Abstract
Background
The aims of the present analysis are to estimate the prevalence of five key chronic cardiovascular, metabolic and renal conditions at the population level, the prevalence of renin–angiotensin–aldosterone system inhibitor (RAASI) medication use and the magnitude of potassium (K+) derangements among RAASI users.
Methods and Results
We used data from more than 375,000 individuals, 55 years of age or older, included in the population-based healthcare database of the Catalan Institute of Health between 2015 and 2017. The conditions of interest were chronic heart failure (CHF), chronic kidney disease (CKD), diabetes mellitus, ischemic heart disease and hypertension. RAASI medications included angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, mineralocorticoid receptor antagonists (MRAs) and renin inhibitors. Hyperkalemia was defined as K+ levels >5.0 mEq/L and hypokalemia as K+ <3.5 mEq/L. The prevalence of chronic cardiovascular, metabolic and renal conditions was high, and particularly that of hypertension (prevalence ranging from 48.2% to 48.9%). The use of at least one RAASI medication was almost ubiquitous in these patients (75.2–77.3%). Among RAASI users, the frequency of K+ derangements, mainly of hyperkalemia, was very noticeable (12% overall), particularly in patients with CKD or CHF, elderly individuals and users of MRAs. Hypokalemia was less frequent (1%).
Conclusion
The high prevalence of K+ derangements, and particularly hyperkalemia, among RAASI users highlights the real-world relevance of K+ derangements, and the importance of close monitoring and management of K+ levels in routine clinical practice. This is likely to benefit a large number of patients, particularly those at higher risk.
Introduction
Potassium (K+) is the most common cation in the human body. K+ is the main intracellular electrolyte, having several important physiological functions including maintenance of the electrical action potential across cell membranes, particularly in the myocardium; cell metabolism, glycogen and protein synthesis.Citation1 Hypokalemia and hyperkalemia are both common K+ homeostasis disorders, with the potential to cause life-threatening arrhythmiasCitation2 via electrophysiological perturbations of the cell membrane. Hypokalemia – defined as serum K+ <3.5 mEq/L – is typically the result of extracellular changes in K+ levels due to excessive excretion, i.e., diarrhea or diuretic treatment, while hyperkalemia – serum K+ >5 mEq/L – results from extracellular shifts of K+, excessive ingestion of this electrolyte and/or impaired kidney function.Citation3
In patients with chronic cardiovascular disorders, K+ homeostasis is often frail, particularly in the presence of renin–angiotensin–aldosterone system inhibition (RAASI) therapies,Citation3 such as angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), renin inhibitors (RIs; e.g., aliskiren), angiotensin II receptor blocker neprilysin inhibitors (ARNIs) and mineralocorticoid-receptor antagonists (MRAs). On the other hand, loop or thiazide diuretics, which are also frequently used in these patients, can reduce hyperkalemia and even cause hypokalemia.Citation2,Citation4-Citation6 In this context, current clinical practical guidelines for the management of these patients recommend close monitoring of their renal function and K+ levels, particularly in the presence of pharmacological therapy changes or drug titration,Citation6–Citation8 although these recommendations are not always implemented.Citation9
Hyperkalemia and hypokalemia have been associated with higher all-cause mortality in observational studies.Citation2,Citation10 In addition, K+ derangements may limit the use and titration of key, class I medications in these patients, including RAASI therapies.Citation11 Nevertheless, very limited epidemiological data on their importance at a population level are available.Citation12 Characterizing the real-world epidemiology of K+ derangements may be particularly important in patients with conditions such as chronic heart failure (CHF), chronic kidney disease (CKD), diabetes mellitus (DM), ischemic heart disease (IHD) and hypertension (HTN), in all of which RAASI therapies are often used.Citation6–Citation8
The purpose of this study was thus to gain a better understanding, from a population-level, real-world perspective, of 1) the prevalence of these five conditions in the general population; 2) the frequency of RAASI use among those patients; and 3) the magnitude of K+ derangements among individuals with these conditions, particularly among patients using RAASI medications.
Materials and Methods
Data Source
We used the population-based healthcare database of the Catalan Institute of Health (Institut Catala de Salut, ICS). This is a longitudinal database that includes detailed healthcare information from primary care, as well as from eight large hospitals, for all residents of Catalonia (Spain). Specifically, the database comprises exhaustive, quality-controlled, detailed, individual-level data on sociodemographic characteristics, medical conditions and medication dispensing, among other variables. Medical conditions are coded using either the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) coding system for in-hospital diagnoses or the International Classification of Diseases, 10th Revision (ICD-10) coding system for primary care diagnoses, and the Clinical Classifications Software (CCS) for both. Medication dispensing is coded using the Anatomical Therapeutic Chemical (ATC) coding system.
For the present analysis, this clinical database was linked to a laboratory test database, which includes any routine care laboratory test results from both hospital care and primary care settings generated since January 1st 2015.
Study Population
Analyses were restricted to the Metropolitana Sud (MetroSud) healthcare area of Barcelona (Catalonia, Spain) (), which comprises approximately 1.2 million individuals and has Bellvitge University Hospital as the tertiary reference hospital. The healthcare-related information of these individuals is captured automatically and exhaustively by the database. Inclusion criteria for the present analysis were age 55 years or older and being alive at study entry. No exclusion criteria were applied.
Study Period
For the purposes of the present analysis, the study period was set between January 1st 2015 and December 31st 2017. The start of the study period was defined by the date on which laboratory test results became available in the database. For primary care diagnoses, any historical information available in the database was used; for hospital diagnoses, we used the information for the years 2014–2017.
Definitions and Data Collection
The chronic cardiovascular, metabolic and renal conditions of interest were CHF, CKD, DM, IHD and HTN. Evidence of each of these diagnoses was searched for in the database using operational definitions combining ICD-9-CM, ICD-10 and CCS codes (see Supplementary Tables S1–S3).
The drugs of interest were RAASI medications, which include ACEIs, ARBs, MRAs and RIs. Use of these medications was identified in the pharmacy dispensing component of the database, using operational definitions combining ATC codes (Table S4). Specifically, the recent drug combination sacubitril/valsartan was assessed as part of the ARB group.
For the analyses, hyperkalemia was defined as evidence of serum K+ levels >5.0 mEq/L and hypokalemia as serum K+ levels <3.5 mEq/L.
Statistical Analyses
To assess the frequency of CHF, CKD, DM, IHD and HTN in the general population, three retrospective assessments, on January 1st 2015, January 1st 2016 and January 1st 2017, were performed. At each time point, the proportion of individuals with each of these diagnoses, as well as the proportion of individuals with at least one of the diagnoses, was calculated using all the historical information available in the database at that time point, among individuals included in the study population for each study period.
A retrospective approach was also used to assess the frequency of RAASI medication use among patients with the conditions of interest. Specifically, at each time point (January 1st 2015, January 1st 2016 and January 1st 2017), the proportion of individuals using, during the preceding year, any (i.e., at least one) RAASI medication, and each RAASI medication subgroup, was calculated among individuals with recorded evidence of at least one of the relevant chronic conditions of interest.
To evaluate the frequency of K+ derangements among individuals with chronic cardiovascular, metabolic and renal conditions, at each time point, the occurrence of at least one episode of hyperkalemia, hypokalemia or any K+ derangement, as well as the occurrence of two or more episodes of hyperkalemia (“recurrent hyperkalemia”), were assessed by evaluating any laboratory test results of serum K+ levels available during the preceding year, among individuals with recorded evidence of CHF, CKD, DM, IHD and HTN. Individuals with no available data on serum K+ levels during the respective period of evaluation were managed in two ways: assuming normokalemia (i.e., included in the denominator of the prevalence calculations) and as missing data (i.e., excluded from the denominator).
To evaluate the frequency of K+ derangements specifically among individuals with any of the relevant conditions and using RAASI medications, the preceding analysis was repeated but restricted to individuals with CHF, CKD, DM, IHD and HTN and using at least one RAASI medication during the preceding year.
To identify determinants of prevalent hyperkalemia, a multivariable analysis was performed, with log-binomial regression for each year (2015, 2016 and 2017). The factors analyzed were age, sex, prevalence of CHF, CKD, DM, IHD and HTN, and treatment with ACEIs, ARBs and MRAs in the previous year. After that, the risk ratios for prevalence of hyperkalemia among these variables were calculated. The models of the multivariable analysis were presented as the risk ratio with corresponding 95% confidence interval.
In post hoc analyses, all calculations were repeated specifically for the subgroup of patients with CHF and either CKD or DM.
Ethics in Research
The present study was approved by the Ethics in Research Committee of the Bellvitge University Hospital and Bellvitge Biomedical Research Institute (IDIBELL).
Results
Study Population
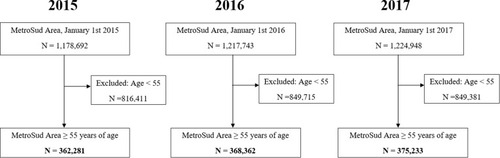
Between January 1st 2015 and January 1st 2017, there was a slight growth in the population of the MetroSud Area (). Consistent with this, the study population included in the analyses also experienced a slight increase, comprising 362,281 individuals on January 1st 2015 and 375,233 on January 1st 2017, all of whom were 55 years of age or older at study entry.
Prevalence of CHF, CKD, DM, IHD and HTN
HTN was very frequent, and affected almost half of the study population, with a prevalence ranging from 48.2% to 48.9% (). The prevalence of DM was the second highest, ranging from 14.6% to 14.8%. On the other hand, CHF was the least frequent of the five conditions, affecting 2.2–3.2% of the study population. The proportion of individuals with any (i.e., at least one) of the relevant conditions was approximately 54%. The prevalence of CHF, CKD and IHD increased over time.
Table 1 Prevalence of CHF, CKD, DM, IHD and HTN Among Adults ≥55 Years of Age, MetroSud Area, Catalonia, January 1st 2015 to January 1st 2017
Among individuals with CHF, the frequency of CKD and of DM was very high (Table S5), ranging from 49.8% to 54.2%.
Frequency of RAASI Medication Use Among Individuals with CHF, CKD, DM, IHD or HTN
During the study period, the use of ACEIs among individuals with any of the relevant conditions was very high, with a 5% increase in this proportion between 2015 (52.9%) and 2017 (57.5%) (). The use of ARBs was also high, with a slight increase over time (31.2% in 2015, 32.4% in 2017). On the other hand, the use of RIs was consistently marginal in the three study periods. The frequency of use of at least one RAASI medication ranged between 75.2% in 2015 and 77.3% in 2017.
Table 2 Prevalence of Use of ACEI, ARB, MRA and RI Among Adults ≥55 Years of Age with at Least One of the Relevant Conditions (CHF, CKD, DM, IHD, HTN), MetroSud Area, Catalonia, January 1st 2015 to January 1st 2017
Prevalence of K+ Derangements Among Individuals with CHF, CKD, DM, IHD or HTN
Hyperkalemia was a very frequent event, ranging from 10.6% when all individuals with at least one relevant condition were included in the calculations (i.e., regardless of whether they had had their K+ levels assessed during the period of evaluation) (, left columns) to 12.8% when only patients with at least one K+ measurement during the period of evaluation were considered (, right columns). On the other hand, hypokalemia was a less frequent event, ranging from 1.0% to 1.2%. The frequency of at least one episode of any K+ derangement (i.e., either hyperkalemia or hypokalemia) was up to 14% among individuals with K+ data available. The prevalence of recurrent hyperkalemia ranged from 1.8% 2.6%. There was a trend towards an increase over time in the occurrence of K+ derangements.
Table 3 Prevalence of Potassium Derangements Among Adults ≥55 Years of Age with at Least One Relevant Condition (CHF, CKD, DM, IHD, HTN), MetroSud Area, Catalonia, January 1st 2015 to January 1st 2017
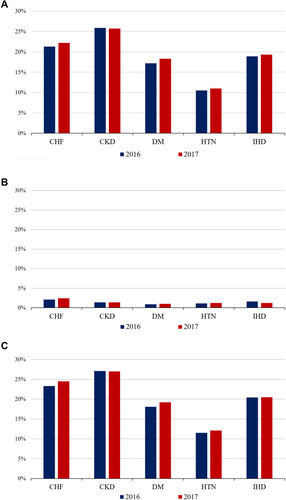
displays the prevalence of K+ derangements among individuals with each of the relevant conditions. Although hyperkalemia was highly prevalent across all of them, the highest frequencies were 26% for CKD, followed by CHF (panel A). The highest frequency of hypokalemia was also observed in patients with CHF.
Figure 3 Prevalence of hyperkalemia, hypokalemia and any potassium derangement (hyperkalemia or hypokalemia) among individuals ≥55 years of age with each of the relevant chronic conditions, MetroSud Area, Catalonia, January 1st 2016 and January 1st 2017. (A) Hyperkalemia. (B) Hypokalemia. (C) Any (hyperkalemia or hypokalemia). For each year, prevalence was assessed between January 1st of the preceding year and January 1st of the corresponding year. Calculations included all individuals with recorded evidence of each of the relevant diseases, regardless of whether they had their K+ levels assessed during the relevant study period.
The frequency of K+ derangements among individuals with CHF and CKD simultaneously is presented in Table S6. In this subgroup of patients, hyperkalemia was even more frequent than in the overall studied population (23.3–24.6%). The prevalence of recurrent hyperkalemia was also very high in this subgroup (6.7–8.0%).
Prevalence of K+ Derangements Among Individuals with CHF, CKD, DM, IHD or HTN and Using RAASI Medications
The frequency of hyperkalemia among patients with at least one of the relevant chronic conditions and using RAASI medications was slightly more frequent (12%) () than when the RAASI use criterion was not considered. On the other hand, the frequency of hypokalemia remained low. In analyses stratified by age, all K+ derangements were more frequent with older age (Table S7).
Table 4 Prevalence of Potassium Derangements Among Adults ≥55 Years of Age with at Least One Relevant Condition (CHF, CKD, DM, IHD, HTN) and Taking at Least One Relevant Drug (ACEIs, ARBs, MRAs, RIs), MetroSud Area, Catalonia, January 1st 2015 to January 1st 2017
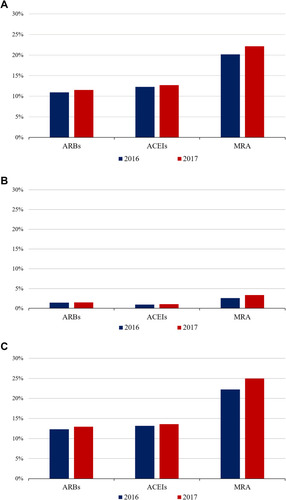
displays the frequency of K+ derangements by RAASI medication subgroup. All K+ disorders were more frequent in patients treated with MRAs, hyperkalemia being present in 20–22% of MRA users.
Figure 4 Prevalence of hyperkalemia, hypokalemia and any potassium derangement (hyperkalemia or hypokalemia) in adults ≥55 years of age with at least one of the relevant chronic conditions, by RAASI drug subgroups, MetroSud Area, Catalonia, January 1st 2016 and January 1st 2017. (A) Hyperkalemia. (B) Hypokalemia. (C) Any (hyperkalemia or hypokalemia). Data are presented as prevalence (in %). For each year, prevalence was assessed between January 1st of the preceding year and January 1st of the corresponding year (e.g., for 2016, prevalence of hyperkalemia was assessed between January 1st 2015 and January 1st 2016), among individuals from the study population with recorded evidence of at least one of the conditions of interest (regardless of whether they had their K+ assessed during the relevant study period). Serum K+ levels were not recorded before January 1st 2015. Patients using each drug could also be using the other study drugs (i.e., groups were not mutually exclusive).
Risk Factors for Hyperkalemia
shows the models of the multivariable analysis. The models display risk ratios (RRs) with corresponding 95% confidence intervals (CIs). All the variables analyzed were related to a higher prevalence of hyperkalemia. The strongest estimators for hyperkalemia were CKD (RR 1.8–1.9; p<0.001) and DM (RR 1.85–1.9; p<0.001), among the chronic conditions. Between the RAASI medications, the highest magnitude relation was with ACEIs (RR 1.39–1.5; p<0.001) and MRA (RR 1.34–1.4; p<0.001). Male gender and age were slightly related to the prevalence of hyperkalemia between 2015 and 2017.
Table 5 Multivariate Log-Binomial Regression Analysis. Risk Factors for the Prevalence of Hyperkalemia Among Adults ≥55 Years of Age with at Least One Relevant Condition (CHF, CKD, DM, IHD, HTN) or Taking at Least One Relevant Drug (ACEIs, ARBs, MRAs), MetroSud Area, Catalonia, January 1st 2015 to January 1st 2017
Prevalence of Hyperkalemia, Defined as K+>5.5 mEq/L
Among patients with at least one relevant condition and taking at least one RAASI drug, the prevalence of K+ >5.5 mEq/L was 2.0–2.1% (Table S8).
Discussion
In this very large, population-based analysis including the whole population from the MetroSud Area (Catalonia) 55 years of age or older, the prevalence of CHF, CKD, DM, IHD and HTN was high. Among these patients, the use of at least one RAASI medication was almost ubiquitous; and among those users, the frequency of K+ derangements and particularly of hyperkalemia was very noticeable, especially in patients with CKD, patients with CHF, elderly individuals and users of MRAs. We identified factors related to the prevalence of hyperkalemia, notably elderly age and male sex, and with higher risk for the presence of CKD or DM, and the use of ACEIs and MRAs. These findings highlight the real-world importance of K+ derangements among these patients, and stress the importance of close monitoring and management of K+ levels in routine clinical practice (particularly in subgroups at higher risk of dyskalemia), which are likely to benefit a large number of patients.
To our knowledge, this is the first study to evaluate the real-world importance of K+ abnormalities (detected in both hospital and primary care settings) across several chronic cardiovascular and metabolic conditions, diagnosed in both ambits. Prior research using alternative study designs and data sources, and including disparate study populations in terms of age and comorbidities, have yielded very heterogeneous findings. For example, the frequency of hyperkalemia in some clinical trials has been reported to be as low as 2–3%, although it increases up to 32% in some patients treated with RAASI medications.Citation12–Citation15
Similarly, large variability has been reported in observational studies, where the prevalence of hyperkalemia has been reported to be around 3% in the general population,Citation16 reaching up to 25% in patients with CHF, DM or CKD,Citation17 and up to 50% in some cohorts of patients with severe CKD.Citation18 The variability across studies in terms of the threshold used to define hyperkalemia (typically ranging from 5.0 to 6.0 mEq/L)Citation12–Citation18 may have also contributed to the heterogeneous estimations. Consistent with this, in our study the prevalence of hyperkalemia decreased when an alternative, more restrictive cut-off point (>5.5 mEq/L) was used.
Focusing specifically on heart failure, in a Danish cohort almost 40% of CHF patients developed at least one episode of hyperkalemia during a mean of 2.2 years of follow-up. Recurrent hyperkalemia was also a very frequent finding.Citation19 There was also a recent study in our environment from Spanish hospitals included in the registry of the European Society of Cardiology, with a prevalence of hyperkalemia (defined as K+ >5.4 mEq/L) of 4.3% and 8.3% in patients with chronic and acute heart failure, respectively; other conditions and hypokalemia were not evaluated.Citation20 In our study, hyperkalemia was highly frequent among patients with CHF, the prevalence becoming even higher in the presence of concurrent predisposing conditions such as CKD or RAASI medication. In the multivariable analysis, CHF was associated with the prevalence of hyperkalemia, although not achieving statistical significance in the years 2016 and 2017. When a higher threshold was implemented, the prevalence of hyperkalemia was still significant, but lower compared with CHF patients in the Spanish study;Citation20 this may be due to the hospital-based characteristic of this registry, in contrast to our wider, population-based data, coming from both hospital and primary care databases.
We also observed a trend towards a higher prevalence of hyperkalemia with older age. This was expected, as elderly patients are frail and are often treated with several co-medications, both of which increase the likelihood of K+ abnormalities. Also, hyperkalemia was more frequent in patients under MRA therapy. This may be the consequence of the fact that MRA is a second-step pharmacotherapy in the management of conditions such as CHF or HTN.Citation7,Citation21,Citation22 This means that an important proportion of these individuals were also being treated with ARBs or ACEIs simultaneously with MRA, increasing the risk of developing hyperkalemia.
Our study has important implications. Hyperkalemia and hypokalemia have both been associated with increased all-cause mortality in observational studies and meta-analyses, emphasizing the value of maintaining normokalemia.Citation10,Citation11 In our environment, Nuñez et al observed that abnormal kalemia was independently associated with higher risk of death in a large clinical cohort of patients with CHF.Citation23 Consequently, clinical practice guidelines already recommend close monitoring of K+ levels in patients with any of these conditions, particularly among those using RAASI medications.Citation6–Citation8,Citation21,Citation22 In this context, our findings provide further evidence on the actual, real-world epidemiological importance of this phenomenon, and suggest that actions and therapies aimed at maintaining normal K+ levels may be relevant to a very large number of patients. Our work gives an extensive view of the magnitude of chronic cardiovascular, metabolic and renal conditions, how we treat them and their interrelation with K+ derangements.
Study Strengths
The present analysis has important strengths. The population-based nature of the study allowed a real-world analysis to be conducted on the epidemiological importance of K+ derangements, and minimized selection bias. Also, we were able to obtain exhaustive information on serum K+ determinations generated in both primary and hospital care settings – a type of information that is usually not available in large healthcare databases, making the present analysis unique.
Study Limitations
Some limitations must also be discussed. First, as for any retrospective analysis using information generated from routine clinical practice and recorded in large administrative healthcare databases, underrecording of health conditions is possible. This may have resulted in an underestimation of the actual prevalence of each of the relevant conditions assessed. Also, the validity of some of the diagnoses, particularly those generated in primary care settings, may be limited.Citation24 On the other hand, the information related to drug dispensing and to laboratory test results is expected to be very exhaustive and highly valid.
Second, K+ derangements were identified from laboratory test results generated during routine clinical practice. Therefore, underdetection of mild, asymptomatic derangements is a possibility. Consequently, a conservative bias is possible, i.e., the frequency of K+ derangements, although already high, may be even higher in real life.
Third, information on serum K+ levels was available only since 2015; therefore, the study period could not be extended before January 1st 2015. Nevertheless, given the very large sample size of the study as well as its population-based nature, we believe that the present set of analyses is sufficiently informative about the population-level prevalence of K+ abnormalities among RAASI users.
Finally, our choice of age threshold for study entry (55 years of age or older) was arbitrary, and other age criteria could have been used. It is important to note that lower age thresholds would have resulted in a lower prevalence of chronic conditions, as younger individuals would be included in the denominator. On the other hand, older thresholds would have resulted in a higher prevalence not only of the conditions of interest but also of K+ derangements, as a consequence of selecting an older, typically frail population. For these reasons, we believe that the age threshold used was reasonable.
Conclusions
In this very large, population-based analysis including the whole population from the MetroSud Area in Catalonia, 55 years of age or older, the frequency of K+ derangements and particularly of hyperkalemia was high among patients with chronic cardiovascular, metabolic and renal conditions such as CHF, CKD, DM, IHD or HTN, and even higher among those using at least one RAASI medication. CKD, DM and the use of ACEIs or MRAs were factors related to the prevalence of hyperkalemia in the multivariable analysis. The present findings highlight the epidemiological importance of K+ derangements among individuals treated with RAASI therapies, and point to the potential importance of interventions aimed at stabilizing serum K+ levels in these patients, which are likely to benefit large numbers of patients.
Abbreviations
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNI, angiotensin II receptor blocker neprilysin inhibitor; ATC, Anatomical Therapeutic Chemical (coding system); CCS, Clinical Classifications Software; CHF, chronic heart failure; CKD, chronic kidney disease; DM, diabetes mellitus; HTN, hypertension; ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification; ICD-10, International Classification of Diseases, 10th Revision; ICS, Catalan Institute of Health (Institut Catala de Salut); IHD, ischemic heart disease; K+, potassium; MetroSud, Metropolitana Sud healthcare area; MRA, mineralocorticoid receptor antagonist; RAAS, renin–angiotensin–aldosterone system; RAASI, RAAS inhibitor; RI, renin inhibitor.
Declaration of Authorship
All authors listed take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Disclosure
Josep Comin-Colet had received speaker fees from Vifor Pharma. The authors report no other potential conflicts of interest for this work.
Additional information
Funding
References
- Rosano GMC, Tamargo J, Kjeldsen KP, et al. Expert consensus document on the management of hyperkalaemia in patients with cardiovascular disease treated with renin angiotensin aldosterone system inhibitors: coordinated by the working group on cardiovascular pharmacotherapy of the European Society of Cardiology. Eur Heart J Cardiovasc Pharmacother. 2018;4:180–188. doi:10.1093/ehjcvp/pvy01529726985
- Collins AJ, Pitt B, Reaven N, et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46:213–221. doi:10.1159/00047980228866674
- De Nicola L, Di Lullo L, Paoletti E, Cupisti A, Bianchi S. Chronic hyperkalemia in non-dialysis CKD: controversial issues in nephrology practice. J Nephrol. 2018;31(5):653–664. doi:10.1007/s40620-018-0502-629882199
- Sarwar CM, Papadimitriou L, Pitt B, et al. Hyperkalemia in heart failure. J Am Coll Cardiol. 2016;68:1575–1589. doi:10.1016/j.jacc.2016.06.06027687200
- Bandak G, Sang Y, Gasparini A, et al. Hyperkalemia after initiating renin-angiotensin system blockade: the Stockholm Creatininemeasurements (SCREAM) project. J Am Heart Assoc. 2017;6:e005428. doi:10.1161/JAHA.116.00542828724651
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi:10.1093/eurheartj/ehw12827206819
- Yancy CW, Januzzi JL Jr, Allen LA, et al. 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology task force on expert consensus decision pathways. J Am Coll Cardiol. 2018;71:201–230. doi:10.1016/j.jacc.2017.11.02529277252
- Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2017b;138:e426–e483.
- Cooper LB, Hammill BG, Peterson ED, et al. Consistency of laboratory monitoring during initiation of mineralocorticoid receptor antagonist therapy in patients with heart failure. JAMA. 2015;314:1973–1975. doi:10.1001/jama.2015.1190426547470
- Kovesdy CP, Matsushita K, Sang Y, et al.; CKD Prognosis Consortium. Serum potassium and adverse outcomes across the range of kidney function: a Ckd prognosisconsortium meta-analysis. Eur Heart J. 2018;39:1535–1542. doi:10.1093/eurheartj/ehy10029554312
- Beusekamp JC, Tromp J, van der Wal HH, et al. Potassium and the use of renin-angiotensin-aldosterone system inhibitors in heart failure with reduced ejection fraction: data from BIOSTAT-CHF. Eur J Heart Fail. 2018;20:923–930. doi:10.1002/ejhf.107929327797
- Brenner BM, Cooper ME, de Zeeuw D, et al.; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi:10.1056/NEJMoa01116111565518
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation Study Investigators. N Engl J Med. 1999;341(10):709–717. doi:10.1056/NEJM19990902341100110471456
- Eschalier R, McMurray JJ, Swedberg K, et al.; EMPHASIS-HF Investigators. Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function: analyses of the EMPHASIS-HF study subgroups (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure). J Am Coll Cardiol. 2013;62:1585–1593. doi:10.1016/j.jacc.2013.04.08623810881
- McMurray JJ, Packer M, Desai AS, et al.; PARADIGM-HF Investigators And Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi:10.1056/NEJMoa140907725176015
- Hughes-Austin JM, Rifkin DE, Beben T, et al. The relation of serum potassium concentration with cardiovascular events and mortality in community-living individuals. Clin J Am Soc Nephrol. 2017;12(2):245–252. doi:10.2215/CJN.0629061628143865
- Jain N, Kotla S, Little BB, et al. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol. 2012;109:1510–1513. doi:10.1016/j.amjcard.2012.01.36722342847
- Sarafidis PA, Blacklock R, Wood E, et al. Prevalence And Factors Associated With Hyperkalemia In Predialysis Patients Followed In A Low-Clearance Clinic. Clin J Am Soc Nephrol. 2012;7:1234–1241. doi:10.2215/CJN.0115011222595825
- Thomsen RW, Nicolaisen SK, Hasvold P, et al. Elevated potassium levels in patients with congestive heart failure: occurrence, risk factors, and clinical outcomes: a Danish Population-Based Cohort Study. J Am Heart Assoc. 2018;7. doi:10.1161/JAHA.118.008912
- Crespo-Leiro MG, Barge-Caballero E, Segovia-Cubero J, et al. Hyperkalemia in heart failure patients in Spain and its impact on guidelines and recommendations: ESC-EORP-HFA heart failure long-term registry. Rev Esp Cardiol. 2019;S1885-5857(19)30285–3.
- Williams B, Mancia G, Spiering W, et al.; ESC Scientific Document Group. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104.30165516
- Ketteler M, Block GA, Evenepoel P, et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) guideline update: what’s changed and why it matters. Kidney Int. 2017;92(1):26–36. doi:10.1016/j.kint.2017.04.00628646995
- Núñez J, Bayés-Genís A, Zannad F, et al. Long-term potassium monitoring and dynamics in heart failure and risk of mortality. Circulation. 2018;27(137):1320–1330. doi:10.1161/CIRCULATIONAHA.117.030576
- Verdu JM, Comın-Colet J, Domingo M, et al. Rapid point-of-care NT-proBNP optimal cut-off point for heart failure diagnosis in primary care. Rev Esp Cardiol. 2012;65:613–619. doi:10.1016/j.recesp.2012.01.01922541282