Abstract
Activation of the signal transducer and activator of transcription 3 (STAT3) is observed in multiple cancer types, including gastric cancer, and represents a potential drug target for chemotherapy. Currently, clinically available small-molecule inhibitors targeting STAT3 are lacking. Here, we report that nifuratel, an antiprotozoal and antifungal drug, is a potent inhibitor of STAT3. We found that nifuratel significantly suppressed proliferation and induced apoptosis of gastric cancer cells. Studies of the mechanism of action of nifuratel indicated that it acts by inhibiting the constitutive and interleukin-6-induced STAT3 activation. Taken together, our findings demonstrate that nifuratel may be a novel, clinically accessible STAT3 inhibitor in gastric cancer cells.
Introduction
Gastric cancer is one of the most common malignancies in China.Citation1 Moreover, it is the fifth most frequently occurring cancer and the third leading cause of cancer deaths worldwide, especially in China, Japan, South America, and Eastern Europe, which also report the highest incidence of gastric cancer.Citation2,Citation3 The average 5-year survival rate for gastric cancer ranges from 50% to 70% in Japan, and only from 10% to 30% in the other countries.Citation4,Citation5 Thus, novel therapeutic approaches for the effective treatment of gastric cancer patients are much needed given their current prognosis.
Current methods for treating gastric cancer include the traditional surgical resection, radiotherapy, and chemotherapy. However, these methods are followed by many adverse reactions.Citation4,Citation6 Signal transducer and activator of transcription 3 (STAT3) is constitutively activated in various human cancer cells, such as gastric cancer cells, colon cancer cells, and breast cancer cells.Citation7 STAT3 has been found to play an important role in preventing cell apoptosis and stimulating cell proliferation in tumor progression.Citation8,Citation9 Specifically, STAT3 and their downstream signaling molecules are critical to the growth of gastric cancer cells.Citation10 Therefore, intensive efforts have been spent on developing drugs that target the STAT3 pathway for the treatment of cancer.Citation11,Citation12 Napabucasin, a STAT3 inhibitor, has been granted orphan drug status for the treatment of gastric cancers, including gastroesophageal junction cancer.Citation13–Citation15 Overall, studies have identified the STAT3 pathway as a target for the effective prevention and treatment of gastric cancer, but clinically available small-molecule inhibitors of the STAT3 pathway are still lacking.
Nifuratel, an analogue of nifuroxazide (), has been clinically proven to be safe and effective in the treatment of bacterial vaginosis (BV), Candida, or Trichomonas vaginalis.Citation16,Citation17 Previous studies found have that nifuroxazide exerts potent antitumor and antimetastatic activities by inhibiting the STAT3 pathway.Citation18,Citation19 The aim of this study was to explore whether nifuratel exerts antitumor growth activity by inhibiting cell proliferation and inducing cell apoptosis of gastric cancer cells through downregulation of the STAT3 signaling pathway. Our results suggest that nifuratel inhibited the proliferation and induced apoptosis of gastric cancer cells. Further investigation revealed that nifuratel blocked the interleukin-6 (IL-6)-mediated activation of the STAT3 signaling pathway. This might be one of the possible mechanisms by which the expression of proapoptotic proteins was upregulated and the proliferation of gastric cancer cells was inhibited.
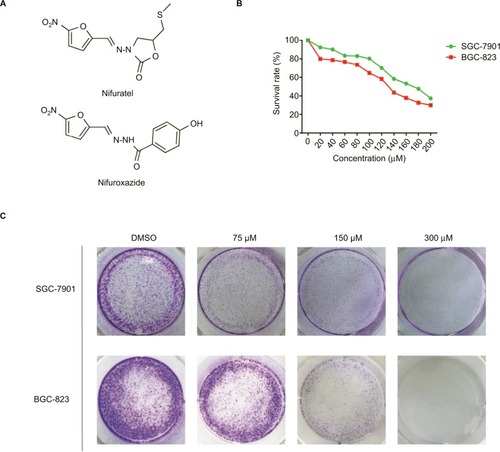
Figure 1 Nifuratel inhibits viability and colony formation of gastric cancer cells.
Abbreviation: DMSO, dimethyl sulfoxide.
In the present study, the results of the MTT assay revealed that nifuratel decreased the viability of SGC-7901 and BGC-823 cells with IC50 values of 169.7 and 133.7 μM, respectively. Western blot analysis showed that nifuratel significantly reduced the expression of P-STAT3, without affecting the expression of P-STAT1. Additionally, nifuratel-induced apoptosis in gastric cancer cells and arrested the cell cycle in the G2/M phase. After treatment with nifuratel, the expression of Bax protein was significantly upregulated, while Bcl-2 protein was decreased in SGC-7901 and BGC-823 cells. In conclusion, our findings suggest that nifuratel can act as an anticancer agent in gastric cancer by inhibiting STAT3 signaling.
Materials and methods
Cell lines and antibody
Human gastric cancer cell lines SGC-7901 and BGC-823 were obtained from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) was obtained from Thermo Fisher Scientific (Waltham, MA, USA). The cells were cultured in a humidified cell incubator with an atmosphere of 5% CO2 at 37°C. The antibodies against P-STAT3, STAT3, P-STAT1, STAT1, Bcl-2, Bax, GAPDH, horseradish peroxidase (HRP)-conjugated donkey anti-rabbit IgG and HRP-conjugated goat anti-mouse IgG were purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). The Annexin V-FITC apoptosis Detection Kit I and propidium iodide (PI) were purchased from BD Pharmingen (Franklin Lakes, NJ, USA).
MTT cell viability assay
Cells were seeded into 96-well plates at a density of 8000 cells per well and cultured in RPMI 1640 medium containing 10% heat-inactivated FBS overnight. Nifuratel was dissolved in DMSO to a final concentration of 10, 20, 40, 60, 80, 100, 120, 150, 200, and 300 μM and gastric cancer cell lines were incubated with nifuratel for 24 hours; the IC50 values were determined by the MTT assay.
Cell cycle analysis
Cells were seed into six-well plates for 24 hours, and then treated with DMSO or different concentrations of nifuratel for 24 hours. Cells were then labeled with PI, and the cell cycle was analyzed by flow cytometry analysis (BD Biosciences, San Jose, CA, USA).
Cell apoptosis analysis
SGC-7901 and BGC-823 cells were seeded in six-well plates and grown overnight, and then treated with different concentrations of nifuratel for 24 hours. The cells were harvested and washed twice with ice-cold PBS, and stained with FITC-conjugated Annexin V and PI in the binding buffer for 30 minutes. The stained cells were evaluated for apoptosis by flow cytometry analysis (FACSCalibur flow cytometer; BD Biosciences).
Western blot analysis
Cells were lysed in protein lysis buffer and centrifuged at 12,000 rpm for 10 minutes at 4°C to remove nuclei and cell debris. The cell extract concentrations were determined with the Bradford protein assay kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). After addition of sample loading buffer, proteins were separated by electrophoresis and the proteins were then electroblotted to poly-vinylidene difluoride transfer membranes. The blots were blocked for 2 hours with fresh 5% non-fat milk in Tris-buffered saline with Tween 20 (TBST) buffer at room temperature, followed by incubation with primary antibody in TBST overnight at 4°C. After washing three times with TBST, the blots were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 hour. After three washes with TBST, the antibody staining was visualized using the ECL kit (Bio-Rad Laboratories Inc.). Then, the images were analyzed using the Image J computer software (National Institute of Health, Bethesda, MD, USA).
Results
The effect of nifuratel on gastric cancer cell proliferation by the MTT and colony formation assays
To investigate the effect of nifuratel on human gastric cancer cell lines, cell viability was evaluated by the MTT assay. The SGC-7901 and BGC-823 cell lines were treated with increasing doses of nifuratel for 48 hours, and the cell viability was evaluated by the MTT assay. The results in show that cell viability was suppressed in a dose-dependent manner in the SGC-7901 and BGC-823 cell lines, with IC50 values of 169.7±2.2 μM in SGC-7901 cells and 133.7±0.85 μM in BGC-823cells.
Also, as shown in , compared with control cells, a dose-dependent decrease in colony formations by SGC-7091 and BGC-823 cells was detected using a colony formation assay. These results suggest that nifuratel significantly inhibited the growth and the proliferation of human gastric cancer cells, inducing cell death in a dose-dependent manner.
Nifuratel induces G2/M cell cycle arrest in human gastric cancer cells
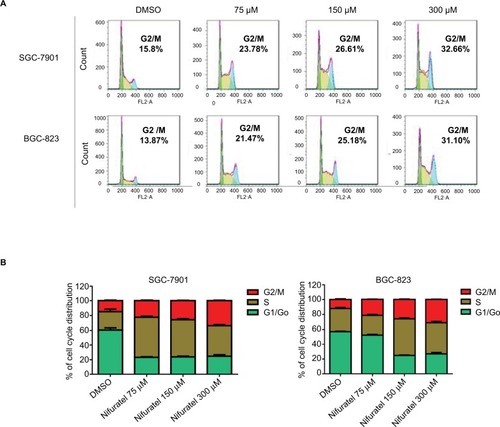
The cell cycle is a process involving a series of cellular events that lead to cell division and ultimately to proliferation. The entire cell cycle can be divided into four stages including the G1 phase, S phase, G2 phase, and M phase (the division stage).Citation20 To evaluate the cell cycle arrest associated with nifuratel, SGC-7901 and BGC-823 cell lines were treated with nifuratel for 24 hours. The percentage of nifuratel-treated and untreated gastric cancer cells in the G2/M phase was measured by flow cytometry analysis. As show in , nifuratel significantly induced the arrest of gastric cancer cells in the G2/M phase of the cell cycle compared with the untreated control group.
Figure 2 Nifuratel induces G2/M cell cycle arrest in human gastric cancer cell lines.
Abbreviation: DMSO, dimethyl sulfoxide.
Nifuratel induces apoptosis in human gastric cancer cells
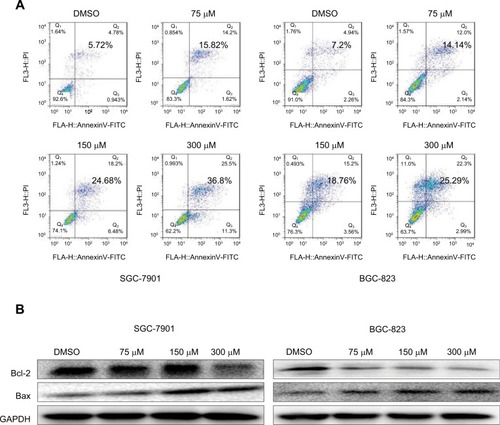
To evaluate the apoptosis-inducing effect of nifuratel in human gastric cancer cell lines, the cancer cells were treated with nifuratel for 24 hours, stained with Annexin V-FITC and PI, and apoptotic cells were evaluated by flow cytometry analysis. The data showed that nifuratel dose-dependently induced cell apoptosis (). In addition, Western blot analysis data further revealed that nifuratel increased the expression of the proapoptotic protein Bax and decreased the level of the antiapoptotic protein Bcl-2 in human gastric cancer cells in a dose-dependent manner (). Our results also revealed that nifuratel could regulate the expression of cell death-related proteins, which blocks the process of human gastric cancer cell proliferation.
Figure 3 Nifuratel-induced apoptosis of gastric cancer cells.
Abbreviation: DMSO, dimethyl sulfoxide.
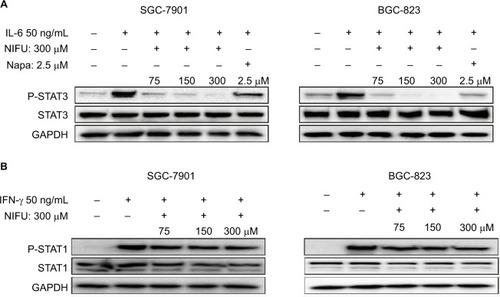
Nifuratel inhibits the STAT3 signaling pathway
A number of studies have shown that overexpression of the STAT3 protein may be associated with the progression of gastric cancer.Citation21 Our results indicated that treatment with 300 μM nifuratel markedly reduces the phosphorylated STAT3 (P-STAT3) protein level in SGC-7901 and BGC-823 cell lines. As show in , nifuratel decreased P-STAT3 protein expression in SGC-7901 and BGC-823 cell lines in a concentration-dependent manner after 24-hour treatment. Notably, the 300 μM dose of nifuratel also markedly decreased P-STAT3 protein expression after 6 hours in the SGC-7901 cell lines and after 3 hours in the BGC-823 cell lines.
Figure 4 Western blot analysis of total STAT3 and P-STAT3 in SGC-7901 and BGC-823 cells.
Abbreviations: DMSO, dimethyl sulfoxide; NIFU, nifuratel; STAT3, signal transducer and activator of transcription 3; P-STAT3, phosphorylated STAT3.
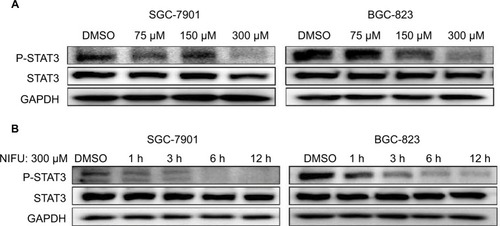
Importantly, the previous study found that the IL-6-induced phosphorylation of STAT3 in neoplastic gastric tissue positively correlated with tumor progression.Citation22 It has been established that IL-6-mediated activation of STAT3 signaling pathway can promote the progression of other cancers.Citation23,Citation24 The STAT3 inhibitor inhibited the activation of STAT3, blocking the IL-6 signaling pathway which downregulated cancer cell proliferation, and downregulated the antiapoptotic protein Bcl-2.Citation25 Thus, to further clarify whether nifuratel can block the IL-6-induced phosphorylation of STAT3, we combined the treatment with nifuratel and IL-6. In addition, napabucasin, an orally available STAT3 inhibitor, was used as a positive control group. The results indicated that nifuratel could block the IL-6-induced activation of the STAT3 signaling pathway, but had no effect on the activation of STAT1 mediated by interferon-γ (IFN-γ) (). Our results also revealed that nifuratel regulated the expression of the cell death-related protein Bcl-2, induced cell cycle arrest, and inhibited the proliferation of human gastric cancer cells by blocking the IL-6-induced activation of the STAT3 signaling pathway.
Figure 5 Nifuratel blocked IL-6-induced STAT3 phosphorylation.
Abbreviations: NIFU, nifuratel; Napa, napabucasin; P-STAT3, phosphorylated STAT3; STAT3, signal transducer and activator of transcription 3.
Discussion
A tumor develops from cell differentiation abnormalities, excessive proliferation, inhibition of apoptosis, and other factors.Citation2 The abnormalities of signal transduction pathways play an important role in these processes.Citation26,Citation27 Several studies have reported that STAT3 is continuously activated in most human primary cancer foci and tumor cell lines, such as human astrocytoma, multiple osteosarcoma, prostate cancer, colon cancer, gastric cancer, etc. Citation8,Citation10,Citation28
Our study showed that nifuratel can reduce the viability of gastric cancer cells and stimulate apoptosis in human gastric cancer cells in a dose-dependent manner. In addition, nifuratel induced the arrest of gastric cancer cells in the G2/M phase of the cell cycle. Western blot analysis results suggest that the antitumor activity of nifuratel might be mediated via the STAT3 inhibition. Furthermore, nifuratel can block the IL-6-induced activation of the STAT3 signaling pathway, thereby inhibiting the proliferation of tumor cells. Our study further confirmed that the treatment with nifuratel can upregulate the expression of the proapoptotic protein Bax and downregulate the expression of the antiapoptotic protein Bcl-2. We have demonstrated that nifuratel inhibited the IL-6-induced activation of the STAT3 signaling pathway and thereby induced apoptosis and reduced the growth of human gastric cancer cells.
Nifuratel is used to effectively and safely treat bacterial vaginitis, Chlamydia trachomatis, and Mycoplasma spp., as well as fungal infections by Candida spp., for several decades.Citation16 Additionally, though to a lesser extent, it has been reported to possess anticancer properties. Our study confirms that the clinical drug nifuratel possesses anticancer properties against tumor cells. We explored the novel antitumor effects of nifuratel and tried to repurpose it for treating gastric cancer. Additionally, we plan to enhance the selectivity and reduce the toxicity effects of nifuratel on normal cells. We will also investigate the combination of nifuratel and other clinical cancer drugs to improve the anticancer potency of nifuratel and verify its effect on human gastric cancer in vivo.
Acknowledgments
This research was supported by the Zhejiang Provincial Natural Science Fund (LY16H160052), Medical Scientific Research Fund of Zhejiang Province (2017192276), and Wenzhou Science and Technology Project (Y20160052). We also thank the Chinese Society of Clinical Oncology (CSCO Y-MX2016-058) and Zhejiang Anticancer Association.
Disclosure
The authors report no conflicts of interest in this work.
References
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- SiegelRLMillerKDJemalACancer Statistics, 2017CA Cancer J Clin201767173028055103
- SiegelRLMillerKDJemalACancer statistics, 2016CA Cancer J Clin201666173026742998
- ShitaraKKadowakiSNishinaTSafety, pharmacokinetic, and clinical activity profiles of ramucirumab in combination with three platinum/fluoropyrimidine doublets in Japanese patients with chemotherapy-naive metastatic gastric/gastroesophageal junction cancerGastric Cancer Epub2017630
- KoderaYFujitaniKFukushimaNSurgical resection of hepatic metastasis from gastric cancer: a review and new recommendation in the Japanese gastric cancer treatment guidelinesGastric Cancer201417220621224022130
- Thuss-PatiencePCShahMAOhtsuATrastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 studyLancet Oncol201718564065328343975
- YuHPardollDJoveRSTATs in cancer inflammation and immunity: a leading role for STAT3Nat Rev Cancer200991179880919851315
- YuHLeeHHerrmannABuettnerRJoveRRevisiting STAT3 signalling in cancer: new and unexpected biological functionsNat Rev Cancer2014141173674625342631
- ZhaoCLiHLinHJYangSLinJLiangGFeedback activation of STAT3 as a cancer drug-resistance mechanismTrends Pharmacol Sci2016371476126576830
- HuynhJEtemadiNHollandeFErnstMBuchertMThe JAK/STAT3 axis: a comprehensive drug target for solid malignanciesSemin Cancer Biol201745132228647610
- LinLHutzenBZuoMNovel STAT3 phosphorylation inhibitors exhibit potent growth-suppressive activity in pancreatic and breast cancer cellsCancer Res20107062445245420215512
- RathKSNaiduSKLataPHO-3867, a safe STAT3 inhibitor, is selectively cytotoxic to ovarian cancerCancer Res20147482316232724590057
- HubbardJMGrotheyANapabucasin: an update on the first-in-class cancer stemness inhibitorDrugs201777101091110328573435
- JonkerDJNottLYoshinoTA randomized phase III study of napabucasin [BBI608] (NAPA) vs placebo (PBO) in patients (pts) with pretreated advanced colorectal cancer (ACRC): the CCTG/AGITG CO.23 trialAnn Oncol2016276149206
- GrotheyATebbuttNVan CutsemECanStem303C trial: a phase III study of BBI-608 (napabucasin) in combination with 5-fluorouracil (5-FU), leucovorin, irinotecan (FOLFIRI) in adult patients with previously treated metastatic colorectal cancer (mCRC)Ann Oncol2016276149206
- PolattiFBacterial vaginosis, Atopobium vaginae and nifuratelCurr Clin Pharmacol201271364022082330
- BrumfittWReynoldsAVHamilton-MillerJMLetter: activity of nitrofurantoin and nifuratel against anaerobic Gram-negative bacilliLancet197517904460
- YeTHYangFFZhuYXInhibition of Stat3 signaling pathway by nifuroxazide improves antitumor immunity and impairs colorectal carcinoma metastasisCell Death Dis201781e253428055016
- NelsonEAWalkerSRKepichANifuroxazide inhibits survival of multiple myeloma cells by directly inhibiting STAT3Blood2008112135095510218824601
- JangWKimTKooJSKimSKLimDSMechanical cue-induced YAP instructs Skp2-dependent cell cycle exit and oncogenic signalingEMBO J201736172510252828673931
- WangTTZhaoYLPengLSTumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathwayGut Epub201738
- HowlettMMenheniottTRJuddLMGiraudASCytokine signalling via gp130 in gastric cancerBiochim Biophys Acta20091793111623163319665497
- LongKBTookerGTookerEIL-6 receptor blockade enhances chemotherapy efficacy in pancreatic ductal adenocarcinomaMol Cancer Ther20171691898190828611107
- BhartiRDeyGBanerjeeISomatostatin receptor targeted liposomes with Diacerein inhibit IL-6 for breast cancer therapyCancer Lett201738829230228025102
- BhartiRDeyGOjhaPKDiacerein-mediated inhibition of IL-6/IL-6R signaling induces apoptotic effects on breast cancerOncogene201635303965397526616855
- MedlockJDasAAKMaddenLAAllsupDJPaunovVNCancer bioimprinting and cell shape recognition for diagnosis and targeted treatmentChem Soc Rev201746165110512728660268
- MillerMAWeisslederRImaging of anticancer drug action in single cellsNat Rev Cancer201717739941428642603
- JungKHYooWStevensonHLMulti-functional effects of a small-molecule STAT3 inhibitor on NASH and HCC in miceClin Cancer Res201723185537554628533225
