Abstract
Aim of database
The aim of the database is to monitor and improve the treatment and survival of melanoma patients.
Study population
All Danish patients with cutaneous melanoma and in situ melanomas must be registered in the Danish Melanoma Database (DMD). In 2014, 2,525 patients with invasive melanoma and 780 with in situ tumors were registered. The coverage is currently 93% compared with the Danish Pathology Register.
Main variables
The main variables include demographic, clinical, and pathological characteristics, including Breslow’s tumor thickness, ± ulceration, mitoses, and tumor–node–metastasis stage. Information about the date of diagnosis, treatment, type of surgery, including safety margins, results of lymphoscintigraphy in patients for whom this was indicated (tumors > T1a), results of sentinel node biopsy, pathological evaluation hereof, and follow-up information, including recurrence, nature, and treatment hereof is registered. In case of death, the cause and date are included. Currently, all data are entered manually; however, data catchment from the existing registries is planned to be included shortly.
Descriptive data
The DMD is an old research database, but new as a clinical quality register. The coverage is high, and the performance in the five Danish regions is quite similar due to strong adherence to guidelines provided by the Danish Melanoma Group. The list of monitored indicators is constantly expanding, and annual quality reports are issued. Several important scientific studies are based on DMD data.
Conclusion
DMD holds unique detailed information about tumor characteristics, the surgical treatment, and follow-up of Danish melanoma patients. Registration and monitoring is currently expanding to encompass even more clinical parameters to benefit both patient treatment and research.
Introduction
The Danish Melanoma Group (DMG), which is part of the Danish Multidisciplinary Cancer Groups,Citation1 has since 1985 on a national basis collected detailed clinical, surgical, pathological, and follow-up information on Danish patients suffering from cutaneous melanoma, including in situ lesions, in the Danish Melanoma Database (DMD). The DMG is an organization focusing on improving care for Danish melanoma patients. All medical specialists with interest in melanoma treatment are involved, and organization of the database, supporting research, and developing clinical guidelines are important parts of the activity.Citation2 Until 2011, the database was a private initiative, driven by dedicated specialists and without stable funding. In 2011, the database was approved as a clinical quality database under the support of the Danish Regions’ Clinical Quality Program, and the first annual quality report was published in 2014 based on 2013 data. Since 2011, data registration has been performed online, and data from 2000 and onward have been entered in the current version. A revision of the registration forms/online modules has just been completed, and the nature and number of quality indicators included in the annual reports are an evolving process. In previous publications, slightly different names for the database have been used over the years: DMG Database, Danish Melanoma Registry, and now the DMD.
Cutaneous melanoma is a potentially deadly disease, generally with a high capacity for metastatic spread. The prognosis primarily depends on tumor thickness at the time of diagnosis. Ulceration of the epithelium and mitotic activity within the dermal component of the tumor along with spread to the draining sentinel node(s) are the other most important negative prognostic variables.Citation3 More than half of the tumors currently diagnosed are thin (≤1.00 mm) with a very good prognosis.Citation4 Treatment of melanoma is surgical with local wide excision to secure safety margins; sentinel node staging is performed for tumors with a ≥10% risk of (micro)metastases to regional lymph nodes (T1b tumors or higher: thickness >1.00 mm or if ulceration or mitotic activity is present).Citation5 Radical lymph node dissection is generally performed in case of spread to the regional lymph nodes. Solitary metastases are generally treated surgically; however, when this is no longer feasible, systemic treatment is offered.Citation2
Aim of database
The aim of the database is to monitor and thereby improve treatment and survival for melanoma patients. More specifically, the purpose of the DMD is to systematically collect key clinical parameters on all incident cases of invasive or in situ melanoma in Denmark for health care monitoring, quality improvement, and research.
Study population
The study population includes all Danish patients with cutaneous melanoma, including in situ lesions. New patients are registered at the time of diagnosis; for patients already in the database, follow-up information including recurrence is recorded, in general until 5 years after initial treatment or last recurrence. In case of late recurrence, the registration is resumed. The incidence of melanoma has increased at the rate of 4%–5% per year, and in situ lesions perhaps even more; however, the preliminary result for 2015 for the first time in the history of the database shows a decrease of 4.2% for invasive melanoma, while the in situ lesions still increase with 3.6%.Citation6 The 2014 annual report included 2,525 patients with invasive melanoma and 780 with in situ lesions,Citation4 corresponding to a crude incidence of 44.6/100,000 and 13.8/100,000, respectively, and in the 2013 report, 2,325 patients had invasive melanoma and 505 had in situ melanomas. The database has included ~30,000 patients since 1985.
Main variables
The main variables are listed in . The pathologist performs the primary entry to the DMD. All relevant tumor characteristics are registered. The Department of Plastic Surgery is responsible for recording the treatment. Currently, physicians in primary health care have removed 38% of tumors at the time of referral.Citation4 The following information is recorded at the first visit: brief information on the history, any familial melanoma, location and clinical description of the tumor, and details of the bioptic basis for the diagnosis (if the tumor is still present, an excisional biopsy is performed at this stage). Later, data on the definite surgery and sentinel node biopsy procedure (if performed) are registered, including information on method and results of lymphoscintigraphy. Pathological evaluation of the sentinel node(s) is recorded with detailed information about location and size of metastases. The tumor–node–metastasis stage is recorded. In case of metastases at the time of diagnosis or later, the clinical information on nature of and location of metastases is recorded, as well as overall information about treatment for this. Follow-up information including status at the time of follow-up (recurrence free, [sign of] recurrence, etc) is entered. The DMD currently does not hold data on outcomes of systemic treatment; these are registered in a private oncologic research database. Information about adjuvant treatment has been added by January 2016. In case of death, the patient is registered off study with date and cause of death. All data have so far been entered manually; however, considerable data catchment from the existing registries (the Danish Pathology Register, the Danish Civil Registration System, and the National Hospital Discharge Register) is planned to commence during 2016.
Table 1 Main variables included in the DMD
Data quality
Prior to the online registration, all registration forms were entered into a database centrally, and missing variables were identified and corrections pursued. After the electronic online registration was introduced, lists of missing data have been produced on key variables, and this list is still expanding in order to include all indicators.
In the annual quality reports, performance on selected key indicators is presented by region and by department. is an example of the small regional variation observed for the current key indicators (). The annual quality results give a very strong incitement for improving registration. Generally, data about on-study information, clinical stage at diagnosis, definite treatment, and the concurrent pathological variables are registered in 94.5%–98.7% of the cases.Citation4 Reporting of recurrence has not yet been included in the quality report, but we know that the quality of data on this is still suboptimal, however improving over time. No formal studies on the exact numbers of missing variables have been made. The coverage of all melanomas is now evaluated by comparison to the Danish Pathology Register,Citation7 and the national coverage is 93%.Citation4 In a recent scientific study on the incidence from 1985 to 2012, data from the DMD were compared with melanomas registered in the Danish Cancer Registry.Citation6 This study revealed that the DMD over the years has constantly captured ~80%–90% of cases ().Citation6 The Cancer Registry does not hold detailed information about location of tumor, tumor characteristics of prognostic significance (thickness, ulceration, mitoses), treatment, recurrence, follow-up, and in situ cancers and has no information about family history.
Figure 1 Regional performance regarding indicator 3 (excisional margins according to guidelines).
Abbreviation: DMD, Danish Melanoma Database.
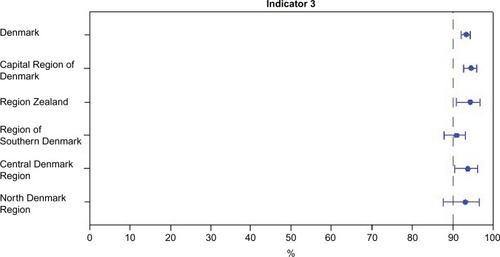
Figure 2 Age-standardized incidence rates from the DMD (full lines) compared with age-standardized incidence rates from the NORDCANa (dotted lines).
Abbreviations: DMD, the Danish Melanoma Database; py, per year; DMG, Danish Melanoma Group.
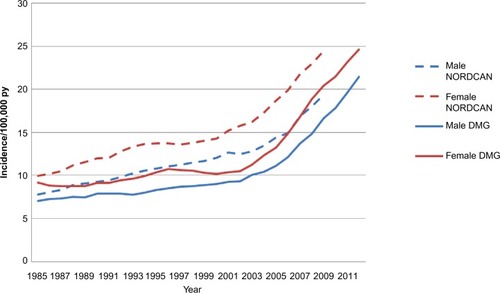
Table 2 Current key indicators for the DMD
Follow-up
Follow-up is primarily clinical and includes examination of the operated area and the rest of the patient’s skin and thorough palpation of lymph node regions as well as the area in between, searching for in-transit metastases (lymphatic spread is the most common route for metastases in most cases). When internal metastases are suspected, imaging (primarily positron emission tomography–computed tomography) is applied, and ultrasonography-guided fine needle aspiration is the method of choice for diagnosis. The use of imaging has increased, but not systematically, in patients with intermediate to thick tumors where risk of recurrence is not negligible and also without actual suspicion of metastases.
The follow-up program has been revised and fully implemented (by January 2016) toward a more intensive program for patients with the poorest prognosis and a less frequent follow-up for patients with better prognosis.Citation8 Patients with T1a tumors and without highly increased risk of second melanomas will not be followed up, except for a visit of 3 months postoperatively. Patients with tumors of intermediate thickness, with a negative sentinel node examination (stage IB, IIA), will be followed up clinically as described with visits twice annually for 5 years. Patients with thick tumors and patients with regional metastases at the time of diagnosis (clinical stages IIB, IIC, III) will be followed up with a clinical examination of four times a year for the first 2 years and then twice a year for the next 3 years. Positron emission tomography–computed tomography will be performed after 6 months, 12 months, 24 months, and 36 months on a routine basis. In all stages, further examinations, imaging, etc will be applied when indicated. Besides status at the time of follow-up (recurrence free, [sign of] recurrence, etc), database data about follow-up will also include information about who suspected the recurrence, how it was diagnosed (clinically, due to symptoms, or with use of imaging), and which kind of imaging was used. The new follow-up program will be monitored in a prospective study. Within the next year, linkage to the Danish Civil Registry will be implemented to achieve complete follow-up for survival on all registered melanoma patients.
Examples of research
Data from the DMD have been used for epidemiological, clinical, genetic, pathological, and molecular studies.Citation6,Citation9–Citation14 The recent study on the development in incidence is a good example of an epidemiological study with use of the data, and this allows for monitoring of the development in incidence and mortality (a study in preparation) over time.Citation6 Advanced molecular and pathology studies have used detailed information from the DMD and provided important new knowledge on tumor profiles and pathogenesis.Citation11–Citation14 In Denmark, we have unique possibilities for high-quality register-based scientific studies because all inhabitants are assigned a unique personal identification number (civil personal registration number). With this number, it is possible to link to a number of validated health and administrative registries.
Administrative issues and funding
The Danish Health Authority and the Danish Data Protection Agency have approved the DMD, and the registration is mandatory. The DMD is funded by the Danish RegionsCitation15 and is administered by the Danish Clinical Registries (RKKP),Citation16 which is the Danish Regions’ Clinical Quality Program. The budget allows for the daily maintenance and administration of the database, but not for research. The participating departments collect and report data as part of the clinical work without additional financial support. Budget and other report drafting for the Danish Regions is maintained with the help from the Registry Support Centre (East) – Clinical Quality Improvement & Health Informatics.
Conclusion
The DMD is an old database, but new as a clinical quality register under the Danish Regions’ Clinical Quality Program. Registration is integrated in the daily clinical activities in pathology departments as well as in the plastic surgery and oncology departments, when responsible for follow-up. The DMD holds unique detailed information about tumor characteristics, surgical treatment, and follow-up. The production of the annual quality reports has clarified the need for revision of the registration forms/electronic modules to ensure the ability to expand the monitoring to encompass even more clinical parameters. In this way, also the basis for research in the field is improved.
Acknowledgments
This article was funded by the Program for Clinical Research Infrastructure established by the Lundbeck Foundation and the Novo Nordisk Foundation and administered by the Danish Regions.
The electronic database is developed and managed by Aleksandar Jovanovic of Carma Group ApS, and without his steady strive for completeness, the database would not be in the current good shape. The DMG includes dedicated specialists all collaborating for the benefit of the melanoma patients. The DMD is controlled by the Steering Committee, which includes the members of the DMG’s Board as well as specialists in plastic surgery and pathology from regions, not represented in the Board. The annual reports are produced by Registry Support Centre (East) – Epidemiology and Biostatistics where data managers and epidemiologists in collaboration with the DMG present the data.
Disclosure
The DMD cooperates with the medical industry in development and research. This includes testing of oncologic products, clinical tests, participation in advisory boards, meetings, and teaching. There are no financial disclosures in relation to this work. The authors report no other conflicts of interest in this work.
References
- Danish Multidisciplinary Cancer Groups (DMCG.dk) [homepage on the Internet] Available from: www.dmcg.dkAccessed March 13, 2016
- Danish Melanoma Group [homepage on the Internet] Available from: www.melanoma.dkAccessed May 2, 2016
- BalchCMGershenwaldJESoongSJFinal version of 2009 AJCC melanoma staging and classificationJ Clin Oncol200927366199620619917835
- Dansk Melanom Database [webpage on the Internet]National Årsrapport2014 Available from: https://www.sundhed.dk/content/cms/30/57130_%C3%A5rsrapport_melanomer_2014_endelig.pdfAccessed July 29, 2015
- ChakeraAHHesseBBurakZEuropean Association of Nuclear Medicine-European Organisation for ResearchEANM-EORTC general recommendations for sentinel node diagnostics in melanomaEur J Nucl Med Mol Imaging200936101713174219714329
- HelvindNMHölmichLRSmithSIncidence of in situ and invasive melanoma in Denmark from 1985 through 2012 a national database study of 24 059 melanoma casesJAMA Dermatol2015151101087109526061591
- BjerregaardBLarsenOBThe Danish pathology registerScand J Public Health2011397 suppl727421775357
- Sundhedsstyrelsen [The Danish Health Authority] [webpage on the Internet]Opfølgningsprogram for modermærkekræft (melanom) [Follow-up program for melanoma patients] Available from: https://sundhedsstyrelsen.dk/da/udgivelser/2015/~/media/06A4C4DB820A49F19311C5879672BE7D.ashxAccessed May 2, 2016 Danish
- ChakeraAHDrzewieckiKTEigtvedAJuhlBRSentinel node biopsy for melanoma: a study of 241 patientsMelanoma Res200414652152615577324
- JensenTOSchmidtHMøllerHJMacrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanomaJ Clin Oncol200927203330333719528371
- WeischerMHeerfordtIMBojesenSECHEK2 *1100delC and risk of malignant melanoma: Danish and German studies and meta-analysisJ Invest Dermatol2012132229930321956126
- Lade-KellerJRiber-HansenRGuldbergPSchmidtHHamilton-DutoitSJSteinicheTE- to N-cadherin switch in melanoma is associated with decreased expression of phosphatase and tensin homolog and cancer progressionBr J Dermatol2013169361862823662813
- AntonioNBønnelykke-BehrndtzMLWardLCThe wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancerEMBO J201534172219223626136213
- WadtKAAoudeLGKroghLMolecular characterization of melanoma cases in Denmark suspected of genetic predispositionPLoS One2015103e012266225803691
- Danish Regions [homepage on the Internet] Available from: http://www.regioner.dkAccessed March 14, 2016
- The Danish Clinical Registries (RKKP) [homepage on the Internet] Available from: http://www.rkkp.dkAccessed March 14, 2016
- EngholmGFerlayJChristensenNhomepage on the InternetNORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 7.2 (16.12.2015)Association of the Nordic Cancer Registries. Danish Cancer Society Available from: http://www.ancr.nuAccessed February 2, 2016
