Abstract
Purpose
The efficacy of osteoporosis medication on reducing the risk of non-trauma fracture (NTFx) among adults with cerebral palsy (CP) has not been comprehensively investigated. There are many logistical and biological factors that may reduce this efficacy, and therefore requires attention. The purpose of this propensity score-matched, observational cohort study was to determine if osteoporosis medication was associated with NTFx risk attenuation among adults with CP and compared to adults without CP.
Materials and Methods
Data from 07/01/2011 to 09/30/2015 were extracted from Optum Clinformatics® Data Mart. Claims identified adults (≥18 years), CP, osteoporosis medication, pre-index NTFx (6-months), and post-index NTFx (12-months). CP without osteoporosis medication (CPMeds-) and without CP with Meds (non-CPMeds+; reflects “background” population) served as controls and were matched (6:1 ratio) to adults with CP with Meds (CPMeds+; n=306). The Meds groups were further stratified by the initiation of their medication as new users or consistent users. Changes in the prevalence of NTFx from pre- to post-index periods were examined with risk ratios (RR) and the change was compared among groups using the ratio of the RR (RRR) via difference-in-difference analysis.
Results
New users with CP had: a larger risk attenuation of any NTFx compared to CPMeds- (RRR=0.39; 95% CI=0.22–0.71), which was consistent for vertebral column/hip and lower extremities; a larger risk attenuation for NTFx of the lower extremities compared to consistent users with CP (RRR=0.22; 95% CI=0.05–0.93); and a similar risk attenuation of any NTFx compared to new users without CP (RRR=0.81; 95% CI=0.45–1.43), which was consistent for vertebral column/hip and lower extremities.
Conclusion
The findings suggest that osteoporosis medication is associated with clinically meaningful risk attenuation of NTFx, especially for new users with CP.
Introduction
Cerebral palsy (CP) is a chronic condition that arises from damage to or malformation of the infant brain and affects approximately 3.1 per 1000 children in the United States.Citation1,Citation2 CP presents as a heterogeneous group of fine and gross motor disorders that can range from mild (independent ambulation) to severe (dependent on wheelchair for mobility).Citation3 Children with CP have impaired neurological, skeletal muscle, and neuromuscular mechanics,Citation4–Citation6 low levels of physical activity,Citation7,Citation8 and suppressed musculoskeletal acquisition,Citation7–Citation10 regardless of motor impairment severity, which is accompanied by elevated musculoskeletal fat infiltration.Citation7,Citation8,Citation11 These factors, in addition to altered nutrition and fall risk, heighten susceptibility for non-trauma fracture (NTFx) for children with CP.Citation12
Unfortunately, the problems with skeletal fragility for children with CP get worse as they age into and throughout their adult years. Previous work has shown that the age-standardized prevalence of fracture is more than double for women and men with vs without CP,Citation13,Citation14 and that nearly half of adults ≥18 years of age are medically diagnosed with low bone mass.Citation15 Emerging evidence using nationwide administrative claims data suggests that NTFx is a risk factor for mortalityCitation16 and incidence of cardiovascularCitation17,Citation18 and respiratoryCitation19 diseases among adults with CP, which are among the leading causes of premature mortality for this population.Citation20,Citation21 Further, the relative risk of post-NTFx adverse outcomes was greater for <65 year olds, suggesting an earlier than expected burden of NTFx for adults with CP prior to reaching the elderly years.Citation18
There is a critical need to identify interventions that optimize skeletal health across the lifespan for individuals with CP. However, given the medical and functional complexity of CP, traditional interventions to augment bone accretion during growth or preserve skeletal integrity in the adult years, such as exercise and medications, may be less effective as compared to the general population. The potential for reduced intervention efficacy on skeletal fragility could stem from logistical or implementation issues, differences in physiology (eg, altered hormonal milieu), or differences in biological responsiveness. For example, sclerostin levels, an osteocyte-secreted protein, are higher for nonambulatory than ambulatory adults with CPCitation22 and bone marrow fat is higher for children with vs without CP,Citation7 which can suppress the anabolic responsiveness of bone.Citation23
Osteoporosis medications are prescribed to reduce the risk of NTFx and augment skeletal robustness and are associated with a lower risk of mortality,Citation24 which is important for adults with CP given the premature mortality burdenCitation20,Citation25 that may be exacerbated by NTFx.Citation16–Citation19 Preliminary studies from children with CP have shown the efficacy of bisphosphonates, which are anti-resorption osteoporosis medications, on reducing fracture risk.Citation26–Citation28 However, these few studies had small sample sizes. Further, no comprehensive investigations have been conducted to determine the efficacy of osteoporosis medication on NTFx risk for adults with CP. To address this knowledge gap, the objective of this propensity score-matched, observational cohort study was to determine if osteoporosis medication was associated with NTFx risk attenuation among adults with CP and compared to adults without CP.
Materials and Methods
Data Source
Data from July 1, 2011 to September 30, 2015 were ascertained from the Optum Clinformatics® Data Mart Database (OptumInsightTM, Eden Prairie, MN, USA)- a national single private payer administrative claims database containing information from privately insured or Medicare Advantage members in the United States, as previously described.Citation14 The data are de-identified and the University of Michigan Institutional Review Board (IRBMED) approved this study as non-regulated. The investigator (DGW) has a data use agreement to analyze this database.
Participant Selection
All medical conditions (eg, CP, NTFx) were identified using the International Classification of Diseases (ICD), Ninth Revision, Clinical Modification codes. The end study date of September 30, 2015 was selected to limit bias because of the switch in ICD-9 to ICD-10 codes on October 1, 2015.
Adults ≥18 years of age with CP were identified by searching for at least one claim for CP which covered all diagnostic CP types (eg, quadriplegia), as previously described, including codes.Citation16–Citation19 Data about the severity of CP using common clinical measures (eg, gross motor function classification system) are not available in insurance claims. Further, >70% of the CP sample had “other” or “unspecified” CP.Citation29 Therefore, clinical CP types were unable to be stratified by or statistically adjusted for.
Between January 1, 2012 and September 30, 2014, an affirmative to osteoporosis medication was defined by at least one outpatient pharmacy claim for any one of the following osteoporosis medications, as guided by previous studies:Citation30,Citation31 alendronate, ibandronate, risedronate, pamidronate, denosumab, zoledronic acid, teriparatide, raloxifene, etidronate, and calcitonin. The index date for those prescribed an osteoporosis medication was the first date within the time frame. The time frame was selected to account for at least 6-months of a “look back” period for individuals with an index osteoporosis medication date from January 1, 2012 to June 30, 2012 to ascertain pre-index fracture and comorbidity data, and up to 12-months of follow up for individuals with an index osteoporosis medication date from October 1, 2013 to September 30, 2014 for the outcome.
The osteoporosis medication (med) groups were further categorized as “new users” and “consistent users”, as the chronicity of medication exposure may have differential effects on 1-year NTFx risk. New users were defined as individuals that had at least 12 continuous months of enrollment prior to their first identified outpatient pharmacy claim for an osteoporosis medication.Citation31 Consistent users were defined as individuals that did not have a full 12 months of continuous enrollment prior to their first identified outpatient pharmacy claim for an osteoporosis medication. These cases may have been prescribed osteoporosis medication prior to the study time period.
Groups included adults with CP and adults without CP. The group of adults without CP was used to reflect the associations among the general population (or “background” population) to determine if there are different or unique associations with the CP groups. Groups were allocated based on the status of CP and osteoporosis medication: (1) with CP and prescribed an osteoporosis medication (CPMeds+); (2) with CP and not prescribed an osteoporosis medication (CPMeds-); and (3) without CP and prescribed an osteoporosis medication (non-CPMeds+). For the main analysis, the primary group of interest was CPMeds+. The start date of follow-up was defined as the index date of the first osteoporosis medication claim for CPMeds+ and non-CPMeds+ or a randomly assigned date within the study time frame for CPMeds-, as previously described.Citation18
Individuals were excluded if they did not have at least 6 continuous months prior to their start date of follow-up, defined as the pre-index time period, and 12 continuous months after their index date, defined as the post-index time period.
Fracture
Guided by the literature,Citation32–Citation34 fracture of the vertebral column, hip (including proximal femur), non-proximal femur, tibia/fibula, humerus, ulna/radius, or unspecified location without trauma codes (eg, vehicle accident) 7 days before to 7 days after the index fracture date was defined as NTFx, as previously described,Citation16–Citation19 and all other fractures were considered trauma fractures. Fractures in the pre-index time period was identified as trauma or non-trauma and by each site. Only NTFx, and not trauma fractures, were examined in the post-index time period. The first NTFx in the post-index period was identified. To ensure that the post-index NTFx event was indeed an incident event, we required this NTFx event to be at a new site, or if at the same site, a gap of at least 6 months from the previous claim. The gap of 6 months is longer than what has been used in previous claims-based studies.Citation30,Citation31 The rationale for this conservative approach is that adults with CP may have longer recovery periods and require more checkups for a fracture than the general population.
Covariates
Covariates were selected based on their relevance to CP or NTFx, and availability and reliability in administrative claims data. Sociodemographic variables included age, sex, race, US region of residence, and insurance type (ie, commercial vs Medicare Advantage plan). Glucocorticoid medications and anti-epileptic medications were defined in the pre-index time period by at least one outpatient pharmacy claim for relevant medications. A dichotomous variable was constructed for the presence of a neurological disability other than CP, including epilepsy, intellectual disabilities, autism spectrum disorders, or spina bifida. A modified version of the Elixhauser comorbidity index was included. The original Elixhauser comorbidity index includes a score (yes or no) for 30 comorbidities.Citation35 However, since there is some overlap with CP, paralysis and other neurological disorders were omitted for this study, making the total count up to 28. Comorbid neurological disabilities is a better measure to capture other neurological conditions that may impact osteoporosis, medication adherence, and NTFx risk for adults with CP.Citation13,Citation32
Propensity-Score Matching
Several covariates may be required to account for group differences that accompany clinical decision-making for osteoporosis treatment, especially for observational study designs. As 1-year fracture incidence is a relatively rare outcome and the primary group of interest (CPMeds+) had a relatively small sample size, regression models adjusting for several covariates may limit the interpretation due to bias of parameter estimates. Therefore, to account for covariates that may influence the associations of interest without the need for statistical adjustment of several covariates in a single model, the comparison groups (ie, CPMeds- and non-CPMeds+) were matched to the primary group of interest, CPMeds+, using a propensity score via the PSMATCH procedure in SAS version 9.4 (SAS Institute, Cary, NC, USA), as previously described.Citation36 Given the rare outcome, the goal of this matching was to provide a ratio of 1:x (case:control) that maximized the number of comparators per case in order to increase the confidence in the analyses using a standard caliper of ≤0.50, without losing any participants from the CPMeds+ group to limit bias, and while achieving balance in matching. We employed the greedy nearest neighbor method after randomizing the order of the comparator groups, and therefore, the comparators were selected at random. For both comparison groups, all covariates were initially included to create the propensity score. If covariates were unable to achieve balance in matching, as determined using standard procedures, statistical models were developed before and after adjusting for those covariates to determine if any unbalance in covariates impacted the associations of interest.
Statistical Analysis
Descriptive characteristics were presented for each group. Logistic regression models were developed to compare the odds of pre- and post-index NTFx, as any NTFx and by NTFx site, comparing CPMeds+ to CPMeds- and non-CPMeds+. When post-index NTFx was the outcome, the models were adjusted for pre-index NTFx corresponding to the same site. For example, post-NTFx of the lower extremities was adjusted for pre-index NTFx of the lower extremities.
To determine the change in NTFx risk from pre- to post-index time periods for each group, risk ratios (RR) and 95% confidence intervals (CI) were estimated. To determine if the change in NTFx risk from pre- to post-index time periods were different across groups, a difference-in-difference analysis was conducted using a generalized linear model with repeated measures and a log link function. Each participant contributed to two observations in the difference-in-difference analysis (ie, pre- and post-index NTFx), and the interpretation was focused on the relative change, which is assessed by taking the ratio of the RR (RRR) from the groups being compared, which approximates the interaction of the time by exposure variable. Therefore, the logistic regression models identified if there were group differences in the odds of NTFx at the pre- and post-index time periods separately, while the RRR determined if there were group differences in the change in NTFx risk from pre- to post-index time periods. One major advantage of following the same participants over time in the difference-in-difference analysis is that they serve as their own internal control, thus limiting confounding.
As there might be differential skeletal effects over time with osteoporosis medication among adults with and without CP, the RR and RRR were estimated after stratifying the osteoporosis medication groups (ie, CPMeds+ and non-CPMeds+) into their new and consistent user strata. The comparisons of interest were: new vs consistent users with CP; new users with vs without CP; and consistent users with vs without CP. Since the propensity score-matching was for the whole groups, covariates were compared among the stratified groups using the Chi-square test for categorical variables and the independent t-test for continuous variables. If statistically different, then the difference-in-difference analyses were performed after adjusting for these covariates.
Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and P≤0.05 (two-tailed) was considered statistically significant.
Results
Prior to matching, 306 out of 7027 (4.4%) adults with CP that met eligibility criteria were prescribed an osteoporosis medication. Baseline descriptive characteristics of propensity-matched participants (1:6 matching ratio) CPMeds+ (n=306), CPMeds- (n=1836), and non-CPMeds+ (n=1836) are presented in . The matches for CPMeds+ and CPMeds- were well balanced. However, the propensity-score matching was unsuccessful for non-CPMeds+. Upon further examination, age and comorbid neurodevelopmental disabilities were omitted from the matching procedure and successful matching between CPMeds+ and non-CPMeds+ was achieved. As secondary analyses, differences between CPMeds+ and non-CPMeds+ were performed before and after statistically adjusting for age and comorbid neurodevelopmental disabilities. Notably, the CP groups were approximately 10 years younger on average compared to non-CPMeds+. Among individuals that were prescribed osteoporosis medication, the distribution of new users (34.3%, 35.0%) and consistent users (65.7%, 65.0%) was similar for CPMeds+ and non-CPMeds+.
Table 1 Baseline Descriptive Characteristics of Propensity-Matched Participants (1:6) by Status of Cerebral Palsy (CP) and Prescribed Osteoporosis Medication (Meds)
Prevalence and Odds of Pre- and Post-Index NTFx
The prevalence of NTFx is presented in and the OR of NTFx is presented in . CPMeds+ had a higher prevalence and OR of pre-and post-index any NTFx compared to CPMeds-. CPMeds+ had a similar prevalence and OR of pre- and post-index any NTFx compared to non-CPMeds+, but after further adjustment for age, comorbid neurodevelopmental disabilities (due to omission of these variables in the matching procedure), and pre-index any NTFx, the OR was elevated for post-index any NTFx (OR=1.43; 95% CI=0.97–2.13); although, this was marginally statistically insignificant (P=0.073).
Table 2 Prevalence and Unadjusted Risk Ratio (RR) of Pre- and Post-Index Non-Trauma Fracture (NTFx) Among Propensity-Matched Participants (1:6) by Status of Cerebral Palsy (CP) and Prescribed Osteoporosis Medication (Meds)
Table 3 Odds Ratio (OR) of Non-Trauma Fracture (NTFx) Among Propensity-Matched Participants (1:6) by Status of Cerebral Palsy (CP) and Prescribed Osteoporosis Medication (Meds)
Due to the small number of outcome cases, NTFx of the vertebral column and hip were combined and adjusted analysis of the upper extremities was not possible. For the comparison of CPMeds+ and CPMeds-, results were similar for NTFx of the lower extremities, while the OR of NTFx of the vertebral column/hip was significantly elevated in the pre-index period, but not in the post-index period before and after adjusting for pre-index NTFx. For the comparison of CPMeds+ and non-CPMeds+, the OR was elevated for pre- and post-index NTFx of the lower extremities.
Change in Pre- to Post-Index NTFx Risk
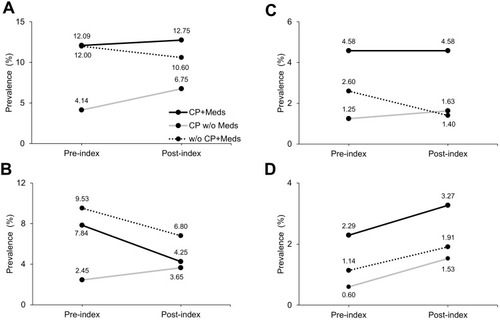
The pre- and post-index prevalence of NTFx is visually presented in and the unadjusted RR of NTFx is presented in . The change in prevalence of any NTFx from the pre- to post-index period showed no change for CPMeds+, increased for CPMeds-, and showed a slight non-significant decrease for non-CPMeds+. For NTFx of the vertebral column/hip, the change decreased for CPMeds+ (P=0.067), increased for CPMeds-, and decreased for non-CPMeds+. For NTFx of the lower extremities, the change decreased for non-CPMeds+. For NTFx of the upper extremities, the change increased for CPMeds- and non-CPMeds+ (P=0.062).
Figure 1 Unadjusted prevalence of pre- and post-index non-trauma fracture (NTFx) as (A) any NTFx, (B) NTFx of the vertebral column or hip, (C) NTFx of the lower extremities, and (D) NTFx of the upper extremities for propensity-matched adults (1:6) by status of cerebral palsy (CP) as with CP (CP) or without CP (w/o CP), and prescribed osteoporosis medication (Meds).
The RRR results for all comparisons are presented in . Comparing CPMeds+ to CPMeds-, the RRR of any NTFx was 0.65 (95% CI=0.41–1.04), suggesting an attenuated risk of post-index any NTFx; although, this was marginally statistically insignificant (P=0.070). When the change in NTFx risk by the group was examined by the NTFx site, there was a stronger and statistically significant risk attenuation of post-index NTFx of the vertebral column/hip for CPMeds+ compared to CPMeds- (RRR=0.36; 95% CI=0.18–0.74), but not for the lower extremities (RRR=0.77; 95% CI=0.33–1.77).
Table 4 The Ratio of the Risk Ratio (RRR) of Pre- to Post-Index Non-Trauma Fracture (NTFx) Among Participants by Status of Cerebral Palsy (CP), Prescribed Osteoporosis Medication (Meds), and Whether the Medication Group Was a New or Consistent User
Comparing CPMeds+ to non-CPMeds+ (and adjusting for age and comorbid neurodevelopmental disabilities), the relative change in NTFx risk by group went in opposite directions. The RRR was lower for NTFx of the vertebral column/hip (RRR=0.76; 95% CI=0.40–1.45), suggesting a larger risk attenuation effect for CPMeds+, but this finding was not statistically significant (P=0.404). Conversely, the RRR was elevated, and to a greater extent, for NTFx of the lower extremities (RRR=1.89; 95% CI=0.84–4.24), suggesting a larger risk attenuation effect for non-CPMeds+; although, this finding was marginally statistically insignificant (P=0.123).
Effect of New vs Consistent Users Change in Pre- to Post-Index NTFx Risk
The pre- and post-index prevalence and unadjusted RR of NTFx for new and consistent users is presented in . visually represents the change in pre- to post-index prevalence of any NTFx for new and consistent users. The number of NTFx cases for the upper extremities was too few to perform analyses.
Figure 2 Unadjusted prevalence of pre- and post-index any non-trauma fracture (NTFx) for participants by status of cerebral palsy (CP) as with CP (CP) or without CP (w/o CP), prescribed osteoporosis medication (Meds), and whether the medication group was a new med or consistent med user.
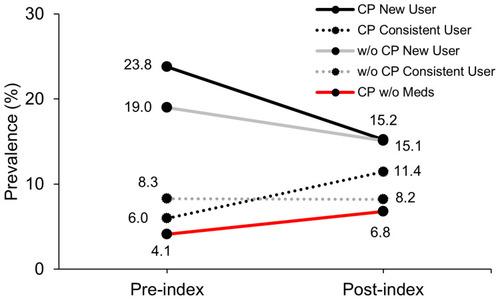
Table 5 Prevalence and Unadjusted Risk Ratio (RR) of Pre- and Post-Index Non-Trauma Fracture (NTFx) Among Participants by Status of Cerebral Palsy (CP) and Whether the Osteoporosis Medication Group Was a New or Consistent User
New users with CP had a higher prevalence of glucocorticoid medication prescription compared to consistent users with CP and CPMeds- (both P<0.005), but no other differences in descriptive characteristics were observed across the five groups. For any NTFx, the RRR compared to CPMeds- was significantly lower for new users with CP and not different for consistent users with CP, and significantly lower for new vs consistent users with CP (). Notably, for NTFx of the lower extremities, the RRR compared to CPMeds- was non-significantly lower for new users with CP and non-significantly elevated for consistent users with CP, and significantly lower for new vs consistent users with CP.
After adjusting for age and comorbid neurodevelopmental disabilities (due to omission of these variables in the matching procedure), the RRR of any NTFx was not different for new users with CP compared to new users without CP, but the RRR was elevated for consistent users with vs without CP; although, the latter finding was marginally statistically insignificant (P=0.059). The RRR was much larger for NTFx of the lower extremities comparing consistent users with vs without CP (RRR=5.19; 95% CI=1.42–18.85).
Discussion
The findings from the current investigation suggest that while the risk of NTFx was elevated in the post-index period, osteoporosis medication was associated with a clinically meaningful 1-year NTFx risk attenuation for adults with CP, especially of the vertebral column/hip and lower extremities for new users with CP. Further, the beneficial effect of osteoporosis medication on 1-year NTFx risk attenuation was stronger for new med vs consistent med users with CP, and similar to the benefit observed in adults without CP. We have previously reported that NTFx of the vertebral column, hip, and lower extremities increases the risk for incident cardiovascular disease among adults with vs without CP, but the risk was highest for NTFx of the lower extremities compared to the other sites.Citation18 Therefore, reducing NTFx among adults with CP, especially of the lower extremities, may lead to improvements in healthful aging by delaying the onset of costly and burdensome diseases. It is important to note that because this study is observational, causation cannot be determined, especially for investigating the effectiveness of medication due to unmeasured confounding. Also, this study did not examine minor and major adverse events or medical side effects of osteoporosis medication. When taken together, this study provides real-world evidence about the effectiveness of osteoporosis medication on NTFx risk attenuation among privately insured adults with CP. However, future work is needed to determine the safety and dosing of osteoporosis medication considering the medical and pharmacological complexities associated with CP prior to changing clinical practice.
In the current sample of privately insured adults, only 4.4% of the total CP group (n=7027) was prescribed osteoporosis medication, which is likely not addressing the pharmacological needs to bolster skeletal integrity for this adult population. However, vitamin D and calcium were not assessed in the current work as many individuals can get these supplements over the counter, which claims data does not capture. While vitamin D and calcium are often recommended to manage skeletal fragility, a meta-analysis of 33 randomized trials with >51,000 older adults found no association between risk of fractures with supplementation of vitamin D, calcium, or both.Citation37 No studies have examined the effect of vitamin D and/or calcium supplementation for adults with CP in regards to fracture risk reduction. Nevertheless, previous studies have shown the age-standardized prevalence of fracture to be 6.7% for men and 8.5% for women with CPCitation13 and that nearly half of adults ≥18 years of age have osteopenia or osteoporosis.Citation15,Citation38 It is well known that care coordination and healthcare quality are suboptimal and fragmented for adults with CP.Citation39–Citation41 Further, adults with CP often seek medical care from physicians that lack the necessary expertise to treat adult patients with CP, which is likely due to inadequate availability of physicians with sufficient knowledge of the life-course health/disease development for this population and the absence of care pathways to guide appropriate treatment. This can result in less comprehensive medical examinations and screening for medical conditions,Citation42,Citation43 such as bone mineral density via dual-energy x-ray absorptiometry, despite this test being feasible for adults with CP.Citation38 Importantly, there is a need to assess skeletal fragility at a much younger age for individuals with CP compared to the general population.Citation14 Therefore, the low prevalence of osteoporosis treatment for adults with CP observed in this study may be due to a lack of awareness of the profound and earlier than the expected burden of skeletal fragility, leading to missed opportunities to clinically assess for and treat skeletal fragility among adults with CP.
In the current study, the prevalence of pre- and post-index NTFx was elevated compared to CPMeds- and non-CPMeds+. However, the change in NTFx risk differed among adults with CP and even among new and consistent users with CP. Compared to CPMeds-, CPMeds+ showed a large effect for NTFx risk attenuation of the vertebral column/hip (RRR=0.36). The extent of NTFx risk attenuation at this site mirrored that of the non-CPMeds+ group, suggesting a similar effect of osteoporosis medication at the vertebral column/hip. While not presented, an exploratory analysis revealed the same trend between CPMeds+ vs CPMeds- when NTFx was examined separately at the vertebral column (RRR=0.38; 95% CI=0.14–1.06) and hip (RRR=0.34; 95% CI=0.12–0.96).
At first glance, osteoporosis medication appeared to be potentially less effective at reducing the risk of NTFx at the lower extremities, which is the most commonly fractured region for individuals with CP.Citation13,Citation44 After further investigation, new users with CP exhibited a clinically meaningful NTFx risk attenuation at the lower extremities compared to CPMeds- (RRR=0.39) and consistent users with CP (RRR=0.22), which mirrored the change in NTFx risk from the group without CP. Surprisingly, the change in NTFx risk of the lower extremities from consistent users with CP was elevated compared to consistent users without CP (RRR=5.19); although, the number of NTFx events at this site was very low and we urge caution for interpretation. Nevertheless, the increased NTFx risk of the lower extremities among consistent users with CP may be due to several factors. First, there may be differences in medication persistence,Citation31 adherence,Citation45 and chronicity, which is associated with fracture risk, but unfortunately was not able to be examined in the current study. Second, adults with vs without CP may be more likely to be taking several medications (eg, polypharmacy) to manage their greater disease profiles,Citation29 which may lead to drug–drug interactions and reduce the long-term efficacy of osteoporosis medication. Third, given the poorly developedCitation7,Citation8,Citation46 and preservedCitation13–Citation15,Citation47 musculoskeletal tissue throughout the lifespan, it is possible that the needs of the musculoskeletal system from adults with CP over time exceed the skeletal benefit of osteoporosis medication alone in the long term.
It is important to note that very little is known about the bone turnover profiles for individuals with CP, and whether the skeletal pathology is due to excess bone resorption, inadequate bone formation, or a unique combination of both. This has important clinical implications because some medications target anti-resorption (eg, bisphosphonates), while other medications target enhanced bone formation (eg, anabolic agents) or both (eg, romosozumab). Unfortunately, the sample size in the current study was too small to examine differential effects by drug classes. The Fracture Study in Postmenopausal Women with Osteoporosis provides unique evidence of the benefit of building the skeletal foundation prior to switching to anti-resorption medications.Citation48 This approach may be attractive as there are longstanding issues with building the skeletal network present in early childhood. Given the promising results of this observational study, future studies are needed to determine the safety, efficacy, and timing of osteoporosis medication and time-sequential coupling of osteoporosis medications for adults with CP accounting for the medical complexity and heterogeneity that accompanies a CP diagnosis.
The limitations of this study must be discussed. First, the number of NTFx events was few for the CPMeds+ group, especially when stratified by NTFx location and new/consistent med users. We, therefore, urge extreme caution with interpretation. Second, based on differences in enrollment criteria for health insurance and our previous studies, our privately insured sample likely reflects a healthierCitation32,Citation49 and less skeletally fragileCitation47 segment of the population with CP. Even with a potentially more skeletally robust sample of adults with CP, the prevalence of osteoporosis medication is still inadequate for the skeletal fragility needs of privately insured adults with CP.Citation13,Citation47 Further, detection of fractures (especially hip fractures) among individuals with CP is slightly lower compared to the general population due to a variety of factors, including motor and cognitive impairment.Citation50 It is not uncommon for an incidental finding of a current or previously unhealed fracture in the clinical setting, which is speculated to be non-traumatic. The NTFx event may not be noticeable, but may subsequently lead to pain, which can be dismissed as pain associated with CP (eg, pain from muscle spasticity or contractures). Third, claims data provides information on whether or not a prescription was filled, but not whether the patient actually took the medication and adhered to the correct dosage and timing. Further, there may have been additional interventions around the time of osteoporosis medication prescription by the healthcare provider(s) that we are unable to account for, such as nutrition counseling, exercise and rehabilitation consultations, or avoidance of risky behaviors that could lead to an NTFx event. Fourth, over the counter medications are not able to be tracked in claims data, such as vitamin D or calcium supplementation. Fifth, the specific class or type of osteoporosis medications were not examined in this study, which may impact results if the distribution differed across groups. However, a recent study using the same database and a similar methodology reported that among adults with or without a neurological condition prescribed an osteoporosis medication, >95% were prescribed an anti-resorptive medication with no difference between new and consistent users.Citation36
Conclusion
Osteoporosis medication was associated with a clinically meaningful 1-year NTFx risk attenuation for adults with CP, especially for new users. The extent of NTFx risk attenuation associated with osteoporosis medication was similar to adults without CP. Future studies are needed to identify why consistent med users with CP have an increased risk of NTFx over time, and if alternative pharmacological treatment strategies (eg, time-sequential coupling of osteoporosis medications) or multi-modal intervention strategies (eg, osteoporosis medication and exercise) are feasible and effective at reducing NTFx risk for adults with CP.
Accessibility of Protocol, Raw Data, and Programming Code
As part of the Date Use Agreement, authors are not allowed to provide raw data. Upon a reasonable request, the corresponding author will provide statistical programming code used to generate results.
Acknowledgments
This research was developed in part under grants from the University of Michigan Office of Health Equity and Inclusion Diversity Fund and American Academy for Cerebral Palsy and Developmental Medicine. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
- Christensen D, Van Naarden Braun K, Doernberg NS, et al. Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning – autism and developmental disabilities monitoring network, USA, 2008. Dev Med Child Neurol. 2014;56(1):59–65. doi:10.1111/dmcn.1226824117446
- Maenner MJ, Blumberg SJ, Kogan MD, Christensen D, Yeargin-Allsopp M, Schieve LA. Prevalence of cerebral palsy and intellectual disability among children identified in two U.S. national surveys, 2011–2013. Ann Epidemiol. 2016;26(3):222–226. doi:10.1016/j.annepidem.2016.01.00126851824
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy april 2006. Dev Med Child Neurol Suppl. 2007;109:8–14.17370477
- Rose J, McGill KC. Neuromuscular activation and motor-unit firing characteristics in cerebral palsy. Dev Med Child Neurol. 2005;47(5):329–336. doi:10.1017/S001216220500062915892375
- Wiley ME, Damiano DL. Lower-extremity strength profiles in spastic cerebral palsy. Dev Med Child Neurol. 1998;40(2):100–107. doi:10.1111/j.1469-8749.1998.tb15369.x9489498
- Elder GC, Kirk J, Stewart G, et al. Contributing factors to muscle weakness in children with cerebral palsy. Dev Med Child Neurol. 2003;45(8):542–550. doi:10.1111/j.1469-8749.2003.tb00954.x12882533
- Whitney DG, Singh H, Miller F, et al. Cortical bone deficit and fat infiltration of bone marrow and skeletal muscle in ambulatory children with mild spastic cerebral palsy. Bone. 2017;94:90–97. doi:10.1016/j.bone.2016.10.00527732905
- Johnson DL, Miller F, Subramanian P, Modlesky CM. Adipose tissue infiltration of skeletal muscle in children with cerebral palsy. J Pediatr. 2009;154(5):715–720. doi:10.1016/j.jpeds.2008.10.04619111321
- Modlesky CM, Whitney DG, Singh H, Barbe MF, Kirby JT, Miller F. Underdevelopment of trabecular bone microarchitecture in the distal femur of nonambulatory children with cerebral palsy becomes more pronounced with distance from the growth plate. Osteoporos Int. 2015;26(2):505–512. doi:10.1007/s00198-014-2873-425199575
- Al Wren T, Lee DC, Kay RM, Dorey FJ, Gilsanz V. Bone density and size in ambulatory children with cerebral palsy. Dev Med Child Neurol. 2011;53(2):137–141. doi:10.1111/j.1469-8749.2010.03852.x21166671
- Whitney DG, Peterson MD, Devlin MJ, Caird MS, Hurvitz EA, Modlesky CM. Bone marrow fat physiology in relation to skeletal metabolism and cardiometabolic disease risk in children with cerebral palsy. Am J Phys Med Rehabil. 2018;97(12):911–919. doi:10.1097/PHM.000000000000098129894311
- Stevenson RD, Conaway M, Barrington JW, Cuthill SL, Worley G, Henderson RC. Fracture rate in children with cerebral palsy. Pediatr Rehabil. 2006;9(4):396–403. doi:10.1080/1363849060066806117111566
- Whitney DG, Caird MS, Jepsen KJ, et al. Elevated fracture risk for adults with neurodevelopmental disabilities. Bone. 2020;130:115080. doi:10.1016/j.bone.2019.11508031655219
- Whitney DG, Alford AI, Devlin MJ, Caird MS, Hurvitz EA, Peterson MD. Adults with cerebral palsy have higher prevalence of fracture compared with adults without cerebral palsy independent of osteoporosis and cardiometabolic diseases. J Bone Miner Res. 2019;34(7):1240–1247. doi:10.1002/jbmr.369430730595
- Whitney DG, Hurvitz EA, Devlin MJ, et al. Age trajectories of musculoskeletal morbidities in adults with cerebral palsy. Bone. 2018;114:285–291. doi:10.1016/j.bone.2018.07.00229981509
- Whitney DG, Bell S, Hurvitz EA, Peterson MD, Caird MS, Jepsen KJ. The mortality burden of non-trauma fracture for adults with cerebral palsy. Bone Rep. 2020;13:100725. doi:10.1016/j.bonr.2020.10072533088868
- Whitney DG, Whitney RT, Prisby RD, Jepsen KJ. Low-trauma fracture increases 12-month incidence of cardiovascular disease for adults with cerebral palsy. J Orthop Res. 2020;38(4):803–810.31710380
- Whitney DG, Bell S, Etter JP, Prisby RD. The cardiovascular disease burden of non-traumatic fractures for adults with and without cerebral palsy. Bone. 2020;136:115376. doi:10.1016/j.bone.2020.11537632335375
- Whitney DG. Nontrauma fracture increases risk for respiratory disease among adults with cerebral palsy. J Orthop Res. 2020.
- Ryan JM, Peterson MD, Ryan N, et al. Mortality due to cardiovascular disease, respiratory disease, and cancer in adults with cerebral palsy. Dev Med Child Neurol. 2019;61(8):924–928. doi:10.1111/dmcn.1417630727025
- Blair E, Langdon K, McIntyre S, Lawrence D, Watson L. Survival and mortality in cerebral palsy: observations to the sixth decade from a data linkage study of a total population register and national death index. BMC Neurol. 2019;19(1):111. doi:10.1186/s12883-019-1343-131164086
- Shin YK, Yoon YK, Chung KB, Rhee Y, Cho SR. Patients with non-ambulatory cerebral palsy have higher sclerostin levels and lower bone mineral density than patients with ambulatory cerebral palsy. Bone. 2017;103:302–307. doi:10.1016/j.bone.2017.07.01528720522
- Tian X, Jee WS, Li X, Paszty C, Ke HZ. Sclerostin antibody increases bone mass by stimulating bone formation and inhibiting bone resorption in a hindlimb-immobilization rat model. Bone. 2011;48(2):197–201. doi:10.1016/j.bone.2010.09.00920850580
- Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799–1809. doi:10.1056/NEJMoa07494117878149
- Crichton JU, Mackinnon M, White CP. The life-expectancy of persons with cerebral palsy. Dev Med Child Neurol. 1995;37(7):567–576. doi:10.1111/j.1469-8749.1995.tb12045.x7615144
- Kim MJ, Kim SN, Lee IS, et al. Effects of bisphosphonates to treat osteoporosis in children with cerebral palsy: a meta-analysis. J Pediatr Endocrinol Metab. 2015;28(11–12):1343–1350. doi:10.1515/jpem-2014-052726214607
- Sees JP, Sitoula P, Dabney K, et al. Pamidronate treatment to prevent reoccurring fractures in children with cerebral palsy. J Pediatr Orthop. 2016;36(2):193–197. doi:10.1097/BPO.000000000000042125757207
- Henderson RC, Lark RK, Kecskemethy HH, Miller F, Harcke HT, Bachrach SJ. Bisphosphonates to treat osteopenia in children with quadriplegic cerebral palsy: a randomized, placebo-controlled clinical trial. J Pediatr. 2002;141(5):644–651. doi:10.1067/mpd.2002.12820712410192
- Whitney DG, Kamdar NS, Ng S, Hurvitz EA, Peterson MD. Prevalence of high-burden medical conditions and health care resource utilization and costs among adults with cerebral palsy. Clin Epidemiol. 2019;11:469–481. doi:10.2147/CLEP.S20583931417318
- Keshishian A, Boytsov N, Burge R, et al. Examining the effect of medication adherence on risk of subsequent fracture among women with a fragility fracture in the U.S. medicare population. J Manag Care Spec Pharm. 2017;23(11):1178–1190. doi:10.18553/jmcp.2017.1705429083977
- Liu J, Guo H, Rai P, Pinto L, Barron R. Medication persistence and risk of fracture among female medicare beneficiaries diagnosed with osteoporosis. Osteoporos Int. 2018;29(11):2409–2417. doi:10.1007/s00198-018-4630-630022254
- Whitney DG, Whibley D, Jepsen KJ. The effect of low-trauma fracture on one-year mortality rate among privately insured adults with and without neurodevelopmental disabilities. Bone. 2019;129:115060. doi:10.1016/j.bone.2019.11506031494304
- Whitney DG, Whitney RT, Prisby RD, Jepsen KJ. Low-trauma fracture increases 12-month incidence of cardiovascular disease for adults with cerebral palsy. J Orthop Res. 2019;38(4):803–810. doi:10.1002/jor.2451531710380
- Whitney DG, Bell S, McNamara NA, Hurvitz EA. The mortality burden attributable to nontrauma fracture for privately insured adults with epilepsy. Epilepsia. 2020;61(4):714–724. doi:10.1111/epi.1646532108937
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi:10.1097/00005650-199801000-000049431328
- Whitney DG. Effectiveness of osteoporosis medication on site-specific fracture risk attenuation among adults with epilepsy. Epilepsia. 2020;61(11):2583–2592. doi:10.1111/epi.1670033090479
- Zhao JG, Zeng XT, Wang J, Liu L. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318(24):2466–2482. doi:10.1001/jama.2017.1934429279934
- Marciniak C, Gabet J, Lee J, Ma M, Brander K, Wysocki N. Osteoporosis in adults with cerebral palsy: feasibility of DXA screening and risk factors for low bone density. Osteoporos Int. 2016;27(4):1477–1484. doi:10.1007/s00198-015-3393-626576540
- Berry JG, Berry SD. Caring for patients with neurological impairment: conversations between a pediatrician and geriatrician. JAMA Pediatr. 2018;172(9):795. doi:10.1001/jamapediatrics.2018.107929971353
- Vohra R, Madhavan S, Sambamoorthi U, St Peter C. Access to services, quality of care, and family impact for children with autism, other developmental disabilities, and other mental health conditions. Autism. 2014;18(7):815–826. doi:10.1177/136236131351290224353274
- Aisen ML, Kerkovich D, Mast J, et al. Cerebral palsy: clinical care and neurological rehabilitation. Lancet Neurol. 2011;10(9):844–852. doi:10.1016/S1474-4422(11)70176-421849165
- Horner-Johnson W, Dobbertin K, Andresen EM, Iezzoni LI. Breast and cervical cancer screening disparities associated with disability severity. Womens Health Issues. 2014;24(1):e147–153. doi:10.1016/j.whi.2013.10.00924439941
- McColl MA, Forster D, Shortt SE, et al. Physician experiences providing primary care to people with disabilities. Healthc Policy. 2008;4(1):e129–147.19377334
- Presedo A, Dabney KW, Miller F. Fractures in patients with cerebral palsy. J Pediatr Orthop. 2007;27(2):147–153. doi:10.1097/BPO.0b013e318031740317314638
- Chen Q, Guo M, Ma X, Pu Y, Long Y, Xu Y. Adherence to teriparatide treatment and risk of fracture: a systematic review and meta-analysis. Horm Metab Res. 2019;51(12):785–791. doi:10.1055/a-1062-944731826274
- Henderson RC, Kairalla JA, Barrington JW, Abbas A, Stevenson RD. Longitudinal changes in bone density in children and adolescents with moderate to severe cerebral palsy. J Pediatr. 2005;146(6):769–775. doi:10.1016/j.jpeds.2005.02.02415973316
- French ZP, Caird MS, Whitney DG. Osteoporosis epidemiology among adults with cerebral palsy: findings from private and public administrative claims data. JBMR Plus. 2019;3(11):e10231. doi:10.1002/jbm4.1023131768490
- Cosman F, Crittenden DB, Ferrari S, et al. FRAME study: the foundation effect of building bone with 1 year of romosozumab leads to continued lower fracture risk after transition to denosumab. J Bone Miner Res. 2018;33(7):1219–1226. doi:10.1002/jbmr.342729573473
- Whitney DG. Prevalence of high-burden medical conditions among young and middle-aged adults with pediatric-onset medical conditions: findings from us private and public administrative claims data. Int J Health Policy Manag. 2019;8(11):629–635. doi:10.15171/ijhpm.2019.6231779288
- Toro G, Moretti A, Paoletta M, De Cicco A, Braile A, Panni AS. Neglected femoral neck fractures in cerebral palsy: a narrative review. EFORT Open Rev. 2020;5(1):58–64. doi:10.1302/2058-5241.5.19001932071774
