Abstract
Renal function is an important consideration in the management of patients with advanced cancer. There is a reciprocal relationship between cancer and the kidney: chronic kidney disease can increase the risk of developing cancer, and patients with cancer often experience renal impairment owing to age, disease-related factors and nephrotoxic treatments. As therapies for cancer continue to improve, patients are living longer with their disease, potentially extending the period over which they are susceptible to long-term complications. Furthermore, secondary symptoms, such as bone metastases or infections, may arise that will require treatment. Certain treatments, including chemotherapy, antibiotics and some bone-targeted agents, are nephrotoxic and may require dose modifications or interruptions to prevent renal injury. Nephrologists should play a key role in the identification and management of renal impairment in patients with cancer. Furthermore, they may be able to provide advice on protecting the kidneys in instances where nephrotoxic agents require dose reductions or interruptions, and when novel therapies or combinations are used. Collaboration between oncologists and nephrologists is important to optimal patient management. This article reviews the relationship between cancer and kidney disease and examines the treatments that may impact kidney function. Considerations for monitoring renal function are also discussed.
Introduction
Advances in health care and treatment options have increased survival times for patients with different solid tumor types. In the UK and the USA, patients with prostate and breast cancer have an estimated 5-year relative survival rate of 28%–100%Citation1,Citation2 and 22%–100%,Citation3,Citation4 respectively, depending on the disease stage at diagnosis. Although the relative survival rate of patients with lung cancer is less than that of those with breast or prostate cancer, up to 49% will survive for 5 years or more.Citation5–Citation7 The consequences of improved survival include the increased incidence of complications associated with the disease process, such as bone metastases, Citation8–Citation11 and adverse events that may arise as a result of long-term cancer treatments,Citation12,Citation13 all of which may increase the risk of renal injury. Kidney function is, therefore, an important consideration when cancer is present, and understanding the connection between the two is important for the optimal disease management. Here, we review the relationship between cancer and kidney disease, and examine the treatments that may impact kidney function. Considerations for monitoring renal function are also discussed.
Cancer and kidney disease
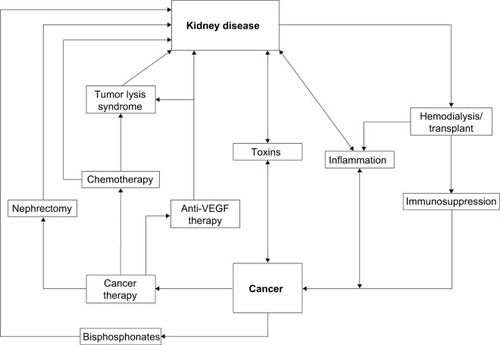
Kidney disease frequently coexists with advanced cancer because of their shared risk factors and bidirectional causal mechanisms. The reciprocal relationship between cancer and kidney disease is summarized in and discussed in detail in the following section.Citation14–Citation16
Figure 1 The reciprocal relationship between cancer and kidney disease.
Abbreviation: VEGF, vascular endothelial growth factor.
Diagnosis of kidney disease
Chronic kidney disease (CKD) is usually asymptomatic in the early stages, but can be detected using laboratory tests. Kidney function is commonly assessed by means of the estimated glomerular filtration rate (eGFR). Measurement of serum creatinine levels, in combination with age, sex, weight or race, is used to calculate eGFR. The formal definition of CKD requires the presence of a reduced eGFR of <60 mL/min per 1.73 m2 for at least 90 days.Citation17 Frequently in the clinical setting, however, a reduced eGFR may be noted on one occurrence without knowledge of its duration – we, hereafter, refer to this as renal impairment (RI), to denote a single reading of eGFR of <60 mL/min per 1.73 m2, without designation of its chronicity.Citation18–Citation20
Two equations are routinely used for the estimation of eGFR based on serum creatinine levels: the Modification of Diet in Renal Disease (MDRD) equation and the recently developed Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, which may be more accurate than the MDRD equation ().Citation20,Citation21 These equations are suitable for use in a broad patient population, including elderly individuals. The Kidney Disease: Improving Global Outcomes (KDIGO) working group does not recommend using one single equation for estimation of renal function, although it recognizes that the CKD-EPI equation may be more precise at higher GFRs and may, therefore, be more applicable than the MDRD equation for day-to-day practice.Citation18 Historically, creatinine clearance (CrCl) has been used as a marker of GFR and is determined from serum creatinine and urine creatinine levels.Citation18 Current product labeling guidance from the US Food and Drug Administration (FDA) for studies of patients with impaired renal function states that dose adjustment categories can be described based on CrCl or eGFR.Citation22
Table 1 Equations used to estimate GFR
Measurement of eGFR alone may be insufficient to determine kidney function because there is often a discrepancy between serum creatinine levels and the CrCl rate, and it should, therefore, be used in conjunction with other renal assessments.Citation23,Citation24 In patients with low body mass index (<18.5 kg/m2), such as cachectic patients with cancer, alternative approaches may be used to provide reliable estimates of renal function; for instance, urine CrCl measured over a 24-hour period or cystatin-based measurements.Citation21 Cystatin C level can be used to estimate GFR; a meta-analysis found it to be superior to using serum creatinine level for this purpose.Citation25 Although cystatin C measurement may offer some advantages over serum creatinine-based evaluations, the assay can be costly and there is a potential lack of standardization across laboratories. Therefore, the KDIGO working group does not currently recommend use of this assay over serum creatinine-based methods.Citation18
Beyond reduction in eGFR, symptoms of kidney disease can include proteinuria, hematuria or albuminuria, or renal tubular abnormalities leading to electrolyte wasting, all of which can be detected using laboratory tests.Citation17
Risk factors in common for kidney disease and cancer
Age is a major risk factor for kidney disease. Decline of renal function is common in the elderly general population; by the age of 70 years, renal function may have declined by up to 40%.Citation26 More than a third of individuals in the healthy population aged over 70 years have moderately impaired renal function.Citation27 The physiological changes associated with aging, including diminished renal mass, reduction in renal blood flow and loss of nephron function,Citation28 contribute toward decreased renal activity. Furthermore, as individuals age, they are likely to acquire an increasing number of comorbidities.Citation29 Thus, it is unsurprising that patients with cancer, who are often elderly, may have some degree of RI.Citation30,Citation31 In the French Renal Insufficiency and Anticancer Medications (IRMA) national, retrospective, observational study of patients with solid tumors, there was a high incidence of RI (65.2%) in a group of 1553 patients aged ≥65 years.Citation31 The prevalence of RI increased with age (65–74 years, 61.7%; 75–84 years, 74.7%).Citation31 RI occurred frequently in a retrospective study of 11,809 USA patients with bone metastases from solid tumors (mean age 66.9 years); the 5-year prevalence of RI was 43%.Citation32 In a retrospective study of elderly Brazilian patients (aged >65 years) with solid tumors, 66% were found to have abnormal renal function.Citation30
Type II diabetes mellitus is one of the most common causes of kidney disease in the USA and is itself a risk factor for several types of cancer.Citation15 The association between diabetes and cancer is further complicated by the fact that the majority of patients with diabetes are overweight or obese,Citation14 which increases their risk of developing several different cancer typesCitation33 and kidney disease.Citation34 Other identified risk factors for CKD include physical inactivity,Citation35 smoking, hypertension,Citation36 cardiovascular disease and being male,Citation34 all of which pose an increased risk of developing certain cancers.Citation15
CKD as a risk factor for cancer
The relationship between cancer and kidney function encompasses more than age and comorbidities (). There is an increased risk of certain cancers in individuals with CKD,Citation16 and the association is most commonly observed in those with renal cell carcinoma or urothelial cancers.Citation37 Patients receiving renal replacement therapies, such as dialysisCitation16,Citation38 or transplantation,Citation16,Citation39 also have an increased risk of developing cancer. In the UK in 2010, cancer was the most common cause of death in patients who had been given a kidney transplant (23%).Citation40 Immunosuppression may contribute to an increased risk of cancer, which can be caused by pre- or posttransplantation treatment with immunosuppressants,Citation41 or by endogenous immunosuppression owing to uremia.
The underlying mechanisms for the association between kidney disease and cancer are not well understood and may be multifactorial. However, CKD can cause an inflammatory microenvironmentCitation42 that may encourage cancer development. Albuminuria, which frequently accompanies inflammatory states, is itself associated with an increased incidence of cancer, in particular urothelial or lung cancer, independent of renal function.Citation43 Vitamin D deficiency is highly prevalent among people with moderately reduced kidney function and, in the general population, has been associated with the risk of developing breast, colon and prostate cancer. The mechanisms to explain this association are, however, yet to be elucidated.Citation44
Cancer as a cause of kidney disease
The natural history of cancer may lead to kidney disease via various mechanisms. Acute kidney injury is common in patients with tumors of the kidney or liver, and in those with multiple myeloma,Citation45 and can be caused by intravascular volume depletion due to sepsis, vomiting or diarrhea, toxins or accumulation of M-protein in multiple myeloma.Citation46 Physical obstruction of the bladder or urethra, often observed in patients with tumors of the bladder, uterus, prostate and cervix, can also result in acute kidney injury.Citation46 Nephrotic syndrome may be caused by the invasion of the glomeruli by cancerous cells.Citation47 Another complication of cancer is tumor lysis syndrome, which results in metabolic disruption and can be caused by rapid tumor cell turnover, or because of chemotherapy-induced cell lysis.Citation48 Cellular release of metabolites, such as potassium, phosphate and citric acid, can lead to the deposition of uric acid and calcium phosphate in the renal tubules, causing acute renal failure, a condition that is further exacerbated in cases of concomitant intravascular volume depletion.Citation48
Patients with advanced cancer often develop bone metastases, particularly those with lung, breast and prostate cancer. Bone metastases disrupt normal bone turnover and can cause an imbalance in calcium levels resulting in hypercalcemia of malignancy, affecting 10%–30% of patients with cancer.Citation49 Prolonged elevated calcium can cause kidney damage and untreated severe hypercalcemia of malignancy can be fatal.Citation49
Hypercalcemia is a key criterion for the diagnosis of multiple myeloma.Citation50 It is caused by tumor-induced bone destruction and the formation of bone lesions, which together contribute to elevated calcium levels.Citation51 Furthermore, the clonal expansion of plasma cells and subsequent excessive production of immunoglobulin light chains can cause cast nephropathy, further damaging the kidney.Citation52
Given that RI is common in patients with advanced cancer, oncologists need to be aware that certain cancer treatments affect the kidney.Citation13 These are discussed in detail in the following section.
Prevalence of kidney disease in advanced cancer
The USA database study found that the 5-year prevalence of RI in patients with solid tumors and bone metastases was 43%, and up to 71% in those with CKD.Citation32 In the large IRMA study of 4684 patients with a range of solid tumors, over half of patients with breast, prostate or lung cancer had abnormal renal function.Citation53–Citation55 Renal failure can occur in patients with advanced cancer, affecting 0.4% of women with breast cancer and bone metastases.Citation55 This is more common in patients with prostate cancer, with one database study reporting that 12% of men with prostate cancer had renal failure in the final year of life.Citation56
Considering renal risk while managing cancer
Assessing renal risk in patients with cancer
It is reasonable to evaluate renal function in all patients with cancerCitation23,Citation24,Citation57 because RI has been detected in almost two-thirds of patients with cancer who have normal serum creatinine levels.Citation24 Given that kidney disease is common in patients with cancer, it is important to understand a patient’s renal function in order to assess the risk of further kidney injury that could occur owing to the nephrotoxic effects of some cancer treatments.
Guidelines recommend regular renal monitoring for patients receiving potential nephrotoxic agents.Citation18,Citation23,Citation58,Citation59 For elderly patients with cancer, the International Society of Geriatric Oncology recommends that renal function should be assessed and hydration should be optimized before drug treatment.Citation23 The IRMA investigators recommend that CrCl is calculated for patients who are receiving nephrotoxic drugs that require dose adjustments in order that their dosage is prescribed according to patients’ levels of renal function. These patients should receive adequate hydration and be monitored closely for a decline in renal function.Citation23,Citation24 The use of antiresorptive therapies for the management of bone metastases is discussed in detail in the following section.
Nephrotoxicity associated with the management of cancer
Diagnosis
Imaging is often used to locate tumors or in the assessment of disease stage. Computed tomography (CT) is the preferred method for identifying renal cell carcinoma and metastases, but is associated with a risk of contrast-induced acute kidney injury, caused by the use of iodinated contrast medium.Citation60 The incidence of kidney injury in this setting is low in the general population (2%); however, in patients with risk factors for kidney disease it increases up to 50%.Citation60 Magnetic resonance imaging is often used to detect tumors; although it is less toxic than CT it also carries a potential risk of kidney injury owing to the use of contrast media often containing gadolinium.Citation61 Therefore, before renal imaging, physicians should consider a patient’s baseline risk of kidney injury and should use prophylaxis with intravenous fluids as an effective intervention. In addition, physicians should consider the use of prophylactic N-acetylcysteine, an agent that has been shown to prevent radiocontrast-induced nephropathy.Citation60
Chemotherapy
Most commonly used chemotherapies are excreted through the kidneys and have known nephrotoxicity.Citation62 Cisplatin is a major antineoplastic alkylating agent used for the treatment of solid tumors. However, acute kidney injury has been reported in 20%–30% of patients receiving cisplatin therapy.Citation63 The nephrotoxicity associated with cisplatin increases in a dose-dependent fashionCitation64 and may be managed with amifostine, an agent that protects tissue from chemotherapy-induced damage.Citation65 Other alkylating agents such as ifosfamide and cyclophosphamide are also nephrotoxic and may have long-term renal effects. For example, survivors of childhood cancers had significantly lower eGFRs in adulthood if they had received high-dose cisplatin (83 vs 101, p=0.004) or high-dose ifosfamide (88 vs 98, p=0.02) than those who had not received such treatments.Citation66 Ifosfamide treatment of solid tumors is also associated with long-term nephrotoxicity in adults.Citation67 Ifosfamide can enter the kidney tubules via organic cation transporters and cause proximal tubular injury. Chloracetaldehyde is a toxic metabolite of ifosfamide that injures kidney tissue.Citation62 Owing to differences in metabolism, cyclophosphamide treatment produces much less chloracetaldehyde and is therefore substantially less nephrotoxic than ifosfamide, although another metabolite, acrolein, can lead to urotoxicity with hemorrhagic cystitis.Citation62 Beyond those issues, cyclophosphamide has also been rarely associated with the syndrome of inappropriate antidiuresis hormone secretion (SIADH).Citation62,Citation68 The mechanism of action of cyclophosphamide-related SIADH has not been determined, but it may be caused by a direct toxic effect of cyclophosphamide or its metabolites on renal collecting tubules, or an antidiuretic hormone-like activity of cyclophosphamide metabolites.Citation68
Methotrexate is another widely used chemotherapy that is associated with nephrotoxicity.Citation32 Indeed, up to 12% of patients who received high-dose methotrexate for the treatment of various cancers have experienced acute kidney injury.Citation62 Furthermore, renal injury can impair clearance of methotrexate, leading to accumulation and further toxicity. Toxicity may be managed using the tissue-protectants leucovorin, or carboxypeptidase-G2, or hemodialysis.Citation69
Melphalan is often administered to patients with multiple myeloma, a disease often associated with RI.Citation70 Melphalan clearance may be decreased in patients with RI and subsequent progression from RI to renal failure has been reported in a patient receiving treatment for multiple myeloma.Citation71 Dose reductions of 50% are recommended for patients with moderate RI (CrCl 30–50 mL/min) who are not scheduled to receive hematopoietic stem cell transplantation (HSCT), but it should not be used in patients with more severe RI (CrCl <30 mL/min; ).Citation72 Nonetheless, high-dose melphalan with HSCT has been used successfully in patients with end-stage renal failure who are receiving dialysis.Citation72,Citation73
Table 2 Label recommendations for common nephrotoxic agents used in cancer therapy
Antivascular endothelial growth factor receptor therapies
The use of agents that target the vascular endothelial growth factor (VEGF) pathway is increasing in the treatment of certain solid tumors, including renal cell carcinoma, non-small cell lung cancer, gastrointestinal stromal tumors and colorectal cancer.Citation74 The humanized monoclonal antibody bevacizumab was one of the first VEGF inhibitors to be used in clinical practice for the treatment of solid tumors, but its use is associated with a significantly increased risk of proteinuria and hypertension.Citation75 In a phase 4, open-label study in patients with lung cancer, grade 3 or higher proteinuria was reported in 3% of patients and hypertension was reported in 6% of patients who received bevacizumab.Citation76 Other VEGF receptor targets, such as the tyrosine kinase inhibitors sorafenib, sunitinib and pazopanib, are used for the treatment of patients with cancer, but are associated with similar adverse vascular effects.Citation74 A meta-analysis of 16 clinical trials of patients with solid tumors has shown that the risk of developing proteinuria was 41% higher with the human recombinant fusion protein aflibercept compared with controls; furthermore, this risk was substantially greater with aflibercept than with bevacizumab (85%).Citation77
Immuno-oncology therapies
The immuno-oncology therapies nivolumab and pembrolizumab are monoclonal antibodies that have both recently been approved to treat advanced melanoma.Citation78,Citation79 Nivolumab is also approved for the treatment of locally advanced or metastatic nonsmall cell lung cancer after prior chemotherapy and as a monotherapy for the treatment of advanced renal cell carcinoma after prior therapy.Citation78 These compounds are currently under investigation in men with prostate cancer. However, in phase 3 studies, the use of nivolumab monotherapy or nivolumab in combination with ipilimumab is associated with immune-related nephritis and renal dysfunction (3.2% [55/1728] and 4.2% [19/448], respectively).Citation78 It is therefore recommended that patients should be monitored for signs and symptoms of nephritis or renal dysfunction. Treatment should be withheld for patients with mild-to-moderate renal dysfunction (from 50% up to 300% increase from a normal baseline serum creatinine) until normalization, with discontinuation recommended in more severe cases.Citation78 Immune-related nephritis has also been reported for a small number of patients in phase 3 studies receiving pembrolizumab (0.4% [7/1567]); patients should be monitored for changes in renal function, with treatment interrupted or discontinued for indications described above.Citation79
Androgen deprivation therapy (ADT)
ADT is commonly used for the treatment of prostate cancer. However, its use has been associated with an increased risk of acute kidney injury; a large database study calculated an odds ratio of sustaining acute kidney injury of 2.48 (95% confidence interval, 1.61–3.82) with ADT treatment versus controls.Citation80 ADT may cause dyslipidemia or result in metabolic syndrome, all of which can contribute to kidney injury.Citation80 Furthermore, patients who received ADT as part of a combination therapy had the highest risk of acute kidney injury, suggesting that ADT may potentiate the nephrotoxicity of other agents.Citation80
Antiresorptive therapies
Bone metastases are common in patients with advanced solid tumors, such as those affecting the breast, prostate or lung (~70%–90% of patients with these cancers have evidence of metastatic bone disease),Citation11,Citation81 therefore patients may require antiresorptive drugs such as bisphosphonatesCitation82,Citation83 and denosumab,Citation84–Citation87 for the prevention of skeletal complications of bone metastases secondary to solid tumors.
The use of the bisphosphonate zoledronic acid 4 mg every 3–4 weeks has been associated with a risk of renal deterioration and, in some rare instances, renal failure.Citation88 However, many patients with kidney disease may be prescribed bisphosphonates, despite the risk of further kidney damage. In a large, retrospective chart review of individuals with bone metastases secondary to solid tumors in the USA, almost half (48%) of patients received bisphosphonates within 12 months of confirmation of CKD.Citation32 Prescribing information stipulates a reduced dose of zoledronic acid for patients with RI and that creatinine monitoring is recommended before each dose. For patients with severe RI, the use of zoledronic acid is not recommended ().Citation88 Despite this, many patients will not undergo proper monitoring of renal function during treatment with bisphosphonates.Citation89 A study of physicians treating patients with bone metastases secondary to solid tumors found that managing the risk of RI was their primary safety concern, but there appears to be a discrepancy between clinical recommendations and real-world practice.Citation90 Treatment with zoledronic acid has been associated with hypocalcemia; some cases have been severe and patients have required hospitalization.Citation88 Prescribing information recommends that serum calcium should be measured and hypocalcemia should be corrected before initiating therapy with zoledronic acid. Patients treated with bisphosphonates should be adequately supplemented with calcium and vitamin D to minimize the risk of hypocalcemia.Citation88
Pamidronate is another bisphosphonate that is associated with nephrotoxicity. Pamidronate is approved at a dose of 90 mg to treat osteolytic bone metastases secondary to breast cancer (2-hour infusion every 3–4 weeks) and osteolytic bone lesions associated with multiple myeloma (4-hour infusion once every 4 weeks).Citation91 Pamidronate has been associated with nephrotoxic syndrome and focal segmental glomerulosclerosis (FSGS), with multiple cases of FSGS reported in the medical literature.Citation92 These nephrotoxic effects appear to be dose-dependent and infusion time-dependent.Citation92 The USA label stipulates that single doses of 90 mg should not be exceeded and serum creatinine should be assessed prior to each treatment. Pamidronate should not be used to treat patients with bone metastases who have severe RI.Citation91
Denosumab 120 mg monthly is used for the prevention of skeletal-related events in patients with bone metastases.Citation93,Citation94 Denosumab is neither excreted through the kidneys nor associated with renal toxicity, so dose adjustment is not required when administered to patients with renal dysfunction.Citation93,Citation94 However, label recommendations state that all patients who are prescribed denosumab should receive daily vitamin D and calcium supplementation, owing to the risk of developing hypocalcemia.Citation93,Citation94 This is particularly important for patients with severe RI (CrCl of <30 mL/min) or for individuals receiving dialysis,Citation87 and in those for whom regular calcium monitoring is required.Citation93,Citation94 This is in line with the European Society for Medical Oncology clinical practice guidelines for managing bone health in patients with cancer.Citation58
Nephrotoxic antibiotics
Patients with cancer, particularly those undergoing chemotherapy, are at increased risk of developing serious infections that are commonly treated using antibiotics. However, the use of certain antibiotics (or combinations of antibiotics) is associated with acute tubular necrosis owing to renal excretion, which can result in cumulative kidney damage.Citation95 Antibiotics associated with acute kidney injury include aminoglycosides, penicillins, cephalosporins and fluoroquinolones. Nephrotoxicity can affect up to 50% of patients receiving these treatments, and the risk of damage increases with advancing age, volume depletion and sepsis. Long treatment courses and high doses of antibiotics can further increase the risk of nephrotoxicity.Citation95 Therefore, careful dose adjustments may be required to ensure that the balance between nephrotoxicity and efficacy is maintained.
Dose adjustments or interruptions for optimal patient management
As discussed, many patients will receive nephrotoxic agents as part of their cancer treatment and may subsequently require dose adjustments to manage kidney-related adverse events. In the large USA study of 11,809 patients with bone metastases secondary to solid tumors, 23.4% of patients received a nephrotoxic agent prior to diagnosis of bone metastases, rising to 69% after diagnosis.Citation32
Many chemotherapeutic agents are metabolized by the kidney, producing toxic by-products in the process. RI can result in delayed drug excretion and local and systemic toxicity may arise.Citation96 Chemotherapy dose adjustments are therefore frequently required when administered to patients with RI. In the IRMA study, most (79.9%) patients who were given an anticancer drug received at least one agent that required a dosage adjustment or an agent for which no data were available on use in patients with RI.Citation24 This affected most patients receiving treatment for prostate cancer (82.9%)Citation53 or breast cancer (90.4%).Citation55 For patients with advanced cancers and bone metastases, dose reductions are recommended in those with mild-to-moderate RI receiving intravenous zoledronic acid.Citation88 In clinical trials, dose adjustments have been reported in approximately one-fifth of patients receiving zoledronic acid.Citation84,Citation97 Common agents used in cancer therapy that require dose adjustments based on renal function are shown in .Citation88,Citation98–Citation107
Mitigating renal complications: role of the nephrologist
An important aspect of minimizing the risk of renal injury includes early recognition of RI, or even prevention. This can be accomplished by routine use of eGFR estimation rather than serum creatinine alone (which may be in the normal range in older patients or those with muscle wasting) and urine protein assessment to identify patients with preexisting RI, or close monitoring of urine output to detect acute reductions in renal function. These scenarios may dictate a need to reduce drug doses, avoid nephrotoxic agents and administer intravenous fluids to correct volume depletion. Perhaps most importantly, early involvement of or routine collaboration with a nephrologist in the care of patients with cancer may improve outcomes.
The nephrologist can assist with proper identification of the presence of preexisting renal disease, or other conditions that put patients at risk of acute kidney injury related to the management of cancer. Acute kidney injury is a common complication among hospitalized patients with cancer and is associated with an increased length of stay and mortality.Citation108,Citation109 One example in which early intervention by a nephrologist may help is in the case of tumor lysis syndrome, the consequences of which include RI, cardiac arrhythmia and death.Citation110 Patients at risk of this condition can be identified, enabling the use of prophylactic measures, such as intravenous fluids or xanthine oxidase inhibitors.Citation111,Citation112 For those with established tumor lysis syndrome, nephrologists can advise on the acceptability of using rasburicase, which helps to clear uric acid from the blood, and in severe cases, of possibly using dialytic therapies.
Another scenario where the assistance of a nephrologist may be beneficial is for the planned administration of potential nephrotoxins, such as intravenous contrast for imaging, certain antibiotics or chemotherapeutic agents.Citation108 In these cases, collaboration between the nephrologist and oncologist may help achieve the best possible benefit:risk ratio when using these nephrotoxic agents. Nephrologists can advise on the need for dose reductions and ensure the proper maintenance of adequate volume balance, which can reduce the risk of renal injury across the spectrum of nephrotoxic exposures.Citation96 In addition, nephrologists can identify and recommend the discontinuation of discretionary medications that are potentially nephrotoxic. Nonsteroidal antiinflammatory agents, for example, can in a certain risk context result in renal injury, even after a modest number of doses.Citation113,Citation114
Finally, nephrologists can play an important role in the diagnosis of renal disease, particularly for rare conditions that are unique to the cancer context, many of which require renal biopsy for the confirmation of the diagnosis. Examples of these include parenchymal invasion of the kidney by tumor cells (usually observed with leukemias or lymphomas), paraneoplastic glomerulonephritides and thrombotic microangiopathies due to certain chemotherapeutic agents, or the cancer itself.Citation115
Given the rapidly evolving field of cancer chemotherapeutics, the prevalence of underlying CKD in elderly patients with malignancies, and the broadening range of renal complications secondary to cancers or their treatment, there has been increasing recognition of the need for specialized knowledge in this clinical context. This has resulted in the development of a new subspeciality of onconephrology.Citation116 The American Society of Nephrology has established an Onconephrology Forum Group,Citation117 and the University of Texas MD Anderson Cancer Center is offering a fellowship training program.Citation118
The partnership between nephrologists and oncologists is important for the optimal management of patients; the identification of onconephrology as an independent medical specialty may help enhance this relationship. The development of this speciality and the enhancement of collaboration between experts have the potential for valuable research advances and to encourage guideline preparation on the management of kidney disease in patients with cancer.Citation119
Conclusion
Improvements in cancer therapy mean that patients are surviving for longer periods of time, with a rapidly expanding range of therapeutic options for management of the primary cancer and for its secondary effects such as bone metastases or infections, many of which have the potential for nephrotoxicity. There is, therefore, increasing recognition of the need for attention to kidney disease in the context of cancer. The balance between cancer therapy and preservation of renal function must be considered carefully by physicians treating patients with advanced cancer, with thoughtful choice and dosing of antineoplastic treatments and bone-targeted agents to minimize nephrotoxicity. Collaboration between nephrologists and oncologists may help ensure that care of patients with cancer and kidney disease is optimized.
Acknowledgments
Medical writing support was provided by Liz Hartfield, PhD, and Sarah Griffiths, PhD, of Oxford PharmaGenesis Ltd, Oxford, UK. Funding for this support was provided by Amgen (Europe) GmbH. Editorial support was provided by Emma Booth and Sarah Petrig of Amgen (Europe) GmbH.
Disclosure
A Bahl: advisory boards and honorarium from Amgen, Bayer, Jansen, Astellas, Sanofi, Novartis. Research grants from Ipsen, Sanofi. VB Shahinian: received payments for consultations from Amgen. V Lorusso: has no disclosures. D Niepel: is an employee of Amgen (Europe) GmbH, Vienna, Austria, and holds stock. The authors report no other conflicts of interest in this work.
References
- American Cancer Society2015Survival rates for prostate cancer Available from: http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-survival-ratesAccessed March 16, 2015
- Cancer Research UK2011Prostate cancer survival statistics Available from: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/prostate/survival/prostate-cancer-survival-statisticsAccessed March 16, 2015
- American Cancer Society2015Breast cancer survival by stage Available from: http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-survival-by-stageAccessed March 16, 2015
- Cancer Research UK2011Breast cancer survival Available from: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/breast/survival/Accessed March 16, 2015
- American Cancer Society2015Non-small cell lung cancer Available from: http://www.cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-small-cell-lung-cancer-survival-ratesAccessed March 16, 2015
- American Cancer Society2015Small cell lung cancer Available from: http://www.cancer.org/cancer/lungcancer-smallcell/detailedguide/small-cell-lung-cancer-survival-ratesAccessed March 16, 2015
- Cancer Research UK2011Lung cancer survival Available from: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/lung/survival/Accessed March 16, 2015
- BlumRHNovetskyDShashaDFleishmanSThe multidisciplinary approach to bone metastasesOncology (Williston Park)2003176845857 discussion 862-863, 86712846127
- ColemanREMetastatic bone disease: clinical features, pathophysiology and treatment strategiesCancer Treat Rev200127316517611417967
- ColemanRERubensRDThe clinical course of bone metastases from breast cancerBr J Cancer198755161663814476
- ParkerCNilssonSHeinrichDAlpha emitter radium-223 and survival in metastatic prostate cancerN Engl J Med2013369321322323863050
- AzimHAJrde AzambujaEColozzaMBinesJPiccartMJLong-term toxic effects of adjuvant chemotherapy in breast cancerAnn Oncol20112291939194721289366
- JanusNLaunay-VacherVByloosECancer and renal insufficiency results of the BIRMA studyBr J Cancer2010103121815182121063408
- Centers for Disease Control and Prevention (CDC)Prevalence of overweight and obesity among adults with diagnosed diabetes – United States, 1988–1994 and 1999–2002MMWR Morb Mortal Wkly Rep200453451066106815549021
- HabibSLRojnaMDiabetes and risk of cancerISRN Oncol2013201358378623476808
- StengelBChronic kidney disease and cancer: a troubling connectionJ Nephrol201023325326220349418
- NICE2015NICE clinical guideline 182 – Chronic kidney disease: early identification and management of chronic kidney disease in adults in primary and secondary care Available from: https://www.nice.org.uk/guidance/cg182/resources/guidance-chronic-kidney-disease-pdfAccessed September 18, 2015
- KDIGOClinical Practice Guideline for the Evaluation and Management of Chronic Kidney DiseaseKidney Int Suppl201331136150
- CockcroftDWGaultMHPrediction of creatinine clearance from serum creatinineNephron197616131411244564
- LeveyASStevensLASchmidCHA new equation to estimate glomerular filtration rateAnn Intern Med2009150960461219414839
- AaproMLaunay-VacherVImportance of monitoring renal function in patients with cancerCancer Treat Rev201238323524021605937
- US Food and Drug Administration. Guidance for industryPharmacokinetics in patients with impaired renal function — study design, data analysis, and impact on dosing and labeling2010 Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM204959.pdfAccessed July 18, 2016
- Launay-VacherVChatelutELichtmanSMWildiersHSteerCAaproMRenal insufficiency in elderly cancer patients: International Society of Geriatric Oncology clinical practice recommendationsAnn Oncol20071881314132117631561
- Launay-VacherVOudardSJanusNPrevalence of Renal Insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) studyCancer200711061376138417634949
- DharnidharkaVRKwonCStevensGSerum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysisAm J Kidney Dis200240222122612148093
- BrennerBMMeyerTWHostetterTHDietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal diseaseN Engl J Med1982307116526597050706
- CoreshJSelvinEStevensLAPrevalence of chronic kidney disease in the United StatesJAMA2007298172038204717986697
- WeinsteinJRAndersonSThe aging kidney: physiological changesAdv Chronic Kidney Dis201017430230720610357
- BergerNASavvidesPKoroukianSMCancer in the elderlyTrans Am Clin Climatol Assoc2006117147155 discussion 155–14618528470
- de Barros PontesLAntunesYPBuganoDDKarnakisTGiglioADKaliksRAPrevalence of renal insufficiency in elderly cancer patients in a tertiary cancer centerEinstein (Sao Paulo)201412330030325295449
- Launay-VacherVSpanoJPJanusNRenal insufficiency and anticancer drugs in elderly cancer patients: a subgroup analysis of the IRMA studyCrit Rev Oncol Hematol200970212413318990585
- ArellanoJHernandezRKWadeSWPrevalence of renal impairment and use of nephrotoxic agents among patients with bone metastases from solid tumors in the United StatesCancer Med20154571372025663171
- BhaskaranKDouglasIForbesHdos-Santos-SilvaILeonDASmeethLBody-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adultsLancet2014384994575576525129328
- AguilarEAAshrafHFrontiniMRuizMReskeTMCefaluCAn analysis of chronic kidney disease risk factors in a Louisiana nursing home population: a cross-sectional studyJ La State Med Soc2013165526026326526724350526
- StengelBTarver-CarrMEPoweNREberhardtMSBrancatiFLLifestyle factors, obesity and the risk of chronic kidney diseaseEpidemiology200314447948712843775
- YamagataKIshidaKSairenchiTRisk factors for chronic kidney disease in a community-based population: a 10-year follow-up studyKidney Int200771215916617136030
- LowranceWTOrdonezJUdaltsovaNRussoPGoASCKD and the risk of incident cancerJ Am Soc Nephrol201425102327233424876115
- ButlerAMOlshanAFKshirsagarAVCancer incidence among US Medicare ESRD patients receiving hemodialysis, 1996–2009Am J Kidney Dis201565576377225662835
- BirkelandSALokkegaardHStormHHCancer risk in patients on dialysis and after renal transplantationLancet200035592181886188710866449
- SteenkampRCastledineCFeestTChapter 6 Survival and causes of death of UK adult patients on renal replacement therapy in 2010: national and centre-specific analysesNephron Clin Pract2012120Suppl 1c10513522964564
- FischerederMCancer in patients on dialysis and after renal transplantationNephrol Dial Transplant20082382457246018398015
- AkchurinOMKaskelFUpdate on inflammation in chronic kidney diseaseBlood Purif2015391–3849225662331
- JorgensenLHeuchIJenssenTJacobsenBKAssociation of albuminuria and cancer incidenceJ Am Soc Nephrol200819599299818256361
- WongGHayenAChapmanJRAssociation of CKD and cancer risk in older peopleJ Am Soc Nephrol20092061341135019406977
- ChristiansenCFJohansenMBLangebergWJFryzekJPSorensenHTIncidence of acute kidney injury in cancer patients: a Danish population-based cohort studyEur J Intern Med201122439940621767759
- CampbellGAHuDOkusaMDAcute kidney injury in the cancer patientAdv Chronic Kidney Dis2014211647124359988
- Wagrowska-DanilewiczMDanilewiczMNephrotic syndrome and neoplasia: our experience and review of the literaturePol J Pathol2011621121821574101
- DavidsonMBThakkarSHixJKBhandarkarNDWongASchreiberMJPathophysiology, clinical consequences, and treatment of tumor lysis syndromeAm J Med2004116854655415063817
- SeccarecciaDCancer-related hypercalcemiaCan Fam Physician2010563244246e290242 French20228307
- MoreauPSan MiguelJLudwigHMultiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-upAnn Oncol201324Suppl 6vi13313723956208
- OyajobiBOMultiple myeloma/hypercalcemiaArthritis Res Ther20079Suppl 1S4
- GoldschmidtHLannertHBommerJHoADMultiple myeloma and renal failureNephrol Dial Transplant200015330130410692511
- Launay-VacherVAyllonJJanusNDrug management of prostate cancer: prevalence and consequences of renal insufficiencyClin Genitourin Cancer200973E838919815487
- Launay-VacherVEtessamiRJanusNLung cancer and renal insufficiency: prevalence and anticancer drug issuesLung20091871697418941834
- Launay-VacherVGligorovJLe TourneauCPrevalence of renal insufficiency in breast cancer patients and related pharmacological issuesBreast Cancer Res Treat2010124374575318704681
- KhafagyRShackleyDSamuelJO’FlynnKBettsCClarkeNComplications arising in the final year of life in men dying from advanced prostate cancerJ Palliat Med200710370571117592982
- National Kidney Foundation2002NKF KDOQI guidelines Available from: http://www2.kidney.org/professionals/KDOQI/guidelines_ckd/toc.htmAccessed March 18, 2015
- ColemanRBodyJJAaproMHadjiPHerrstedtJBone health in cancer patients: ESMO Clinical Practice GuidelinesAnn Oncol201425Suppl 3iii12413724782453
- GralowJRBiermannJSFarookiANCCN Task Force Report: bone health in cancer CareJ Natl Compr Canc Netw201311Suppl 3S150 quiz S51
- GoldfarbSMcCulloughPAMcDermottJGaySBContrast-induced acute kidney injury: specialty-specific protocols for interventional radiology, diagnostic computed tomography radiology, and interventional cardiologyMayo Clin Proc200984217017919181651
- PerazellaMAGadolinium-contrast toxicity in patients with kidney disease: nephrotoxicity and nephrogenic systemic fibrosisCurr Drug Saf200831677518690983
- PerazellaMAMoeckelGWNephrotoxicity from chemotherapeutic agents: clinical manifestations, pathobiology, and prevention/therapySemin Nephrol201030657058121146122
- MillerRPTadagavadiRKRameshGReevesWBMechanisms of cisplatin nephrotoxicityToxins (Basel)20102112490251822069563
- StewartDJMikhaelNZNanjiAARenal and hepatic concentrations of platinum: relationship to cisplatin time, dose, and nephrotoxicityJ Clin Oncol198539125112564040958
- HensleyMLHagertyKLKewalramaniTAmerican Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectantsJ Clin Oncol200927112714519018081
- DekkersIABlijdorpKCransbergKLong-term nephrotoxicity in adult survivors of childhood cancerClin J Am Soc Nephrol20138692292923411430
- FarryJKFlombaumCDLatchaSLong term renal toxicity of ifosfamide in adult patients – 5 year dataEur J Cancer20124891326133122503397
- GilbarPJRichmondJWoodJSullivanASyndrome of inappropriate antidiuretic hormone secretion induced by a single dose of oral cyclophosphamideAnn Pharmacother2012469e2322911342
- WidemannBCBalisFMKempf-BielackBHigh-dose methotrexate-induced nephrotoxicity in patients with osteosarcomaCancer2004100102222223215139068
- RajkumarSVDimopoulosMAPalumboAInternational Myeloma Working Group updated criteria for the diagnosis of multiple myelomaLancet Oncol20141512e53854825439696
- JolivotPAPoinsignonVPaciAA case of melphalan sustained accumulation in an 80-year old patientInt J Clin Pharm201537698498726394785
- EMC+Melphalan summary of product characteristics2014 Available from: https://www.medicines.org.uk/emc/medicine/703Accessed October 1, 2015
- BirdJMFugeRSirohiBThe clinical outcome and toxicity of high-dose chemotherapy and autologous stem cell transplantation in patients with myeloma or amyloid and severe renal impairment: a British Society of Blood and Marrow Transplantation studyBr J Haematol2006134438539016822294
- HaymanSRLeungNGrandeJPGarovicVDVEGF inhibition, hypertension, and renal toxicityCurr Oncol Rep201214428529422544560
- ZhuXWuSDahutWLParikhCRRisks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysisAm J Kidney Dis200749218619317261421
- CrinoLDansinEGarridoPSafety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a phase 4 studyLancet Oncol201011873374020650686
- PengLZhaoQYeXZhouYHuDZhengSIncidence and risk of proteinuria with aflibercept in cancer patients: a meta-analysisPLoS One2014911e11183925365378
- EMC+Opdivo summary of product characteristics2016 Available from: https://www.medicines.org.uk/emc/medicine/30476Accessed August 5, 2016
- EMC+Keytruda summary of product characteristics2016 Available from: https://www.medicines.org.uk/emc/medicine/30602Accessed August 5, 2016
- LapiFAzoulayLNiaziMTYinHBenayounSSuissaSAndrogen deprivation therapy and risk of acute kidney injury in patients with prostate cancerJAMA2013310328929623860987
- ColemanREClinical features of metastatic bone disease and risk of skeletal morbidityClin Cancer Res20061220 Pt 26243s6249s17062708
- BodyJJDielIJLichinitzerMOral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: results from two randomised, placebo-controlled phase III studiesBr J Cancer20049061133113715026791
- RosenLSGordonDTchekmedyianNSLong-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trialCancer2004100122613262115197804
- FizaziKCarducciMSmithMDenosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind studyLancet2011377976881382221353695
- FizaziKLiptonAMarietteXRandomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonatesJ Clin Oncol200927101564157119237632
- LiptonAFizaziKStopeckATSuperiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trialsEur J Cancer201248163082309222975218
- LiptonAStegerGGFigueroaJExtended efficacy and safety of denosumab in breast cancer patients with bone metastases not receiving prior bisphosphonate therapyClin Cancer Res200814206690669618927312
- NovartisZometa® Summary of Product Characteristics2006 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000336/WC500051730.pdfAccessed September 18, 2016
- HoustonSGrieveRJHickishTPercivalFHamiltonERenal function changes and NHS resource use in breast cancer patients with metastatic bone disease treated with IV zoledronic acid or oral ibandronic acid: a four-centre non-interventional studyJ Med Econ201013116216720136578
- ArellanoJHauberABMohamedAFPhysicians’ preferences for bone metastases drug therapy in the United StatesValue Health2015181788325595237
- Ltd. HUPamidronate disodium summary of product characteristics Available from: https://www.medicines.org.uk/emc/medicine/214422014 http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021113s017lbl.pdfAccessed January 9, 2017
- PerazellaMAMarkowitzGSBisphosphonate nephrotoxicityKidney Int200874111385139318685574
- Amgen2015XGEVA Prescribing Information Available from: http://pi.amgen.com/united_states/xgeva/xgeva_pi.pdfAccessed September 18, 2015
- Amgen2014XGEVA Summary of Product Characteristics Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002173/WC500110381.pdfAccessed September 18, 2015
- TarloffJBLashLHToxicology of the Kidney3rd edBoca Raton, FLCRC Press2004
- LameireNNephrotoxicity of recent anti-cancer agentsClin Kidney J201471112225859345
- HenryDHCostaLGoldwasserFRandomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myelomaJ Clin Oncol20112991125113221343556
- RocheAvastin Summary of Product Characterisitcs Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000582/WC500029271.pdfAccessed September 21, 2015
- Astellas Pharma Europe B.V. Xtandi Summary of Product Characteristics Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002639/WC500144996.pdfAccessed September 21, 2015
- EMC+Cisplatin summary of product characteristics2015 Available from: https://www.medicines.org.uk/emc/medicine/623Accessed September 21, 2015
- EMC+Cyclophosphamide summary of product characteristics2014 Available from: https://www.medicines.org.uk/emc/medicine/29592Accessed September 21, 2015
- EMC+Ifosfamide summary of product characteristics2014 Available from: https://www.medicines.org.uk/emc/medicine/30183Accessed September 21, 2015
- EMC+Methotrexate summary of product characteristics2013 Available from: https://www.medicines.org.uk/emc/medicine/12034Accessed September 21, 2015
- Pfizer LtdSunitinib summary of product characteristics Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000687/WC500057737.pdfAccessed September 21, 2015
- EMC+Gentamicin summary of product characteristics2015 Available from: https://www.medicines.org.uk/emc/medicine/21665Accessed September 21, 2015
- EMC+Tobramycin summary of product characteristics2015 Available from: https://www.medicines.org.uk/emc/medicine/6566Accessed September, 21 2015
- Novartis Europharma LtdVotrient summary of product characteristics Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001141/WC500094272.pdfAccessed September 21, 2015
- BenoitDDHosteEAAcute kidney injury in critically ill patients with cancerCrit Care Clin201026115117919944280
- SalahudeenAKDoshiSMPawarTNowshadGLahotiAShahPIncidence rate, clinical correlates, and outcomes of AKI in patients admitted to a comprehensive cancer centerClin J Am Soc Nephrol20138334735423243268
- MirrakhimovAEVoorePKhanMAliAMTumor lysis syndrome: a clinical reviewWorld J Crit Care Med20154213013825938028
- EjazAAPourafsharNMohandasRSmallwoodBAJohnsonRJHsuJWUric acid and the prediction models of tumor lysis syndrome in AMLPLoS One2015103e011949725775138
- WilsonFPBernsJSOnco-nephrology: tumor lysis syndromeClin J Am Soc Nephrol20127101730173922879434
- HaragsimLDalalRBaggaHBastaniBKetorolac-induced acute renal failure and hyperkalemia: report of three casesAm J Kidney Dis19942445785807942813
- WheltonANephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implicationsAm J Med19991065B13S24S10390124
- HumphreysBDSoifferRJMageeCCRenal failure associated with cancer and its treatment: an updateJ Am Soc Nephrol200516115116115574506
- FinkelKWHowardSCOnco-nephrology: an invitation to a new fieldJ Clin Oncol201432222389239024934791
- American Society of NephrologyOnco-nephrology forum2016 Available from: https://www.asn-online.org/about/committees/committee.aspx?panel=OncoNephAccessed June 2, 2016
- University of Texas MD Anderson Cancer CenterOnco-nephrology fellowship2016 Available from: https://www.mdanderson.org/education-and-research/education-and-training/schools-and-programs/graduate-medical-education/residency-and-fellowship-programs/onco-nephrology-fellowship.htmlAccessed June 2, 2016
- AbudayyehAALahotiASalahudeenAKOnconephrology: the need and the emergence of a subspecialty in nephrologyKidney Int20148551002100424786870
- EMC+Zytiga summary of product characteristics2016 Available from: https://www.medicines.org.uk/emc/medicine/24976Accessed May 5, 2016
- EMC+Inlyta summary of product characteristics2016 Available from: https://www.medicines.org.uk/emc/medicine/27051Accessed May 5, 2016
