measures of prevalence and incidence of diseases, risk factors for diseases, and the prognosis following abnormal laboratory test results among patients that are recorded with specific tests. As an example, a series of three studies examined the risk of elevated potassium levels, associated risk factors, and prognosis following hyperkalaemia in patients with diabetes, heart failure, and chronic kidney disease.Citation21–Citation23 The uniqueness of these large population-based studies was their ability to separately appraise the risk of mild, moderate, and severe elevation of plasma potassium levels and to assess the risk of hyperkalaemia as a function of glycaemic control (HbA1c) and kidney function (eGFR). In another series of studies, the association between vitamin B12 levels and cancer was evaluated.Citation24–Citation26 Not only was elevated vitamin B12 levels a marker of occult cancer, it was also associated with poor cancer prognosis and with the risk of venous thromboembolism among cancer patients. Finally, laboratory data can be used to assess the risk of specific drug side effects. A recent study found that the risk of hyponatraemia was higher among users of several different types of antidepressant drugs compared to non-users.Citation27
Strengths and Weaknesses
LABKA and the RLRR are valuable data resources for research. Their geographical and temporal completeness is relatively high, although the completeness varies between the data sources (). They provide population-based data encompassing most routine biomarker results from Danish hospital laboratories. Another strength of the databases stems from standardised quality assurance measures implemented in most Danish hospital laboratories. These standards ensure that erroneous test results caused by interferences, such as haemolysis in the blood tube, endogenous proteins in a patient’s blood, or prescribed medical drugs are systematically detected and expunged so they are not provided to the clinician and not included in the databases. Unambiguous individual-level data linkage among the laboratory databases and other Danish healthcare registries ensures virtually endless opportunities for conducting epidemiological research.
Use of routine laboratory data for research purposes also has some potential limitations. Differences across laboratories in analytical methods and laboratory equipment imply that they do not necessarily produce comparable results and reference ranges for a given biomarker. Moreover, individual laboratories occasionally change their analytical methods, equipment, or units of measurement. Thus, biomarker levels and reference ranges may change over time within a given laboratory. However, the generally high number of observations makes it possible to account for such changes in most analyses. For example, analyses can be stratified by time period or one can combine data from laboratories with comparable analytic methods and reference intervals. Another concern is that the indications for specific requested biomarkers and the reasons for repeated measurements are not readily available. It is beyond the scope of this Data Resource Profile to assess regional differences or temporal changes in the pattern of biomarker results.
Comparison with Other Biomarker Data Sources
Other data sources that include biomarkers have been described in several publications.Citation28–Citation32 They differ in several ways from the data sources described here. Some use the laboratory information systems to sample patients with results of specific markers, such as plasma creatininCitation28,Citation29 differential blood cell counts,Citation30 or thyroid-stimulating hormone.Citation31 Hence, they are less comprehensive than the resources presented here. Others have used data directly from the laboratory information systems to assess research hypothesis.Citation32 This allows for more detailed information on eg specimen handling, but data directly from the laboratory information systems are limited to smaller geographical areas of Denmark.
Other data sources include biomarker results only from general practice, such as the UK-based General Practice Research DatabaseCitation33 and The Health Improvement Network.Citation34
Data Resource Access
Researchers interested in using data from the LABKA database could contact the Department of Clinical Epidemiology, Aarhus University Hospital, Denmark, regarding specific research projects.
The Danish Health Data Authority allows only Danish institutions to access its data, subsequent to approval of a formal application. The application must include the background and purpose of the study, and a description of the data sources and variables needed. Individual study data are available to researchers who successfully complete the application process, but they must access the data through servers behind firewalls. Transfer of individual-level data outside these servers is prohibited. Uploading of external data to the servers is possible. The Danish Health Data Authority ensures anonymization on their servers, and any access to non-anonymized data requires additional special approval. Access to registry data in Denmark for research purposes does not require approval from an ethics committee according to Danish law, but all studies should be approved by the Danish Data Protection Agency.Citation35 Foreign researchers can access data only through collaboration with a Danish institution that is approved for handling and accessing data on the servers of the Danish Health Data Authority. A fee for administration and data access is charged for projects that achieve approval with the Danish Health Data Authority.
Author contributions
JFHA, ATH, HTS, and KAD conceived the idea for this manuscript. JFHA, ATH, and KAD drafted the initial manuscript. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
We thank Pia Buys Petersen from the Danish Health Data Authority for her valuable input regarding the RLRR to the manuscript.
Disclosure
Dr Johan Frederik Håkonsen Arendt has received a lecture fee on one occasion within the last 36 months from Siemens Healthineers, Denmark, and a lecture on one occasion from Teva Denmark A/S within the last 36 months. The lectures had no relation to the present study work; The Department of Clinical Epidemiology, Aarhus University Hospital, receives funding for other studies from companies in the form of research grants to (and administered by) Aarhus University. None of these studies have any relation to the present study. The sponsors of this study had no role in the initiation, planning, design or conduct of the study, data acquisition, management and analyses, interpretation of results, writing and approval of the manuscript, or the decision to submit the manuscript for publication. The researchers involved in this study declare their independence from the sponsors of the study. The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–591. doi:10.2147/CLEP.S17908331372058
- Thygesen LC, Ersboll AK. Danish population-based registers for public health and health-related welfare research: introduction to the supplement. Scand J Public Health. 2011;39(7 Suppl):8–10. doi:10.1177/140349481140965421775344
- Sørensen HT, Pedersen L, Jorgensen J, Ehrenstein V. Danish clinical quality databases - an important and untapped resource for clinical research. care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2016;8:425–427. doi:10.2147/CLEP.S11326527843338
- Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. doi:10.1007/s10654-014-9930-324965263
- Grann AF, Erichsen R, Nielsen AG, Froslev T, Thomsen RW. Existing data sources for clinical epidemiology: the clinical laboratory information system (LABKA) research database at Aarhus University, Denmark. Clin Epidemiol. 2011;3:133–138. doi:10.2147/CLEP.S1790121487452
- The Danish Health Data Authority [homepageontheInternet]. Register of Laboratory Results for Research; [ updated 3 12, 2018]. Available from: https://sundhedsdatastyrelsen.dk/da/registre-og-services/om-de-nationale-sundhedsregistre/doedsaarsager-og-biologisk-materiale/laboratoriedatabasen. Accessed 1016, 2019.
- Pontet F, Magdal Petersen U, Fuentes-Arderiu X, et al. Clinical laboratory sciences data transmission: the NPU coding system. Stud Health Technol Inform. 2009;150:265–269.19745311
- Forrey AW, McDonald CJ, DeMoor G, et al. Logical observation identifier names and codes (LOINC) database: a public use set of codes and names for electronic reporting of clinical laboratory test results. Clin Chem. 1996;42(1):81–90. doi:10.1093/clinchem/42.1.818565239
- Gradel KO, Schønheyder HC, Arpi M, Knudsen JD, Ostergaard C, Søgaard M. The Danish Collaborative Bacteraemia Network (DACOBAN) database. Clin Epidemiol. 2014;6:301–308. doi:10.2147/CLEP.S6699825258557
- Schønheyder HC, Søgaard M. Existing data sources for clinical epidemiology: the North Denmark Bacteremia Research Database. Clin Epidemiol. 2010;2:171–178. doi:10.2147/CLEP.S1013920865114
- Christiansen JU, Maruard CD, Nielsen HC. LABKA. A real-time computer system for the clinical laboratory. Scand J Clin Lab Invest Suppl. 1989;194:57–61.2772556
- Erichsen R, Lash TL, Hamilton-Dutoit SJ, Bjerregaard B, Vyberg M, Pedersen L. Existing data sources for clinical epidemiology: the Danish National Pathology Registry and Data Bank. Clin Epidemiol. 2010;2:51–56. doi:10.2147/CLEP.S990820865103
- Statistics Denmark[homepageontheInternet]. Population figures 2019; [ Updated 1 7, 2019]. Available from: https://www.dst.dk/da/Statistik/emner/befolkning-og-valg/befolkning-og-befolkningsfremskrivning/folketal. Accessed 1016, 2019.
- MedCom [homepageontheInternet]. Laboratoriesvarportalen. Available from: https://www.medcom.dk/opslag/support/laboratoriesvarportalen. Accessed 1016, 2019.
- Danish Healthcare Services [homepageontheInternet]. eHealth in Denmark. Available from: https://www.sundhed.dk. Accessed 1016, 2019.
- Henriksen DP, Rasmussen L, Hansen MR, Hallas J, Pottegard A. Comparison of the five Danish regions regarding demographic characteristics, healthcare utilization, and medication use–a descriptive cross-sectional study. PLoS One. 2015;10:e0140197. doi:10.1371/journal.pone.014019726439627
- International Organization for Standardization [homepageontheInternet]. ISO 15189:2012. Medical laboratories — Requirements for quality and competence: International Organization for Standardization; 2012 Available from: https://www.iso.org/standard/56115.html. Accessed 1016, 2019.
- Danish Accreditation Fund [homepageontheInternet]. Database of accredited companies; 2019 Available from: http://english.danak.dk/database_eng. Accessed 1016, 2019.
- Schmidt M, Schmidt SAJ, Søndergaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi:10.2147/CLEP.S9112526604824
- Holland-Bill L, CF C, Ulrichsen SP, Ring T, Jørgensen JO, Sørensen HT. Validity of the International Classification of Diseases, 10th revision discharge diagnosis codes for hyponatraemia in the Danish National Registry of Patients. BMJ Open. 2014;4(4):e004956. doi:10.1136/bmjopen-2014-004956
- Thomsen RW, Nicolaisen SK, Hasvold P, et al. Elevated potassium levels in patients with congestive heart failure: occurrence, risk factors, and clinical outcomes: a Danish population-based cohort study. J Am Heart Assoc. 2018;7(11):e008912. doi:10.1161/JAHA.118.00891229789332
- Thomsen RW, Nicolaisen SK, Adelborg K, et al. Hyperkalaemia in people with diabetes: occurrence, risk factors and outcomes in a Danish population-based cohort study. Diabet Med. 2018;35(8):1051–1060. doi:10.1111/dme.1368729790603
- Thomsen RW, Nicolaisen SK, Hasvold P, et al. Elevated potassium levels in patients with chronic kidney disease: occurrence, risk factors and clinical outcomes-a Danish population-based cohort study. Nephrol Dial Transplant. 2018;33(9):1610–1620. doi:10.1093/ndt/gfx31229177463
- Arendt JF, Farkas DK, Pedersen L, Nexo E, Sorensen HT. Elevated plasma vitamin B12 levels and cancer prognosis: a population-based cohort study. Cancer Epidemiol. 2016;40:158–165. doi:10.1016/j.canep.2015.12.00726724465
- Arendt JF, Farkas DK, Pedersen L, Sorensen HT. Elevated plasma vitamin B12 levels and risk of venous thromboembolism among cancer patients: A population-based cohort study. Thromb Res. 2017;156:177–183. doi:10.1016/j.thromres.2017.06.02228667844
- Arendt JF, Pedersen L, Nexo E, Sorensen HT. Elevated plasma vitamin B12 levels as a marker for cancer: a population-based cohort study. J Natl Cancer Inst. 2013;105(23):1799–1805. doi:10.1093/jnci/djt31524249744
- Leth-Moller KB, Hansen AH, Torstensson M, et al. Antidepressants and the risk of hyponatremia: a Danish register-based population study. BMJ Open. 2016;6(5):e011200. doi:10.1136/bmjopen-2016-011200
- Runesson B, Gasparini A, Qureshi AR, et al. The Stockholm CREAtinine Measurements (SCREAM) project: protocol overview and regional representativeness. Clin Kidney J. 2016;9(1):119–127. doi:10.1093/ckj/sfv11726798472
- Henriksen DP, Damkier P, Hallas J, Nybo M. Sixteen years of creatinine measurements among 460 000 individuals-The Funen Laboratory Cohort (FLaC), a population-based pharmacoepidemiological resource to study drug-induced kidney disease. Basic Clin Pharmacol Toxicol. 2019;124(9):582–590. doi:10.1111/bcpt.1316730417606
- Andersen CL, Siersma VD, Karlslund W, et al. The copenhagen primary care differential count (CopDiff) database. Clin Epidemiol. 2014;6:199–211. doi:10.2147/CLEP.S6099124966694
- Laulund AS, Nybo M, Brix TH, Abrahamsen B, Jorgensen HL, Hegedus L. Duration of thyroid dysfunction correlates with all-cause mortality. the OPENTHYRO register cohort. PLoS One. 2014;9(10):e110437. doi:10.1371/journal.pone.011043725340819
- Hansen AT, Hoffmann-Lucke E, Nielsen BK, et al. Delayed sample arrival at the laboratory does not lead to more false negatives in the Danish population screening for colorectal cancer. Scand J Clin Lab Invest. 2017;77(8):685–688. doi:10.1080/00365513.2017.137909128933963
- Williams T, van Staa T, Puri S, Eaton S. Recent advances in the utility and use of the General Practice Research Database as an example of a UK Primary Care Data resource. Ther Adv Drug Saf. 2012;3(2):89–99. doi:10.1177/204209861143591125083228
- Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19(4):251–255. doi:10.14236/jhi.v19i4.82022828580
- Ludvigsson JF, Haberg SE, Knudsen GP, et al. Ethical aspects of registry-based research in the Nordic countries. Clin Epidemiol. 2015;7:491–508. doi:10.2147/CLEP.S9058926648756
