Abstract
Introduction
Trophoblast cell surface antigen 2 (TROP2) has been linked to disease prognosis in various human cancers and plays a critical role in tumor development, progression, and metastasis. A number of relevant studies have been published on this topic. A meta-analysis of the latest literature to evaluate the value of TROP2 as a predictive prognosticator of cancer was performed.
Methods
Several online databases were searched, and relevant articles were retrieved. Overall and subcategory meta-analyses were performed, and results were collated.
Results
Twenty-seven articles, including 29 studies, were included, involving 4,852 cancer patients, and results showed that the above-baseline expression of TROP2 was significantly associated with poorer overall survival (OS) (pooled hazard ratio [HR]: 1.84, 95% confidence interval [CI]: 1.45–2.35), disease-free survival (DFS) (pooled HR: 2.77, 95% CI: 1.73–4.42), and progression-free survival (PFS) (pooled HR: 1.71, 95% CI: 1.25–2.35). The following clinical characteristics were also significantly linked with TROP2 overexpression: moderate/poor differentiation (pooled HR: 3.03, 95% CI: 1.99–4.63), distant metastasis (pooled HR: 2.46, 95% CI: 1.05–5.75), lymph node metastasis (pooled HR: 2.47, 95%: CI 1.72–3.56), and advanced TNM stage (pooled HR: 2.02, 95% CI: 1.38–2.95).
Conclusion
TROP2 overexpression was predictive of poor prognosis in human cancers and may be an independent prognostic predictive biomarker. Further studies should be performed to confirm the significance of TROP2 in clinical practice.
Keywords:
Introduction
Cancer is a major disease burden worldwide, with high morbidity and mortality rates compounded by the economic burden of maintaining patient quality-of-life and lengthening survival period.Citation1,Citation2 To date, many predictive biomarkers with excellent prognostic utility have been discovered for various cancers. Targeted molecular therapy and cancer immunotherapy have been introduced to improve disease management.Citation3–Citation6 One such biomarker is a cell surface protein known as trophoblast cell surface antigen 2 (TROP2),Citation7 also called “tacstd2”, “m1s1 protein”, “tumor-associated calcium signal transducer 2”, “tumor-associated antigen ga733-1”, “ga733-1 antigen”, “membrane component 1 surface marker 1”, “epithelial glycoprotein 1”, and “gastrointestinal antigen 733-1”.Citation8 This protein shows relatively low expression in normal epithelial cells and is overexpressed in various types of human cancers.Citation9–Citation23 Overexpression of TROP2 in cancer has been linked to disease aggression and shorter overall survival (OS). Several clinical studies have demonstrated that therapies targeting TROP2-benefited cancer patients by inhibiting TROP2 expressionCitation24–Citation33 and have explored this protein as a potential predictor of cancer prognosis. However, due to small sample size, the results were not categorically conclusive.Citation13,Citation15,Citation23,Citation34–Citation46 The first meta-analysis about TROP2 was published 1 year ago,Citation47 which indicated that TROP2 overexpression was associated with poor survival in human solid tumors. Some new relevant studies have been published since then, therefore, we performed this meta-analysis to systematically review and gather more powerful evidence to verify the relationship between TROP2 overexpression and clinical characteristics/prognosis in patients with a variety of human cancers.
Methods
Search strategy
Articles related to TROP2 and carcinomas were retrieved from online databases: Embase, PubMed, ISI Web of Science, China National Knowledge Infrastructure (CNKI), and Wan-Fang Data Knowledge Service Platform (WanFang Data). The Medical Subject Headings (MeSH) search terms were as follows: “tacstd2” or “m1s1 protein” or “tumor-associated calcium signal transducer 2” or “trop2” or “tumor-associated antigen ga733-1” or “ga733-1 antigen” or “trop-2” or “trophoblast cell surface antigen 2” or “membrane component 1 surface marker 1” or “epithelial glycoprotein 1” or “gastrointestinal antigen 733-1” and “cancer” or “tumor” or “carcinoma” or “neoplasm”. We additionally retrieved references cited in the articles and included them in the study. The last search was performed on September 23, 2017.
Selection criteria
Studies that 1) investigated the relationship between TROP2 and patient prognosis; 2) provided available data to obtain or calculate risk ratio (RR) or hazard ratio (HR) for survival and 95% confidence interval (CI); and 3) had clear statement about TROP2 expression state as “high” and “low” or “positive” and “negative” were included in this meta-analysis.
Exclusion criteria were (1) published letters, editorials, abstracts, reviews, case reports and expert opinions; (2) experiments not performed on patients; and (3) articles without the HRs and 95% CI or K–M survival curves about patients’ prognostic outcomes.
Data extraction
The following data were extracted from each publication: first author, year of publication, country, tumor type, clinical stage, sample size, age of patients, analysis method, follow-up period, outcome, parameter cutoff values, survival analysis, estimates such as HRs or RRs concerning the overexpression of TROP2 in terms of OS, disease-free survival (DFS)/progression-free survival (PFS), disease recurrence (DR), and patient clinical characteristics. The HRs or RRs and their 95% CIs were extracted from the original papers directly if available (23 articles, 25 studies). Otherwise, relevant data such as sample number in test groups, log-rank statistics, and p value were used to calculate the variable (3 studiesCitation48–Citation50). Alternatively, the approximate HRs (1 studyCitation15) were calculated according to the Zhou ZR’s statistical method from the Kaplan–Meier survival curves.Citation51 The Engauge Digitizer version 4.1 was used for this analysis.
Statistical analysis
The extracted HRs/RRs were summarized as pooled HR and 95% CI values, using Stata, version 12.0. The fixed-effects model was used at first to calculate the heterogeneity and construct forest plots. For inconsistency tests, I2 > 50% and p < 0.05 were considered statistically significant. Larger values of I2 indicated higher heterogeneity. The fixed-effects model was subsequently used when heterogeneity was not significant (<50%).Citation52 We conducted subgroup analysis and sensitivity analysis to compensate for statistical heterogeneity. Graphical funnel plots were generated, and Begg’s test and Egger’s test were performed to assess the extent of publication bias by visual inspection or by quantitative evaluation.Citation53,Citation54
Results
Study selection and characteristics
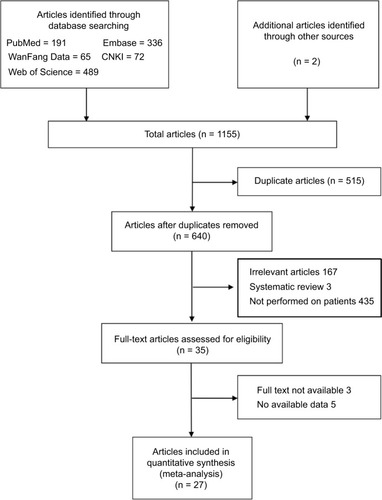
As shown in , a total of 1,155 articles were identified initially. After excluding 515 duplicates, titles/abstracts of 640 studies were reviewed. Of these, 167 articles were not related to the research objective, 435 articles were not performed on patients and 3 were systematic reviews. Thirty-five articles were reviewed further. Three articles were not available to get full text, and five papers did not provide applicable data for meta-analysis. We handpicked the remaining 27 articles eligible for this meta-analysis. The studies by Inamura estimated the roles of TROP2 in cancer prognosis among 3 different lung cancer subtypes (adenocarcinoma, squamous cell carcinoma, and high-grade neuroendocrine tumor), and thus it was regarded as 3 independent studies.Citation55 The main characteristics of these studies are presented in . All included studies were published from 2006 to 2017. There were 17 studies from China, 5 from Japan, 3 from Austria, 3 from Italy, and 1 from South Korea. A total of 4,852 patients were enrolled (sample size: maximum: 702, minimum: 47, and mean: 167), and 16 carcinoma types were analyzed, including lung cancer (6, different subtypes), colorectal cancer (4), bladder cancer (2), breast cancer (2), gallbladder cancer (2), gastric cancer (2), ovarian carcinoma (2), cervical cancer (1), endometrioid endometrial carcinoma (1), extranodal natural killer (NK)/T cell lymphoma/nasal type (1), hilar cholangiocarcinoma (1), laryngeal squamous cell carcinoma (1), nasopharyngeal carcinoma (1), pancreatic cancer (1), pituitary adenomas (1), and squamous cell carcinoma of oral cavity (1). A total of 47 HRs/RRs were extracted from 29 studies, including 26 for OS, 6 for DFS,Citation15,Citation34,Citation35,Citation41,Citation44,Citation56 5 for PFS,Citation13,Citation34,Citation35,Citation39,Citation57 4 for DR,Citation38,Citation49,Citation57,Citation58 3 for CSS,Citation55 and 1 for DFS/PFS.Citation59 Study quality was evaluated by using the Newcastle–Ottawa Scale (NOS), and the quality scores ranged from 6 to 9, suggesting high methodological quality.
Table 1 Main characteristics of the eligible studies in this meta-analysis
Relationship between the expression of TROP2 and patients’ OS
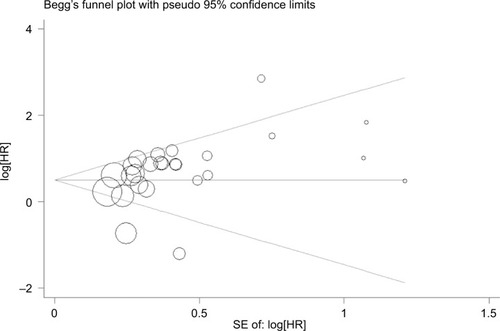
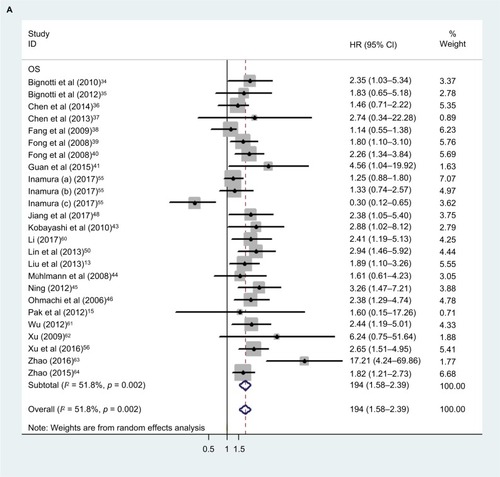
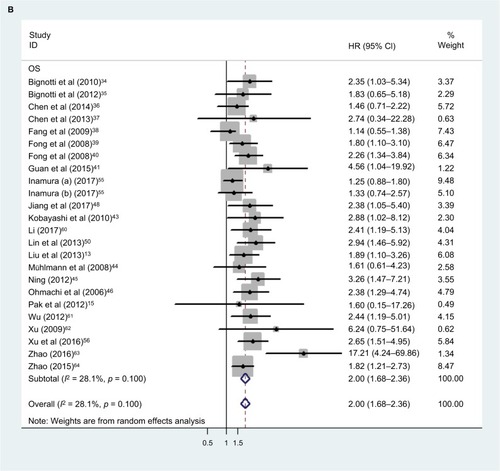
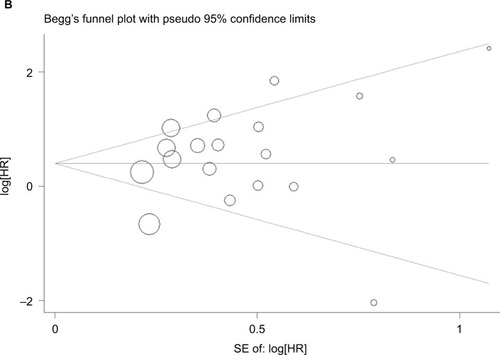
Our analysis revealed a positive link between TROP2 overexpression and OS (pooled HR: 1.84, 95% CI: 1.45–2.35), with heterogeneity (I2 = 67.3%; p = 0.000), indicating that higher level of TROP2 expression could predict shorter OS outcomes ( and ). In subgroup analysis according to geographical location, HRs were greater than 1.0 in the population from China, Austria, with low heterogeneity, in agreement with previous studies (China: I2 = 43.0%, p = 0.044; Austria: I2 = 0.0%, p = 0.762) (). While HRs of Japan and Italy were not statistically significant (), the results of the sensitivity analysis showed that the association between TROP2 and OS was stable, and the studies by Ambrogi et al,Citation49 Inamura et alCitation55 affected results greatly (). After excluding these 2 studies (Ambrogi and Inamura (c)) one by one, the heterogeneity decreased significantly (without Ambrogi: I2 = 51.8%, p = 0.002; without Ambrogi and Inamura (c): I2 = 28.1%, p = 0.100) (). The publication bias evaluation is shown in (Egger’s test: p = 0.048; Begg’s test: p = 0.217). According to Shi’s conclusions,Citation65 we thought that there is no significant publication bias.
Table 2 Results of meta-analysis
Figure 2 Overall analysis and subgroup analysis about patients’ overall survival.
Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not applicable.
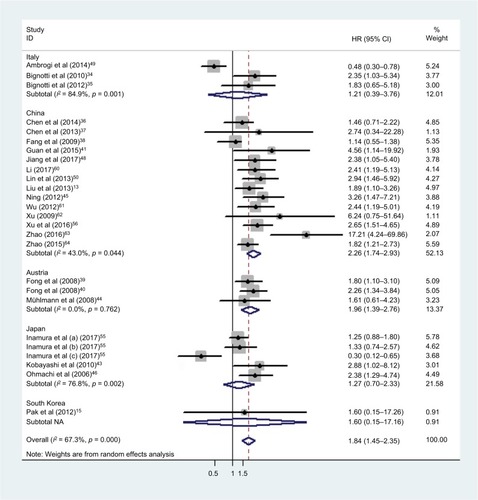
Figure 3 Sensitivity analysis to assess the effect of each study of the meta-analysis about the overall survival (random model).
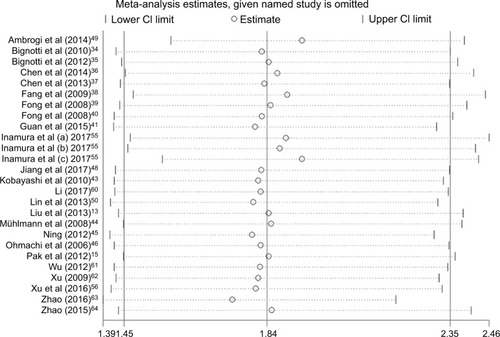
Figure 4 Overall analysis of the correlation between TROP2 expression and patients’ OS after excluding the significant studies which held opposite views.
Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival.
Relationship between TROP2 expression and patient outcomes
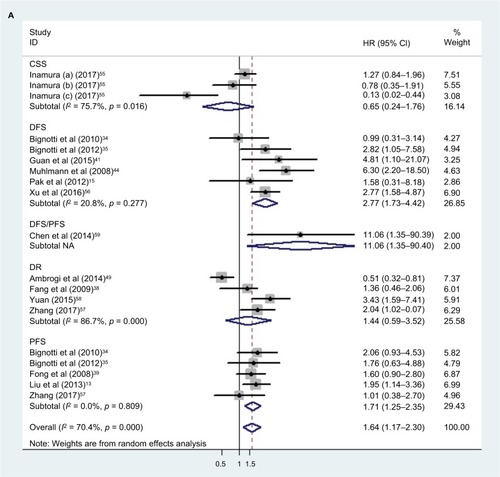
There were 6 studies, 5 studies, 4 studies, 3 studies, and one related to the association between TROP2 expression and DFS, PFS, DR, CSS, and DFS/PFS, respectively. We found that the overexpression of TROP2 was a potential negative prognostic factor for DFS (pooled HR: 2.77, 95% CI: 1.73–4.42) and PFS (pooled HR: 1.71, 95% CI: 1.25–2.35), with low heterogeneity between studies (DFS: I2 =20.8%, p = 0.277; PFS: I2 =0.0%, p = 0.809; random model) (). The association between TROP2 and DR or CSS was not significant (DR: pooled HR: 1.44, 95% CI: 0.59–3.52; I2 =86.7%, p = 0.000; CSS: pooled HR: 0.65, 95% CI: 0.24–1.76; I2 =75.7%, p = 0.016; random model) (). The publication bias analyses were performed, and no significant publication bias was found (Egger’s test: p = 0.297; Begg’s test p = 0.624) ().
Figure 6 The meta-analysis and Begg’s funnel plot of the correlation between TROP2 expression and patients’ DFS/PFS/CSS/DR.
Abbreviations: CSS, cancer-specific survival; DR, disease recurrence; DFS, disease-free survival; PFS, progression-free survival; TROP2, trophoblast cell surface antigen 2; NA, not applicable.
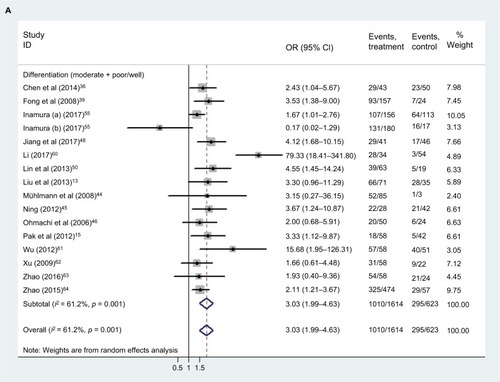
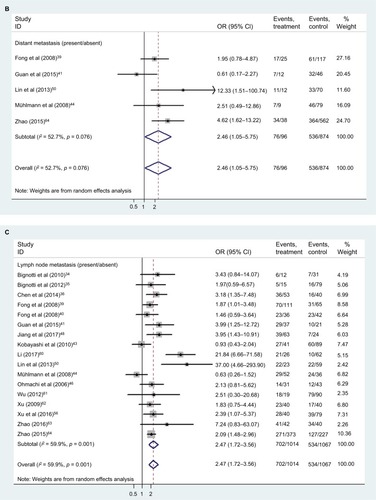
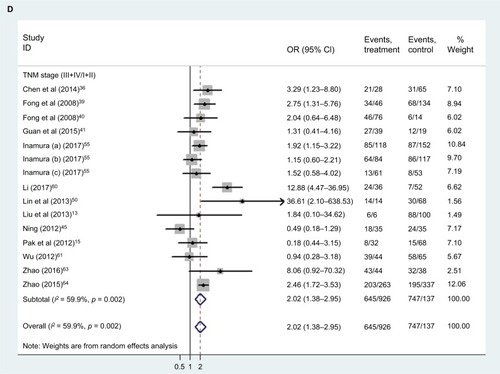
Relationship between TROP2 overexpression and clinical characteristics
shows the patient clinical characteristics, including sex, age, lymph node metastasis, distant metastasis, TNM stage, and differentiation. Our results () showed that TROP2 overexpression correlated with moderate/poor differentiation (pooled HR: 3.03, 95% CI: 1.99–4.63), distant metastasis (pooled HR: 2.46, 95% CI: 1.05–5.75), lymph node metastasis (pooled HR: 2.47, 95%: CI 1.72–3.56), and advanced TNM stage (pooled HR: 2.02, 95% CI: 1.38–2.95) (), with a certain heterogeneity (all: I2 = 52.7–61.2%, p = 0.001–0.076). The sex and age of patients were not significantly linked to the expression level of TROP2 (sex: pooled HR: 1.08, 95% CI: 0.90–1.29; age: pooled HR: 0.94, 95% CI: 0.79–1.11).
Table 3 Relationship between TROP2 overexpression and clinical characteristics
Figure 7 The correlation between TROP2 expression and carcinoma patients’ clinicopathologic features.
Abbreviations: CI, confidence interval; OR, odds ratio; TNM, The TNM Classification of Malignant Tumours; TROP2, trophoblast cell surface antigen 2.
Discussion
This meta-analysis contained data from 4,852 participants, evaluated in 27 articles (29 studies). Overall analysis and subgroup analysis were performed. The results clearly showed that overexpression of TROP2 is significantly associated with poor OS, DFS, PFS, as well as the following clinical characteristics: moderate/poor tumor differentiation, lymph node metastasis, the presence of distant metastasis, and advanced TNM stage. Although some significant heterogeneity was found, the association between TROP2 and cancers was stable, just as sensitivity analysis and publication bias evaluation showed. We found that the studies by Ambrogi et alCitation49 and Inamura et alCitation55 put forward opposite views from the other studies, then we checked them carefully and no obvious error or defect was found. That is why we made this meta-analysis due to the urgent need of further studies with larger sample sizes.
This meta-analysis has both strengths and limitations. A larger sample size compared to a previous studyCitation47 (27 vs 16 articles, 4,852 vs 2,569 patients) powered the study effectively and increased the reliability of the results. However, most of the included papers are retrospective observational studies without control groups. In addition, there were inconsistencies among studies in defining important terms such as: “the overexpression of TROP2”, “the TNM stage”, “differentiation”, and “the cut-off value for age”. Another limitation of this study is that, in some cases, values were indirectly obtained from survival curves or were calculated using related data, probably resulting in some bias because of analytical errors. Furthermore, a wide range of the publication dates meant that other biases may have been introduced due to gradual improvements in detection techniques, surgical efficacy, safety, and medical treatment over time. These limitations were unavoidable and could only be addressed by performing more studies with larger sample sizes.
Currently, the mechanism of TROP2 signaling and its function remain uncertain. The proposed mechanisms of TROP2 action are as follows: regulating calcium levels via protein kinase C (PKC) mitogenic signaling pathway, modulating extracellular regulated protein kinases (ERK) signaling, decreasing cell adhesion to fibronectin via integrin pathway, regulating gene expression via intramembrane proteolysis, causing neuregulin 1 (NRG1) release, and activating the epidermal growth factor family receptor, ErbB3.Citation8 Studies in zebrafish and mice have elucidated the role of TROP2 in the development of lung, intestines, and kidney.Citation66,Citation67 These studies have revealed the role of TROP2 in promoting cell proliferation and organ development. A number of clinical studies overwhelmingly confirmed a strong association between TROP2 expression levels and tumor proliferation, aggressiveness, invasiveness, and metastasis, so they pointed out that TROP2 can be used as a biomarker for clinical diagnosis and to predict prognosis.Citation9,Citation31,Citation35,Citation37,Citation39,Citation42,Citation46,Citation68 Furthermore, recombinant antibodies against TROP2 have been used to treat cancers by inhibiting TROP2 expression or by destroying cancer cells directly. Results from such studies have confirmed the efficacy of TROP2 targeted therapies.Citation24–Citation33 However, normal-born TROP2-knockout mice can survive and grow to adulthood, which means that TROP2 may not be vital for organ and body development, or that its function can be taken over by other proteins.Citation69 In addition, one study has shown that tumorigenesis may result as a consequence of defective TROP2.Citation70
Conclusion
Thus, the function and the mechanisms of action of TROP2 are not clear yet, while the relationship between TROP2 and cell proliferation is complex, possibly determined by tissue type and context.Citation8,Citation55 Further research studies with larger sample sizes should be conducted to learn and confirm its role in cancer occurrence, development, and mechanism of action. In conclusion, the expression of TROP2 is associated with cancer disease, maybe a potential diagnostic indicator and prognostic biomarker.
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- WangCZhangJCaiMDBGC: A Database of Human Gastric CancerPLoS One20151011e014259126566288
- WeitzJKochMDebusJHohlerTGallePRBuchlerMWColorectal cancerLancet2005365945415316515639298
- McWilliamsRRErlichmanCNovel therapeutics in colorectal cancerDis Colon Rectum20054881632165015906130
- WuDHLiuLChenLHAntitumor effects and radiosensitization of cytosine deaminase and thymidine kinase fusion suicide gene on colorectal carcinoma cellsWorld J Gastroenterol200511203051305515918188
- AggarwalSChuECurrent therapies for advanced colorectal cancerOncology (Williston Park, NY)2005195589595
- LipinskiMParksDRRouseRVHerzenbergLAHuman trophoblast cell-surface antigens defined by monoclonal antibodiesProc Natl Acadof Sci U S A198178851475150
- McDougallARTolcosMHooperSBColeTJWallaceMJTrop2: from development to diseaseDev Dyn201524429910925523132
- CubasRZhangSLiMChenCYaoQTrop2 expression contributes to tumor pathogenesis by activating the ERK MAPK pathwayMol Cancer2010925320858281
- FornaroMArcipreteRDStellaMCloning of the gene encoding TROP-2, a cell-surface glycoprotein expressed by human carcinomasInt J Cancer19956256106187665234
- GuerraETrerotolaMDell’ ArcipreteRA bicistronic CYCLIN D1-TROP2 mRNA chimera demonstrates a novel oncogenic mechanism in human cancerCancer Res200868198113812118829570
- JuXJiaoXErtelAv-Src Oncogene Induces Trop2 Proteolytic Activation via Cyclin D1Cancer Res201676226723673427634768
- LiuTLiuYBaoXTianJLiuYYangXOverexpression of TROP2 predicts poor prognosis of patients with cervical cancer and promotes the proliferation and invasion of cervical cancer cells by regulating ERK signaling pathwayPLoS One201389e7586424086649
- NakanishiHTaccioliCPalatiniJLoss of miR-125b-1 contributes to head and neck cancer development by dysregulating TACSTD2 and MAPK pathwayOncogene201433670271223416980
- PakMGShinDHLeeCHLeeMKSignificance of EpCAM and TROP2 expression in non-small cell lung cancerWorld J Surg Oncol2012105322482828
- RipaniESacchettiACordaDAlbertiSHuman TROP-2 is a tumor-associated calcium signal transducerInt J Cancer19987656716769610724
- SawanyawisuthKTantapotinanNWongkhamCSuppression of trophoblast cell surface antigen 2 enhances proliferation and migration in liver fluke-associated cholangiocarcinomaAnn Hepatol2016151718126626643
- StoyanovaTGoldsteinASCaiHDrakeJMHuangJWitteONRegulated proteolysis of Trop2 drives epithelial hyperplasia and stem cell self-renewal via beta-catenin signalingGenes Dev201226202271228523070813
- TrerotolaMCantanelliPGuerraEUpregulation of Trop-2 quantitatively stimulates human cancer growthOncogene201332222223322349828
- TrerotolaMGangulyKKFazliLTrop-2 is up-regulated in invasive prostate cancer and displaces FAK from focal contactsOncotarget2015616143181432826015409
- WangXDWangQChenXLTrop2 inhibition suppresses the proliferation and invasion of laryngeal carcinoma cells via the extracellular signal-regulated kinase/mitogen-activated protein kinase pathwayMol Med Rep201512186587025779928
- WangerTMDewittSCollinsAMaitlandNJPoghosyanZKnauperVDifferential regulation of TROP2 release by PKC isoforms through vesicles and ADAM17Cell Signal20152771325133525817572
- YangJZhuZWangHLiFDuXMaRZTrop2 regulates the proliferation and differentiation of murine compact-bone derived MSCsInt J Oncol201343385986723783522
- WangHLiuQTangXEukaryotic expression of human anti-TROP2 antibody IgG and its inhibitory effect on cell proliferation of pancreatic cancerJ Nanjing Med Univ Nat Sci Ed2014347863869882
- van RijCMFrielinkCSharkeyRMFDG-PET and pretargeted immunoPET of prostate cancer with an anti-TROP2 x anti-HSG bispecific antibody in a nude mouse modelEur J Nucl Med Mol Imaging201239S194S195
- van RijCMFrielinkCGoldenbergDMPretargeted Radioimmunotherapy of Prostate Cancer with an Anti-TROP-2xAnti-HSG Bispecific Antibody and a (177)Lu-Labeled PeptideCancer Biother Radiopharm201429832332925226447
- van RijCMFrielinkCGoldenbergDMPretargeted immunoPET of prostate cancer with an anti-TROP-2 x anti-HSG bispecific antibody in mice with PC3 xenograftsMol Imaging Biol20151719410125060065
- MaoYWangXZhengFThe tumor-inhibitory effectiveness of a novel anti-Trop2 Fab conjugate in pancreatic cancerOncotarget2016717248102482327050150
- LiuXLiSYiFTrop2 gene: a novel target for cervical cancer treatmentJ Cancer Res Clin Oncol201414081331134124816726
- LiuTTianJChenZAnti-TROP2 conjugated hollow gold nanospheres as a novel nanostructure for targeted photothermal destruction of cervical cancer cellsNanotechnology2014253434510325102337
- LinHZhangHWangJA novel human Fab antibody for Trop2 inhibits breast cancer growth in vitro and in vivoInt J Cancer201413451239124923982827
- FarivarTNNajafipourRJohariPNano - drug Delivery of Apoptosis Activator 2 to AGS Cells by Liposomes Conjugated with Anti-TROP2 AntibodyN Am J Med Sci201241158258523181231
- AlbertiSTrerotolaMDell’ARSelective killing of human cancer cells by targeting a fusion mRNA between CYCLIN D1 and TROP2J Clin Oncol20092715_supple14569
- BignottiETodeschiniPCalzaSTrop-2 overexpression as an independent marker for poor overall survival in ovarian carcinoma patientsEur J Cancer201046594495320060709
- BignottiEZanottiLCalzaSTrop-2 protein overexpression is an independent marker for predicting disease recurrence in endometrioid endometrial carcinomaBMC Clin Pathol20121212223151048
- ChenM-BWuH-FZhanYPrognostic value of TROP2 expression in patients with gallbladder cancerTumor Biol201435111156511569
- ChenRLuMWangJIncreased expression of Trop2 correlates with poor survival in extranodal NK/T cell lymphoma, nasal typeVirchows Archiv2013463571323979406
- FangYJLuZHWangGQElevated expressions of MMP7, TROP2, and survivin are associated with survival, disease recurrence, and liver metastasis of colon cancerInt J Colorectal Dis200924887588419421758
- FongDMoserPKrammelCHigh expression of TROP2 correlates with poor prognosis in pancreatic cancerBr J Cancer20089981290129518813308
- FongDSpizzoGGostnerJMTROP2: a novel prognostic marker in squamous cell carcinoma of the oral cavityMod Pathol200821218619118084248
- GuanGFZhangDJWenLJPrognostic value of TROP2 in human nasopharyngeal carcinomaInt J Clin Exp Pathol201589109951100426617817
- HaoWHuiming XuMSShuZMPotential therapeutic target and independent prognostic marker of TROP2 in laryngeal squamous cell carcinomaHead Neck201335101373137822987366
- KobayashiHMinamiYAnamiYExpression of the GA733 gene family and its relationship to prognosis in pulmonary adenocarcinomaVirchows Archiv20104571697620473768
- MuhlmannGSpizzoGGostnerJTROP2 expression as prognostic marker for gastric carcinomaJ Clin Pathol200962215215818930986
- NingSGuoSXieJXuYLuXChenYTROP2 correlates with microvessel density and poor prognosis in hilar cholangiocarcinomaJ Gastrointest Surg201317236036823207686
- OhmachiTTanakaFMimoriKInoueHYanagaKMoriMClinical significance of TROP2 expression in colorectal cancerClin Cancer Res200612103057306316707602
- ZengPChenMBZhouLNTangMLiuCYLuPHImpact of TROP2 expression on prognosis in solid tumors: A Systematic Review and Meta-analysisSci Rep201663365827645103
- JiangAGaoXZhangDZhangLLuHExpression and clinical significance of the Trop-2 gene in advanced non-small cell lung carcinomaOncol Lett20136237538024137332
- AmbrogiFForniliMBoracchiPTrop-2 Is a Determinant of Breast Cancer SurvivalPLos One201495e9699324824621
- LinHHuangJFQiuJRSignificantly upregulated TACSTD2 and Cyclin D1 correlate with poor prognosis of invasive ductal breast cancerExp Mol Pathol2013941737823031786
- ZhouZRZhangTSBoLIMaoZZengXTLiuSXExtracting and transforming of appropriate data of Meta-analysis in survival curveChin J Evid-Based Cardiovasc Med201463243247
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBr Med J2003327741455756012958120
- BeggCBMazumdarMOperating characteristics of a rank correlation test for publication biasBiometrics1994504108811017786990
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- InamuraKYokouchiYKobayashiMAssociation of tumor TROP2 expression with prognosis varies among lung cancer subtypesOncotarget2017817287252873528404926
- XuNZhangZZhuJOverexpression of trophoblast cell surface antigen 2 as an independent marker for a poor prognosis and as a potential therapeutic target in epithelial ovarian carcinomaInt J Exp Pathol201697215015827127000
- ZhangLYangGJiangHTROP2 is associated with the recurrence of patients with non-muscle invasive bladder cancerInt J Clin Exp Med201710116431650
- YuanGExpression and significance of TROP2 in the non-muscle-invasive bladder transitional cell Cancer [Master Thesis]Soochow University2015
- ChenXPangBLiangYOverexpression of EpCAM and Trop2 in pituitary adenomasInt J Clin Exp Pathol20147117907791425550831
- LiXXTengSFXuKExpression of trophoblast cell-surface antigen 2, phosphorylated extracellular signal-regulated kinase 1/2, and cyclin D1 in gallbladder carcinoma tissue and related clinical significanceJ Clin Hepatol2017335909914
- WuHXuHZhangSPotential therapeutic target and independent prognostic marker of TROP2 in laryngeal squamous cell carcinomaHead Neck201335101373137822987366
- XuKYGuJExpression of TROP2 mRNA in left-sided and right-sided colon cancer and its clinical significanceZhonghua Wei Chang Wai Ke Za Zhi2009123285289 Chinese19434540
- ZhaoPChenJCaoWThe expression and clinical significance of TROP-2 protein in colon cancerChongqing Med2016453549634966
- ZhaoWZhuHZhangSTrop2 is overexpressed in gastric cancer and predicts poor prognosisOncotarget2016756136614526716416
- ShiXComparison of the Power Difference of Egger’s Test and Begg’s Test and the Reason AnalysisActa Med Univ Sci Et Technologiae Huazhong20093819193
- TsukaharaYTanakaMMiyajimaATROP2 expressed in the trunk of the ureteric duct regulates branching morphogenesis during kidney developmentPLos One2011612e2860722194864
- MustataRCVasileGFernandezvalloneVIdentification of Lgr5-independent spheroid-generating progenitors of the mouse fetal intestinal epitheliumCell Rep20135242124139799
- TrerotolaMAlbertiSLanguinoLRTrop2 modulates beta1 integrin-mediated adhesion and migration of prostate cancer cellsProceedings of the American Association for Cancer Research Annual Meeting20105112461246
- WangJZhangKGrabowskaDLoss of Trop2 promotes carcinogenesis and features of epithelial to mesenchymal transition in squamous cell carcinomaMol Cancer Res20119121686169521970857
- LinJCWuYYWuJYTROP2 is epigenetically inactivated and modulates IGF-1R signalling in lung adenocarcinomaEMBO Mol Med20124647248522419550