Abstract
Aim of the database
The aim of the Danish National Quality Database for Births (DNQDB) is to measure the quality of the care provided during birth through specific indicators.
Study population
The database includes all hospital births in Denmark.
Main variables
Anesthesia/pain relief, continuous support for women in the delivery room, lacerations (third and fourth degree), cesarean section, postpartum hemorrhage, establishment of skin-to-skin contact between the mother and the newborn infant, severe fetal hypoxia (proportion of live-born children with neonatal hypoxia), delivery of a healthy child after an uncomplicated birth, and anesthesia in case of cesarean section.
Descriptive data
Data have been collected since 2010. As of August 2015, data on women and children representing 269,597 births and 274,153 children have been collected. All data for the DNQDB is collected from the Danish Medical Birth Registry. Registration to the Danish Medical Birth Registry is mandatory for all maternity units in Denmark. During the 5 years, performance has improved in the areas covered by the process indicators and for some of the outcome indicators.
Conclusion
Measuring quality of care during childbirth has inspired and enabled staff to attend to the quality of the care they provide and has led to improvements in most of the areas covered.
Keywords:
Background and aim of the DNQDB database
The Danish National Quality Database for Births (DNQDB) started out as a part of the Danish National Indicator Project (DNIP) in 2010.Citation1,Citation2 The aim of the database is to measure the quality of the care provided during labor and birth through specific indicators. In the context of the database, birth is defined as the time from a woman’s arrival at a maternity unit/labor ward until 2 hours after the birth. Mode of delivery can be either vaginal or by cesarean section.
Study population
All hospital births in Denmark are included in the study’s population. The indicators related both to the woman giving birth and to the child(ren). Births in Denmark are approximately 55,000–60,000 per year, of which 1.5% are home births. The latter are not included in the database at the present time. The accumulated number of births and children included in the database as of August 31, 2015 were 269,597 and 274,153, respectively.
Main variables
Childbirth is a complex clinical situation, sometimes involving specialists from more than one department. Because of this, the indicators and the standards adopted were decided by a group of clinicians that included midwives, obstetricians, a pediatrician, anesthesiologists, and an epidemiologist. To decide which parts of the birth process to cover and include in the DNQDB, a schematic, standardized course of labor and birth () was created. Initially, eight indicators were selected, to fit within the upper limit of 8–10 set by the DNIP. Guiding factors for selection were relevance, scientific evidence, acceptability, and patient safety.Citation1,Citation2 Further details on the indicators selected and the reasons for excluding other potential indicators are available elsewhere.Citation2 Subsequently, some of the indicators were extended, and an additional outcome indicator was included in 2013 concerning the proportion of acute cesarean sections (grade 2) performed under local anesthesia. Data from this indicator are not included in the analyses in and because only 2 years of data have been collected, making it as yet invalid for comparison.
Figure 1 Schematic, standardized course of birth.
Abbreviations: CTG, cardiotocography; STAN, ST analysis; SBE, standard base excess; BW, birth weight.
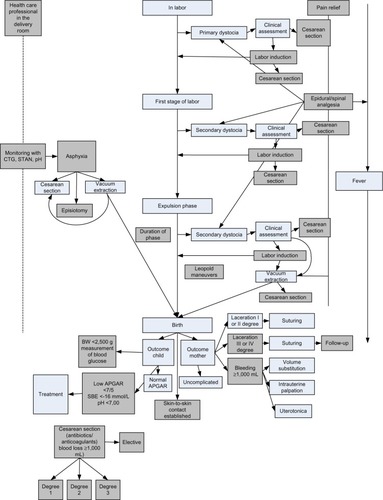
Table 1 Process indicators in the Danish National Quality Database for Birth Comparison of results in 2012 and 2015
Table 2 Outcome indicators in the Danish National Quality Database for Birth
All the quality indicators are process and outcome indicators concerning care during and after labor. The initial eight indicators are listed in and . Further information on the indicators and the data definitions are given in Kesmodel and Jølving.Citation2
All data for the DNQDB are collected from the Danish Medical Birth Registry.Citation3 Registration to the Danish Medical Birth Registry is mandatory for all maternity units in Denmark. After an initial period of inaccuracies in registration and of getting used to new registrations designed for the database, the data completeness is now very high (>90% for the indicators in and with apparently no or little selection bias), and the results are therefore considered reliable.
The maternity units receive monthly reports of their own data/results.
Measuring quality appears to have inspired improvements in performance, locally, within the clinical units, as there has been a great effort to perform well in the areas measured by the database. During the 5 years studied so far, performance has improved in the areas covered by process indicators and also for some of the outcome indicators. The comparison shown in and , between data from the second year of the database (2012) and the latest year (2015), reveals a significant reduction in the proportion of first-time deliveries complicated by third and fourth degree lacerations, as well as a significant increase in both the proportion of cesarean sections carried out within the time recommended and the proportion of epidural/spinal given within 1 hour from prescription. Likewise, significantly more women now have continuous support during the active phase of the labor. The indicators will be validated and reevaluated in 2016.
In addition to reducing complications and improving organizational processes, the act of collecting data for the DNQDB has also resulted in measurements that are more accurate. An example is the exact amount of bleeding during birth. Because of this focus on the accurate measurement of this indicator, we now know that maternal bleeding during birth is more extensive than previously suspected. We assume this not to be due to a real increase but to a new and continuous focus on measuring the bleeding correctly, in this case by objective weighing rather than guessing. Another example, which presents a similar difficulty in the comparison of results from the last 5 years, is the proportion of children with fetal hypoxia. In this indicator, hypoxia is defined as pH <7. 0 or, if pH is not measured, Apgar score <7 after 5 minutes. During the 5-year period, there has been an increase in the proportion of births with umbilical cord pH measurement, which compromises the ability to compare data across the years.
As well as focusing on quality locally, each unit has also been inspired to cooperate with other units and to integrate experience obtained elsewhere. This has been the case when trying to reduce the proportion of severe lacerations. Interestingly, the risk of birth complicated by a severe laceration is now almost the same in all maternity units no matter in which part of Denmark the birth takes place ().
During the 5 years that the DNQDB database has existed, there have been changes in the measurement of quality in childbirth and pregnancy in Denmark. When the database started, the database of the Danish Healthcare Quality Program (DDKM) included indicators concerning pregnancy and maternity.Citation4 However, these DDKM indicators no longer exist. Because of this, new indicators are currently being selected by the group of clinicians behind the DNQDB database. These will measure the quality of care in pregnancy, thereby potentially extending the period covered by this database hitherto. A part of this work is done in cooperation with the National Fetal Medicine Database.Citation5 This new cooperation between the two databases is important and can be extended in time. The measurement of quality during maternity will be a subject for indicators in the future.
Follow-up
The group of clinicians involved in the project currently meet every year before publishing the annual national report (available from http://www.kcks-vest.dk/kliniske-kvalitetsdatabaser/foedsler/ or on request from the authors). Every year all the indicators are reevaluated, and the need for new indicators is considered.
Examples of research
Most epidemiologic research concerning birth in Denmark is by tradition based on data collected from the Danish Medical Birth Registry. The service offered by the Danish Medical Birth Registry has changed in recent years, making it more difficult and time consuming to access data for research and quality improvement. Because of this, using data from the DNQDB can be an advantage for researchers. Access to the DNQDB data via the Registry Support Centers of Epidemiology and Biostatistics (South) is not only quicker and easier but the DNQDB data are also refined and analyzed by an epidemiologist before being delivered to the researchers. After 5 years of collecting data, the DNQDB data are now very comprehensive and reliable. This gives the opportunity to plan large-scale projects based on data and experience from the DNQDB. Currently, a national group of clinicians is planning to coordinate these projects. The first step in this process is to get access to more data from the Danish Medical Birth Registry.
Administrative issues and funding
Like most other national clinical quality databases in Denmark, the DNQDB is funded by the five Danish regions that administer and finance the public health care system in Denmark. Key information is reported immediately after birth by the attending midwife to the (national) Danish Medical Birth Registry, administered by the Danish Health and Medicines Authority. Subsequently, relevant data are drawn from the registry by the Registry Support Centres of Epidemiology and Biostatistics (South), which then refines it and performs relevant analyses based on the fixed formulas decided by the database steering committee.
Acknowledgments
This paper was funded by the Program for Clinical Research Infrastructure (PROCRIN) established by the Lundbeck Foundation and the Novo Nordisk Foundation, and administered by the Danish Regions.
Disclosure
The authors report no conflicts of interest in this work.
References
- KesmodelUSThe Danish National Indicator Project, Deliveries – Documentarist’s Report2010 3rd version 2012. Available from: http://www.kcks-vest.dk/siteassets/de-kliniske-databaser/fodsler/dokumentalistrapport-dkf-januar-2012_211211.pdfAccessed November 26, 2015 Danish
- KesmodelUSJølvingLMeasuring and improving quality in obstetrics – the implementation of national indicators in DenmarkActa Obstet Gynecom Scand20119010295304
- KnudsenLBOlsenJThe Danish Medical Birth RegistryDan Med Bull19984533203239675544
- The Danish Healthcare Quality Programme Available from: http://www.ikas.dk/DDKM.aspxAccessed November 26, 2015
- National Fetal Medicine Database Available from: http://www.dfms.dk/cms/index.php/fagligt/dbAccessed November 26, 2015
