Abstract
Background
Glucocorticoid receptor (GR) activity plays a role in many aspects of human physiology and may play a crucial role in chemotherapy resistance in a wide variety of solid tumors. A novel immunohistochemistry (IHC) based assay has been previously developed and validated in order to assess GR immunoreactivity in triple-negative breast cancer. The current study investigates the standardized use of this validated assay to assess GR expression in a broad range of solid tumor malignancies.
Methods
Archived formalin-fixed paraffin-embedded tumor bank samples (n=236) from 20 different solid tumor types were analyzed immunohistochemically. Nuclear staining was reported based on the H-score method using differential intensity scores (0, 1+, 2+, or 3+) with the percent stained (out of at least 100 carcinoma cells) recorded at each intensity.
Results
GR was expressed in all tumor types that had been evaluated. Renal cell carcinoma, sarcoma, cervical cancer, and melanoma were those with the highest mean H-scores, indicating high levels of GR expression. Colon, endometrial, and gastric cancers had lower GR staining percentages and intensities, resulting in the lowest mean H-scores.
Conclusion
A validated IHC assay revealed GR immunoreactivity in all solid tumor types studied and allowed for standardized comparison of reactivity among the different malignancies.
Impact
Baseline expression levels of GR may be a useful biomarker when pharmaceutically targeting GR in research or clinical setting.
Introduction
Glucocorticoid receptor (GR), a member of the steroid nuclear receptor superfamily, is responsible for modulating many processes, including cell homeostasis, cellular development, metabolic function,Citation1–Citation4 immune function,Citation5 and central nervous system and psychiatric function.Citation6,Citation7 In oncology, GR has been implicated in the development of cancer cell resistance by modulating the intracellular apoptosis balance point and influencing several well-documented cascade mechanisms.Citation8 GR modulation of gene expression is complex and involves the binding of ligand and receptor in the cytoplasm, dissociation of heat shock proteins, and homodimerization and translocation of the ligand/receptor complex to the nucleus.Citation3 The ligand/receptor complex then binds directly to the DNA and acts as a transcription factor for multiple gene products. This activity is further influenced by the complex environment of coactivator and corepressor molecules, which contribute additional effects to gene expression.Citation2,Citation6 Investigators have identified the role of GR in the development of chemotherapy resistance in experimental models involving tumors of epithelial origin, especially triple-negative breast cancer (TNBC), prostate cancer, and ovarian cancer.Citation9–Citation15
The addition of the GR agonist, dexamethasone, to cell line chemotherapy models reduces apoptosis and increases transcription of pro-cell-survival genes. These effects were reversed in the presence of the GR antagonist, mifepristone.Citation15–Citation17 A TNBC cell line MDA-MB-231 xenograft model demonstrated that addition of dexamethasone reduced cell death induced by paclitaxel, whereas the addition of mifepristone enhanced the efficacy of the taxanes.Citation16 The effect of mifepristone has been replicated in in vitro and in vivo experiments across multiple cancer cell lines and in combination with multiple chemotherapeutic agents.Citation14–Citation17
These in vitro data indicate that glucocorticoids, whether endogenous or exogenous, may cause expression of prosurvival/antiapoptotic genes and protect tumor cells from the effects of chemotherapy.Citation18,Citation19 Conversely, benefits of GR antagonists have been shown preclinically in several tumor types expressing GR including TNBC, ovarian, lung, and prostate cancer.Citation9–Citation14,Citation16,Citation17,Citation20 In addition to the preclinical evidence,Citation16 clinical evidenceCitation21–Citation23 suggests that GR expression plays a substantial role in TNBC. These findings have considerable clinical implications as the coadministration of glucocorticoids is common to counteract hypersensitivity to various treatments as well as nausea.
An immunohistochemistry (IHC) assay has been developed and validated for evaluating GR expression in TNBC.Citation24 Several clinical trials are currently evaluating GR antagonists in conjunction with chemotherapy in patients with TNBC.Citation25 These studies further measure GR tumor expression using the validated IHC assay. During development of the previously reported TNBC assay, 50 archival, formalin-fixed paraffin-embedded (FFPE) tissue samples of TNBC from individual treatment-naïve patients, collected as diagnostic tissue blocks and stored in a commercial tissue bank, were evaluated. While the earlier literature suggested rates of GR positivity of ~25%–50% in TNBC samples,Citation26–Citation28 rates based on the IHC assay were ≥80%Citation24 using a minimum cutoff of 10% tumor cells staining positively.
The objective of this exploratory GR expression study was to extend this research beyond TNBC by using the previously developed IHC GR assay to survey the degree of GR expression across other tumor types. Little is known about rates of GR expression in other tumor types even though the role of GR in chemotherapy resistance has been suggested in many tumors of epithelial origin.Citation9–Citation13 As understanding of the role of GR in oncology progresses, the findings from this study may guide decisions about how to successfully introduce GR antagonists into clinical testing.
Methods and materials
GR IHC protocol
For each of the 20 selected tumor types, archival FFPE tumor bank tissues were randomly chosen for this study. With the exception of the pancreatic cancer samples that were purchased from Pantomics Inc. (Richmond, CA, USA), all other tissues samples were obtained from the QualTek tissue bank (QualTek Molecular Laboratories, Newtown, PA, USA). These tumor tissues were ethically acquired from various clinical sites with diverse patient populations with personal identifiers redacted.
The FFPE tumor tissues were sectioned (4–5 μm) onto slides with a positive charge (Fisher ProbeOn Plus™; Thermo Fisher Scientific, Waltham, MA, USA) and dry heated for 1 h at 65°C within 1 week of testing. Deparaffinization included a series of four 100% xylene changes followed by rehydration with a graded series of ethanol (100%, 70%, 30%) to distilled water.
Based on a previous publication, rabbit monoclonal antibody anti-GR (D8H2; Cell Signaling Technology [#3660S], Danvers, MA, USA), was chosen from three different GR antibody candidates for further assay development and validation.Citation24
Antigen retrieval consisted of a 20-min incubation of slides in Citra Plus Target Retrieval Solution (BioGenex [catalog number HK080-9K], Fremont, CA, USA), heated to 98°C with a commercial steamer (Black and Decker HS1000 model steamer; Black and Decker, Baltimore, MD, USA). Following a 5-min cool down, slides were transferred onto an automated IHC platform (TechMate™ 500 or 1000 with WorkMate software version 3.96; Roche Diagnostics, Oro Valley, AZ, USA). All reagent changes were automated, including all detection kit steps (Rabbit Polink2+ HRP and DAB chromogen; Golden Bridge International, [catalog numbers D39-110 and C09-100], Bothell, WA, USA), a protein blocking step (QualTek proprietary reagent), primary antibody incubation (anti-GR [D8H2]; Cell Signaling Technology [catalog number 3660S]; diluted to 1:1,750 in primary antibody diluent [QualTek proprietary]), a peroxidase blocking step (3% United States Pharmacopeia H2O2, with ~0.02% v/v Tween® 20 detergent; Thermo Fisher Scientific), hematoxylin counterstaining and all intervening washes (tris-buffered saline containing 0.02% v/v Tween 20), by a capillary gap processCitation29 at room temperature (25°C) using the previously optimized assay conditions.Citation24 The slides were then dehydrated in a series of ethanol (95%, 100%) and 100% xylene changes, and mounted with a coverslip (Cytoseal™ XYL mounting media, Thermo Fisher Scientific).
Evaluation of GR expression in various tumors
The assay was previously validatedCitation24 in a Clinical Laboratory Investigation Amendment (CLIA)-accredited facility (QualTek Clinical Laboratories). GR expression levels, detected by the validated IHC assay, were evaluated in a panel of 20 tumor types () using archival, treatment-naïve, FFPE tissue samples (individual or within tissue microarrays [TMAs]). Tissues included bladder cancer (n=10), breast cancer (non-TNBC) (n=10), cervical cancer (n=15), colon cancer (n=16), endometrial cancer (n=13), esophageal cancer (n=8), gall bladder cancer (n=10), gastric cancer (n=11), glioblastoma (n=8), head and neck cancer (n=10), hepatocellular carcinoma (n=10), lung cancer (n=17), melanoma (n=11), mesothelioma (n=8, including TMA [n=4; four cores each, 1.5 mm; US Biomax, Inc., catalog number T392a, Rockville, MD, USA]), neuroendocrine cancer (n=11), ovarian cancer (n=11), pancreatic cancer (n=16, including TMA [two cores each, 2 mm]; Pantomics Inc. [catalog number PAC481]), prostate cancer (n=11), renal cell carcinoma (RCC) (n=10), and sarcoma (n=14).
Table 1 Solid tumor types evaluated using glucocorticoid receptor IHC sensitivity screening
GR staining is sensitive to poor tissue fixation, common among nuclear antigens, and may result in a potential false-negative IHC result. However, because GR strongly stains stromal cells (which act as an internal positive control), areas of poor fixation that would contribute to a false-negative result are easily identifiable. In such cases, those regions were not scored or the samples were rejected.
Tissue sections were scored for nuclear staining within tumor cells across the total evaluable area of the tissue. Staining within the cytoplasm of tumor cells or stroma was not scored. Necrotic regions, in situ carcinoma, and poorly fixed regions of the tissue were also excluded from scoring. GR reactivity was scored using differential intensity scores (0, null; 1+, low or weak; 2+, moderate; 3+, high or strong). A total percentage score (% of tumor cells staining ≥1+ intensity; ie, the sum of the percentage of cells at 1+, 2+, and 3+ intensities) was used to semiquantitatively evaluate tumor expression of GR. Only samples with at least 100 invasive carcinoma cells were included. The H-score, which numerates staining ratios with respect to both intensity and frequency, was used to capture the pattern of nuclear staining observed. The H-score was calculated using the following formula: H-score = [(% at 0)×0]+[(% at 1+)×1]+[(% at 2+)×2]+ [(% at 3+)×3]. The H-score produces a continuous variable that ranges from 0 to 300.
Results
GR staining was evaluated in 20 different tumor types () with 236 total samples analyzed and 5–16 samples per tumor type (). Although GR staining is generally broad and is observed in most normal tissue, stromal staining served as an internal positive control to help identify samples to reject or regions to avoid during the scoring process. Positive and negative controls were included in all tests and were evaluated for appropriate reactivity before GR staining was evaluated.
Table 2 Immunohistochemistry scoring for glucocorticoid receptor status in 20 tumor types
GR reactivity was observed in the nuclei of all tumor cell types examined and was highly expressed in the majority of tumors () with little to no cytoplasmic staining. Representative micrographs showing a range of staining intensities are provided in .
Figure 1 Representative micrographs of immunohistochemical GR staining in various tumor types showing variations in levels of staining.
Abbreviation: GR, glucocorticoid receptor; TNBC, triple-negative breast cancer.
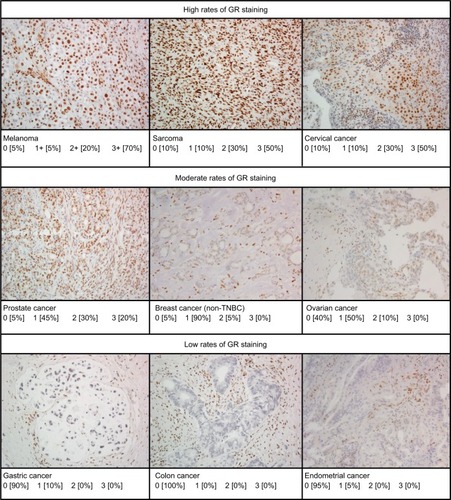
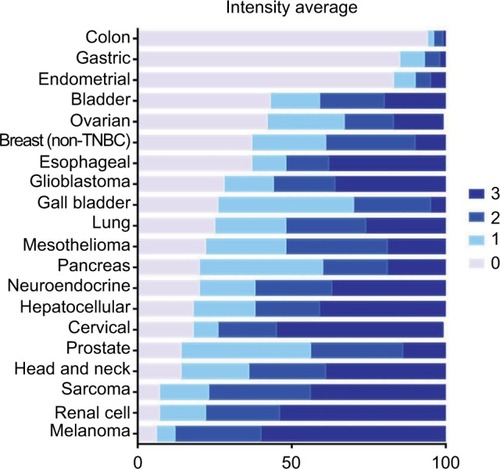
With GR staining depicted as a fraction of cells with 0, 1+, 2+, and 3+ staining, melanoma, RCC, and sarcoma tumor samples show a high fraction of 2+ and 3+ staining, and few samples (<10%) show 0 staining. In contrast, >80% of colon, gastric, and endometrial cancer samples had no GR staining. For the other tumors evaluated, at least 50% of the tumors expressed 1+ to 3+ staining.
Evaluating GR expression of the tumor types by H-score (accounting for both intensity and frequency) versus frequency of GR positivity showed similar results ( and ). Several cancer types have high mean and median H-scores along with high variability of H-scores within tumor types. An exception is seen with melanoma, cervical cancer, RCC, and sarcoma tumor types. Their H-scores were high with little variability and the GR staining was uniformly high. Colon, gastric, and endometrial cancer were distinctly different from the other tumors by virtue of their very low GR staining intensity fraction and H-score.
Figure 2 Glucocorticoid receptor positivity by mean H-score.
Abbreviation: TNBC, triple-negative breast cancer.
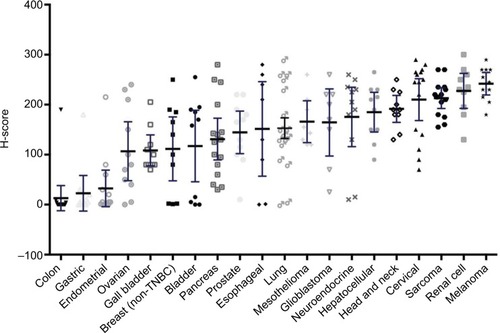
Figure 3 Average percent staining of glucocorticoid receptor at various intensities.
Abbreviation: TNBC, triple-negative breast cancer.
The limited histologic subtypes that were analyzed (data not shown) indicated that GR expression varies by tumor subtype. This analysis showed that small-cell lung cancer (SCLC) has very low GR expression (mean H-score 81, range 0–230), whereas non-small-cell lung cancer (NSCLC) adenocarcinoma and squamous cell carcinoma have high GR expression (mean H-score 193, range 140–290, and 173, range 120–260, respectively). In the samples studied, the subtypes of the RCC were limited to clear cell type, all of high GR staining. The sarcoma tumor group included soft tissue sarcomas only, also all of high GR staining.
Discussion
GRs play an important role in tumor response to microenvironment, and GRs have been implicated in tumor cell survival and response to chemotherapy.Citation12–Citation15,Citation17,Citation18,Citation20 To date, GR expression has been reported only within a limited number of solid tumor types (eg, breast and colon),Citation26–Citation28,Citation30 and assay results have been highly variable. Hence, the clinical relevance of the results in the literature is difficult to interpret. A CLIA-validated,Citation24 IHC-based, GR assay has been developed and assessments of 20 different tumor types have been standardized with more than 200 samples. Using surrounding tissues (ie, positively staining fibroblasts, endothelial cells, and a subset of lymphocytes) as an internal stromal control provides high confidence of antibody specificity to GR protein. The antibody used in this assay, D8H2, is both specific and sensitive to the Leu368 region of both alpha and beta isoforms of the GR proteinCitation24 without cross-reactivity to the mineralocorticoid receptor and nonspecific binding.
GR reactivity was observed in all 20 tumor types that had been evaluated, many of which had no prior published GR expression rates. GR staining was detectable at a wide range of intensities across and within the various tumor types.
Applying the validated GR IHC assay, it was observed that GR expression varies by tumor type. Among the tumors with an overall high degree of GR staining, some tumors were consistently high staining (clear cell RCC, soft tissue sarcoma, melanoma, and cervical cancer), and others showed great variability among individual samples. Colon, gastric, and endometrial cancer have very low GR staining by intensity and H-score, with the majority of samples showing no GR expression. As seen with the lung cancer samples evaluated, GR expression was different for SCLC and NSCLC. Further analyses of GR expression by other tumor subtypes is needed.
As with most established tumor receptors that have been successfully targeted, much effort has been placed on correlating clinical outcomes with target expression levels and activity. The definition of what is considered “positive” for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 researchCitation31,Citation32 has been modified over time. The definition of “positive” for both ER and PR decreased over time from ≥10% of tumor cells staining positive to ≥1%.Citation31 Similar efforts are currently ongoing with programmed cell death protein 1 (PD1) and PD1-ligand 1 (PD-L1) therapies. Thus, the data were presented as intensity and frequency of staining across the 20 tumor types, without applying a threshold cutoff.
The wide range of staining intensity and H-scores seen warrants further research to understand the role of GR expression, both as a prognostic factor and as a predictive factor in the response to chemotherapy. For some tumor types (melanoma, sarcoma, and RCC), GR assessment may not be necessary due to the consistently high staining intensity and H-scores. Research is needed to determine if tumors with low staining and H-scores (colon, gastric, and endometrial cancers) are good candidates for GR-targeted therapeutics.
With the introduction of more selective GR antagonists, GR may become a relevant therapeutic target. Hence, this validated assay may provide new opportunities in developing companion diagnostic and predictive assays for GR expression. Establishing the optimal threshold for GR expression qualitatively or quantitatively that correlates with clinical benefit in patients with specific tumor types will require larger studies with clinical endpoints. An initial assessment of GR as a therapeutic target and predictive factor is ongoing in a clinical trial testing the selective GR modulator CORT125134 in combination with nab-paclitaxel in solid tumors (NCT02762981).
The current assessment of GR positivity include the limited sample size and absence of subtyping within a tumor type. Furthermore, assessment of single sections of an FFPE sample may not completely represent GR distribution within the tumor and may only reflect a portion of a tumor. Also, GR expression may evolve in response to anticancer therapy and be further influenced by the use of concomitant medication, particularly those including steroids. This validated assay provides the first steps of assessing GR as a clinical surrogate and pharmacological target. Further studies will be needed to determine the most robust technical method to prepare samples through the course of treatment and to determine which quantitative assessment provides the strongest correlation with receptor expression (eg, GR protein correlation with mRNA levels) and clinical outcomes.
Conclusion
GR expression using a validated IHC assay showed that GR is expressed in multiple different solid tumor malignancies, and may be a predictive tool to guide the clinical development of GR antagonists. Further clinical correlation will be needed to determine, within individual tumor types, what the optimal thresholds for GR expression will be for patient selection and stratification.
Acknowledgments
The authors thank Ruth Ann Gover of Corcept Therapeutics for administrative assistance in assembling the references; Grayce Fjeld of QualTek Molecular Laboratories for managing project logistics during conduct of the assays and obtaining the IHC images included in this manuscript; and Caren Rickhoff, MWC, of MedGraphica Medical Writing Services for providing medical writing and editorial assistance. This manuscript was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company-Sponsored Medical Research: the GPP3 Guidelines” and the International Committee of Medical Journal Editors’ “Uniform Requirements for Manuscripts Submitted to Biomedical Journals.” Funding for the preparation of this manuscript was provided by Corcept Therapeutics. The authors did not receive any grants in support of writing this manuscript.
Disclosure
TSB and DPN are employees of Corcept Therapeutics, Menlo Park, CA, USA. Corcept Therapeutics sponsored the research reported here. TIM and FJL are employees of QualTek Molecular Laboratories, contracted to perform the assays. The authors report no other conflicts of interest in this work.
References
- SapolskyRMRomeroLMMunckAUHow do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actionsEndocr Rev2000211558910696570
- KadmielMCidlowskiJAGlucocorticoid receptor signaling in health and diseaseTrends Pharmacol Sci201334951853023953592
- RhenTCidlowskiJAAntiinflammatory aciton of glucocorticoids—New mechanisms for old drugsN Engl J Med2005353161711172316236742
- ColeTJBlendyJAMonaghanAPTargeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturationGenes Dev19959160816217628695
- BaschantUTuckermannJThe role of the glucocorticoid receptor in inflammation and immunityJ Steroid Biochem Mol Biol2010120697520346397
- MoraitisAGBlockTNguyenDBelanoffJKThe role of glucocorticoid receptors in metabolic syndrome and psychiatric illnessJ Steroid Biochem Mol Biol201716511412027002803
- JuddLLSchettlerPJBrownESAdverse consequences of glucocorticoid medication: psychological, cognitive, and behavioral effectsAm J Psychiatry2014171101045105225272344
- SchlossmacherGStevensAWhiteAGlucocorticoid receptor-mediated apoptosis: mechanisms of resistance in cancer cellsJ Endocrinol2011211172521602312
- HerrIUcurEHerzerKGlucocorticoid cotreatment induces apoptosis resistance toward cancer therapy in carcinomasCancer Res200363311212012810637
- SchmidtSRainerJPlonerCPresulERimlSKoflerRGlucocorticoid-induced apoptosis and glucocorticoid resistance: molecular mechanisms and clinical relevanceCell Death Differ200411S45S5515243581
- GasslerNZhangCWengerTDexamethasone-induced cisplatin and gemcitabine resistance in lung carcinoma samples treated ex vivoBr J Cancer2005921084108815756274
- PangDKocherginskyMKrauszTKimS-YConzenSDDexamethasone decreases xenograft response to paclitaxel through inhibition of tumor cell apoptosisCancer Biol Ther20065893394016775428
- ConzenSDNuclear receptors and breast cancerMol Endocrinol200822102215222818417735
- IsikbayMOttoKKregelSGlucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancerHormone Cancer2014527289
- ZhangCBeckermannBKallifatidisGCorticosteroids induce chemotherapy resistance in the majority of tumour cells from bone, brain, breast, cervix, melanoma and neuroblastomaInt J Oncol2006291295130117016664
- SkorMNWonderELKocherginskyMGlucocorticoid receptor antagonism as a novel therapy for triple-negative breast cancerClin Cancer Res201319226163617224016618
- Stringer-ReasorEMBakerGMSkorMNGlucocorticoid receptor activation inhibits chemotherapy-induced call death in high-grade serous ovarian carcinomaGynecol Oncol201513865666226115975
- GrossKLLuNZCidlowskiJAMolecular mechanisms regulating glucocorticoid sensitivity and resistanceMol Cell Endocrinol200930071619000736
- SmithLKCidlowskiJAGlucocorticoid-induced apoptosis of healthy and malignant lymphocytesProg Brain Res201018213020541659
- Gruver-YatesALCidlowskiJATissue-specific actions of glucocorticoids on apoptosis: a double-edged swordCells2013220222324709697
- PanDKocherginskyMConzenSDActivation of the glucocorticoid receptor is associated with poor prognosis in estrogen receptor-negative breast cancerCancer Res201171206360637021868756
- NandaRChennamaneniPStringerEA randomized phase I trial of nanoparticle albumin bound paclitaxel (Agraxane) with or without mifepristone for advanced breast cancerSan Antonio Breast Cancer Symposium2013
- NandaRStringer-ReasorEMSahaPA randomized phase I trial of nanoparticle albumin-bound paclitaxel with or without mifepristone for advanced breast cancerSpringer Plus2016594727386391
- BakerGMMurphyTBlockTNguyenDLynchFJDevelopment and validation of an immunohistochemistry assay to assess glucocorticoid receptor expression for clinical trials of mifepristone in breast cancerCancer Manag Res2015736136826673410
- ClinicalTrials.govStudy identifiers: NCT02014337, NCT01493310, NCT02788981, and NCT02046421 Available from: https://clinicaltrials.gov/Accessed October 11, 2016
- AbduljabbarRNegmOHLaiC-FClinical and biological significance of glucocorticoid receptor (GR) expression in breast cancerBreast Cancer Res Treat201515033534625762479
- BuxantFEngohan-AlogheCNoelJ-CEstrogen receptor, progesterone receptor, and glucocorticoid receptor expression in normal breast tissue, breast in situ carcinoma, and invasive breast cancerAppl Immunohistochem Mol Morphol20101825425719875955
- BelovaLDelgadoBKocherginskyMMelhemAOlopadeOIConzenSDGlucocorticoid receptor expression in breast cancer associates with older patient ageBreast Cancer Res Treat2009116444447
- ReedJAManahanLJParkC-SBrigatiDJComplete one-hour immunocytochemistry based on capillary actionBiotechniques19921334344431389176
- LienH-CLuY-SShunC-TYaoY-TChangW-CChengA-LDifferential expression of glucocorticoid receptor in carcinomas of the human digestive systemHistopathology20085231432418269582
- HammondMEHHayesDFDowsettMAmerican Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancerArch Pathol Lab Med201013490792220524868
- WolffACHammondMEHHicksDGRecommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline updateArch Pathol Lab Med2014138224125624099077
