Abstract
Background
The purpose of this study was to clarify whether pretreatment tumor burden-related index, including the gross tumor volume (GTV) of metastatic lymph nodes (VLN) and maximum diameter of metastatic lymph nodes (DLN), and inflammatory markers, consisting of neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), are useful for assessing the therapeutic effects and prognosis with secondary lymph node metastasis (LNM) receiving chemoradiotherapy (CRT) or radiotherapy (RT) alone after resection of esophageal squamous cell carcinoma (ESCC).
Patients and methods
A total of 119 patients with secondary LNM after resection of ESCC were recruited and received curative RT only or CRT. The enrolled patients were grouped according to the median values of NLR, PLR, VLN, and DLN. The relationship between the responsiveness to treatment and these markers was analyzed by logistic analysis. The Kaplan–Meier method and log-rank test were adopted to calculate and compare the overall survival (OS) rates with these markers. The Cox models were used to carry out multivariate analyses.
Results
Univariate logistic regression analysis showed that the responses to treatment were highly associated with treatment method (P=0.011), NLR (P=0.000), PLR (P=0.003), VLN (P=0.000), and DLN (P=0.000). Next, multivariate logistic regression analysis showed that therapeutic method (hazard ratio [HR]=1.225, P=0.032), NLR (HR=2.697, P=0.019), and VLN (HR=4.607, P=0.034) were independent risk factors for tumor response. Additionally, Kaplan–Meier survival analysis of this cohort revealed that NLR (χ2=27.298, P=0.000), PLR (χ2=16.719, P=0.000), VLN (χ2=48.823, P=0.000), DLN (χ2=40.724, P=0.000), and treatment methods (χ2=18.454, P=0.018) were significantly associated with OS. Furthermore, multivariate analysis was performed, and the results showed that therapeutic method (HR=1.223, P=0.048), NLR (HR=2.000, P=0.018), VLN (HR=2.379, P=0.020), and DLN (HR=2.901, P=0.002) were considered independent prognostic factors for OS.
Conclusion
This study found that NLR and VLN were promising as predictive markers for therapeutic effects, and NLR combined with VLN and with DLN might be useful biomarkers in predicting outcomes in patients with secondary LNM receiving CRT or single RT after esophagectomy.
Introduction
Thoracic esophageal squamous cell carcinoma (ESCC) consists of >90% of the esophageal cancer cases in East Asia, and tumors located in the upper and middle thoracic esophagus (Mt) are most commonly observed. Surgery is still a preferred therapeutic strategy for patients with thoracic ESCC. However, the recurrence rate of ESCC is as high as 50% after radical surgery during the follow-up period,Citation1 and locoregional recurrence (especially single-station lymph node recurrence) is the major cause of treatment failure,Citation2,Citation3 which correlated with an unfavorable prognosis.
At present, chemoradiotherapy (CRT) is the main treatment method for the patients with secondary lymph node metastasis (LNM) after esophagectomy; however, the therapeutic effect has not been obviously improved, and this phenomenon may be related to the gross tumor volume (GTV) of metastatic lymph nodes (VLN) and maximum diameter of metastatic lymph nodes (DLN). It is generally recognized that the high tumor burden is correlated with poor sensitivity of CRT, and the long-term prognosis is inferior. Chen et alCitation4 reported that GTV defined on radiotherapy (RT) planning scans may serve as a good prognostic factor for ESCC patients treated with radical RT; however, the prognostic value of the patients with postoperative nodal recurrences who underwent RT or CRT remains unclear.
Recently, some trials reported a close relationship between systemic hematological markers and prognosis in human malignancies,Citation5–Citation9 and the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) have been studied in various malignancies.Citation10–Citation15 These markers, which can be measured easily and inexpensively, are widely used in clinical practice and may contribute to predict an unfavorable prognosis in patients with esophageal carcinoma.Citation10,Citation16,Citation17 This correlation has been well documented in other types of human malignancies, but the combination of tumor burden markers, which are represented by GTV and maximum diameter, and hematological markers has rarely been studied in patients with LNM after resection of ESCC. Therefore, the purpose of this study was to clarify whether pretreatment tumor burden-related index and inflammatory markers, including VLN, DLN, NLR, and PLR, were useful for assessing the therapeutic effects and prognosis with LNM receiving CRT or radiation (RT) alone after resection of ESCC.
Patients and methods
Patients
Between January 2011 and December 2014, a total of 119 esophageal carcinoma patients with secondary LNM after resection of ESCC at the Department of Thoracic Surgery, the Affiliated Taixing People’s Hospital of Yangzhou University were recruited in this retrospective study. The inclusion criteria were as follows: 1) secondary LNM after curative esophagectomy; 2) the diagnosis of LNM was performed by pathologic conformation or short axis of ≥10 mm in mediastinum and cervix or short axis of ≥5 mm in tracheoesophageal groove with enhanced computed tomography (CT) imaging; 3) normal liver and renal function, without severe dysfunction of important organs, and overall performance status of 0 or 1; 4) complete record of pretreatment hematological variables; 5) no presence of distant metastasis; 6) the patients with complete follow-up time ≥1 year; 7) no presence of infection or inflammatory conditions, such as rheumatologic conditions, connective tissue disorders, or heart diseases. Finally, 119 patients were enrolled and analyzed in this study. Clinicopathological features were obtained from the patients’ records. This research was approved by the ethics committee of the Affiliated Taixing People’s Hospital of Yangzhou University. Informed consent was obtained from all individual participants included in this study.
Treatment modalities
All patients were treated with three-dimensional conformal RT (3-DCRT). Definitive RT alone (n=32) or in combination with chemotherapy (n=87) was intended to be administered. All treatments were planned based on CT simulation planning system with 4 mm thickness scan slice throughout the entire neck and thorax. A total dose of up to 60.0–64.0 Gy was delivered by standard fractionated RT in 30–32 fractions (2.0 Gy per fraction; over 6–7 weeks). Concurrent chemotherapy consisted of a daily dose of cisplatin (25 mg/m2, days 1–4) with Paclitaxel (135–175 mg/m2, day1) for 28 days per cycle, for a total of two cycles.
The target volumes were defined as follows: 1) GTV: metastatic lymph node; 2) clinical target volume (CTV): GTV +2 cm margins in the metastatic lymph node long axis, superiorly and inferiorly to encompass potential invasions; and 3) planning target volume (PTV): CTV +0.5 cm margin.
Assessment of therapeutic effect
Clinical responses were assessed by CT scan 1 month after RT with or without two cycles of chemotherapy. Tumor response was assessed by the Response Evaluation Criteria in Solid Tumors (RECIST) guideline (version 1.1).Citation18 Accordingly, tumor response was divided into four groups as follows: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Patients demonstrating CR or PR after treatment were defined as responders, whereas those exhibiting SD or PD were classified as non-responders.
Data collection and follow-up
Images were retrieved from the patients’ database, and the GTV and maximum diameter of metastatic lymph nodes (VLN and DLN) were calculated using the Monaco 5.1 system for each patient.
The following pretreatment hematological parameters were collected within 1 week prior to the initial treatment: neutrophil count, lymphocyte count, monocyte count, and platelet count. The NLR and PLR were calculated by division of the absolute values of the corresponding hematological parameters.
After the completion of treatment, all patients were asked to return to the hospital for examination every 3 months for the first year, every 6 months for the next 2 years, and then annually. The duration of follow-up was calculated from the day of treatment to the day of death or July 2017.
Statistical analysis
VLN, DLN, NLR, and PLR were divided into high/low group by the corresponding median value. Univariate logistic analysis was performed to determine which variables were associated with response to therapy. Variables generating P-values ≤ 0.05 by univariate logistic analysis were subjected to multivariate logistic regression analysis.
The overall survival (OS) curves based on pretreatment VLN, DLN, NLR, and LMR were plotted using the Kaplan–Meier method, and differences were assessed by the log-rank test. Univariate and multivariate analyses of Cox regression proportional hazard model were used to evaluate the influence of each variable on OS with the enter method. Hazard ratio (HR) with 95% confidence interval (CI) was used to quantify the strength of the association between predictors and survival. ROC curves were also plotted to verify the accuracy of VLN, DLN, NLR, and PLR for therapeutic effect and OS prediction. A two-tailed P-value ≤ 0.05 was considered statistically significant. Statistical analyses were performed using the Statistical Package for Social Sciences version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Patients and clinicopathological features
A total of 87 male (73.1%) and 32 female (26.9%) patients were investigated. The median age was 63 years (range: 46–78). Primary tumors were located in the upper thoracic esophagus (Ut) in six patients (5.0%), in the Mt in 78 patients (65.5%), and in the lower thoracic esophagus (Lt) in 35 patients (29.5%). Single RT was administered to 32 patients; concurrent CRT was delivered to 87 patients. With a median follow-up time of 18 months (range: 4–36 months), 94 patients (79%) were dead at the end of follow-up time. The clinical and pathological characteristics of 119 patients are shown in .
Table 1 Clinicopathological features of 119 patients with LNM after esophagectomy.
Correlation between therapeutic efficacy and VLN, DLN, NLR, and PLR
A total of 119 patients with LNM after resection of esophageal carcinoma were grouped according to the median values of NLR, PLR, and the size of LNM, including VLN and DLN, as shown in . The relationship between the responsiveness of treatment and these markers was analyzed by univariate logistic regression analysis. The results demonstrated that responses to treatment were highly associated with treatment method (P=0.011), NLR (P=0.000), PLR (P=0.003), VLN (P=0.000), and DLN (P=0.000). Next, multivariate logistic regression analysis showed that therapeutic method (HR=1.225, 95% CI: 1.085–2.837, P=0.032), NLR (HR=2.697, 95% CI: 1.201–7.429, P=0.019), and VLN (HR=4.607, 95% CI: 1.124–18.889, P=0.034) were independent risk factors for tumor response.
Table 2 Univariate and multivariate logistic regression analyses between tumor response and NLR, PLR, VLN, DLN of 119 patients with LNM after resection of ESCC
Prognostic analysis based on NLR, PLR, VLN, and DLN
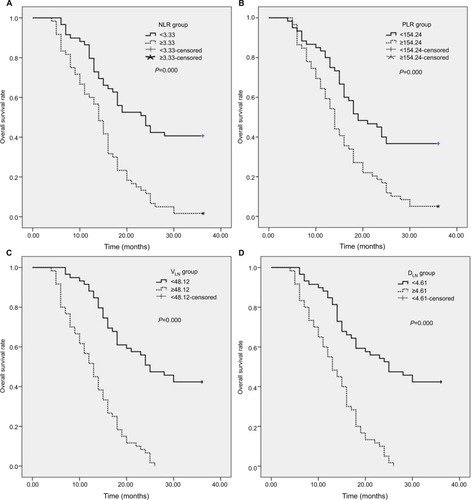
For all patients, the median OS time was 16 months. The OS rates at the 1-, 2-, and 3-year period were 69.7%, 28.6%, and 21.1%, respectively. As shown in , in the NLR < 3.33 group, the 1-, 2-, and 3-year OS rates were 79.7%, 45.5%, and 40.6%, respectively, while in the NLR ≥ 3.33 group, the OS rates were 60.4%, 11.8%, and 2.7%, respectively (; χ2=27.298, P=0.000). In the PLR < 154.24 group, the 1-, 2-, and 3-year OS rates were 80.0%, 40.0%, and 36.7%, respectively, and in the PLR ≥ 154.24 group, the OS rates were 59.3%, 16.9%, and 5.1%, respectively (; χ2=16.719, P=0.000). In the VLN < 48.12 cm3 group, the 1-, 2, and 3-year OS rates were 88.1%, 50.8%, and 42.4%, respectively, while in the VLN ≥ 48.12 cm3 group, the OS rates were 51.7%, 6.7%, and 1.8%, respectively (; χ2=48.823, P=0.000). In addition, in the DLN < 4.61 cm group, the 1-, 2-, and 3-year OS rates were 84.7%, 52.5%, and 42.4%, respectively, while in the DLN ≥ 4.61 cm group, the OS rates were 55.0%, 6.9%, and 1.6%, respectively (; χ2=40.724, P=0.000).
Figure 1 Kaplan–Meier curves for OS stratified according to NLR, PLR, VLN, and DLN median values.
Notes: (A) OS curves grouped by NLR median value (the 3-year OS for low NLR 40.7%, high NLR 2.7%; P=0.000). (B) OS curves stratified according to PLR median value (the 3-year OS for low PLR 36.7%, high PLR 5.1%; P=0.000). (C) OS curves stratified by VLN median value (the 3-year OS for low VLN 42.4%, high VLN 1.80%; P=0.000). (D) OS curves grouped by DLN median value (the 3-year OS for low DLN 42.4%, high DLN 1.60%; P=0.000). VLN, the GTV of metastatic lymph nodes; DLN, the maximum diameter of metastatic lymph nodes
Abbreviations: GTV, gross tumor volume; OS, overall survival; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
shows the OS curves based on pretreatment NLR, PLR, VLN, and DLN. Our results indicated that NLR, PLR, VLN, DLN, and treatment methods were significantly associated with OS using the univariate analysis. Furthermore, multivariate Cox proportional hazard regression model analysis for OS was performed to identify the prognostic factors for enrolled patients treated with RT or CRT. The results showed that NLR, VLN, DLN, and therapeutic method were considered independent prognostic factors for OS, whereas PLR did not indicate a statistical difference associated with OS ().
Table 3 Univariate and multivariate analyses of the NLR, PLR, VLN, and DLN for the prediction of overall survival in patients with LNM after resection of ESCC (N=119)
ROC curve for therapeutic responsiveness and OS prediction
ROC curves for therapeutic efficacy were plotted to verify the median values of NLR, PLR, VLN, and DLN (). As shown in , the area under the curve (AUC) for NLR, PLR, VLN, and DLN was 0.709 (95% CI: 0.583–0.834, P=0.004), 0.636 (95% CI: 0.511–0.781, P=0.061), 0.668 (95% CI: 0.536–0.799, P=0.021), and 0.616 (95% CI: 0.511–0.781, P=0.051), respectively. The results indicated that NLR and VLN were superior to PLR and DLN as a predictive factor for therapeutic responsiveness in patients with LNM after resection of ESCC.
Figure 2 The ROC curves grouped by NLR, PLR, VLN, and DLN.
Notes: (A) ROC curves based on therapeutic effect. NLR is represented by the green line with an AUC=0.709; PLR is represented by the blue line with an AUC=0.636; VLN is represented by the purple line with an AUC = 0.668; and DLN is represented by the red line with an AUC=0.616. (B) ROC curves for OS. NLR is represented by the green line with an AUC=0.767; PLR is represented by the blue line with an AUC=0.633; VLN is represented by the red line with an AUC=0.808; and DLN is represented by the purple line with an AUC=0.817. VLN, the GTV of metastatic lymph nodes; DLN, the maximum diameter of metastatic lymph nodes.
Abbreviations: GTV, gross tumor volume; AUC, area under the curve; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; ROC: receiver operating characteristic.
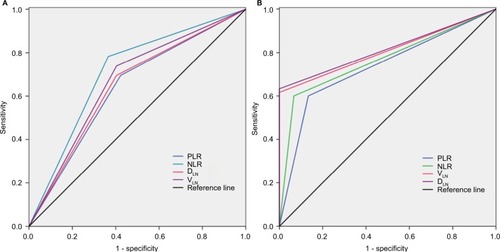
ROC curves for OS were also plotted, the AUC was 0.767 (95% CI: 0.650–0.884, P=0.001) for NLR, 0.633 (95% CI: 0.602–0.724, P=0.053) for PLR, 0.808 (95% CI: 0.712–0.904, P=0.000) for VLN, and 0.817 (95% CI: 0.723–0.910, P=0.000) for DLN, indicating that NLR, VLN, and DLN were superior to PLR as a predictive factor for OS in patients with LNM after esophagectomy ().
Correlations among the NLR and PLR, VLN, and DLN
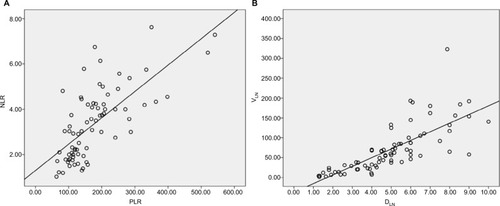
As shown in , univariate analysis showed that a high NLR and PLR and a high VLN and DLN were all individually associated with an unfavorable survival outcome. Furthermore, the correlations between PLR and NLR and between VLN and DLN were examined using Pearson correlation analysis (). The results showed that there were moderate correlations between PLR and NLR and between VLN and DLN (correlation coefficient R2=0.493 and 0.572, respectively).
Figure 3 Correlations among the prognostic markers.
Notes: (A) Correlation chart between NLR and PLR (regression line: Y=1. 301+0.012´X, correlation coefficient: R2=0.493, P=0.000). (B) Correlation chart between VLN and DLN (regression line: Y= −36.990+21.731×X, correlation coefficient: R2=0.572, P=0.000). VLN, the GTV of metastatic lymph nodes; DLN, the maximum diameter of metastatic lymph nodes.
Abbreviations: GTV, gross tumor volume; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
Discussion
There are several clinical data supporting that the survival of cancer patient is determined not only by tumor itself but also by host-related factors, such as the systemic inflammatory status. In this clinical study, we investigated the significance for the tumor treatment response and survival prognosis of inflammatory markers and tumor burden-related index in patients with secondary LNM after resection of ESCC. This study indicated that NLR (P=0.019), treatment strategies (P=0.032), and VLN (P=0.034) were independent risk factors for tumor treatment efficacy. While the NLR, VLN, and DLN were considered independent prognostic factors for OS. To our knowledge, this is the first report to demonstrate the clinical significance of NLR, PLR, VLN, and DLN in patients with LNM receiving CRT or RT after esophagectomy.
At present, surgery is the most important treatment strategy for patients with non-metastatic thoracic ESCC. However, the prognostic outcome is unsatisfactory, and the recurrence rate of ESCC is high after radical surgery. Liu et alCitation1 demonstrated that ~50% of patients with ESCC developed treatment failure during the follow-up period. Single-station LNM was the most common type of failure and the location of metastasis in the bilateral supraclavicular areas as well as the superior mediastinum was more frequent than in other regions. In the clinical practice, the OS rate of patients with locoregional recurrence was worse, and one of the reasons may consist of tumor volume affecting prognostic outcomes. One previous study demonstrated that larger GTV did predict a poorer prognosis in ESCC patients treated with radical radiochemotherapy.Citation4 A large tumor burden means more hypoxic cells, which is resistant to treatment, so the prognosis is worse. The negative impact of tumor hypoxia on survival is related to radiobiological mechanisms caused by hypoxia, which may include 1) the reduced oxygen enhancement effect, 2) increased radioresistance due to expression of genes for cell cycle delay and stress proteins, and/or 3) accelerated tumor progression to more radioresistant and metastatic variants by increased genetic heterogeneity.Citation19 In the present study, we determined that the GTV and maximum diameter of metastatic lymph nodes (VLN and DLN) are useful for assessing the therapeutic effects and prognosis in ESCC patients with LNM after resection. The results found that patients who suffered the large tumor burden (VLN ≥ 48.12 cm3 and DLN ≥ 4.61 cm) had significantly worse therapeutic efficacy and OS than those who suffered small tumor burden (VLN < 48.12 cm3 and DLN < 4.61 cm).
In the case of hematological markers, a high NLR was significantly associated with poor response to treatment and unfavorable OS in patients with LNM after resection of ESCC. Since the pathologist Rudolf Virchow first discovered leukocytes in malignant tissue specimens ~150 years ago,Citation20 the prognostic values of pretreatment hematological markers have been highlighted. Currently, compelling evidence suggested that there were statistically significant differences in the therapeutic response and survival rates grouped by blood inflammatory markers for several types of malignancies.Citation10–Citation15,Citation21,Citation22 Multiple studies have demonstrated that hyperfibrinogenemia and elevated NLR or the combination of NLR with PLR were the predictors of poor therapeutic response before initial treatment.Citation21,Citation22 Duan et alCitation23 reported that preoperative serum NLR is a useful prognostic marker to complement TNM staging for operable ESCC patients, particularly in patients with Stage IIIA disease. In clinical practice, variations in NLR and PLR can reflect changes in the relative number of neutrophils, platelets, and lymphocytes. Tumor cells can produce granulocyte colony-stimulating factor, tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1, and IL-6, which can influence leukocyte and neutrophil counts in the bloodstream.Citation24 Some research also showed that platelets could enhance hematogenous metastasis by stabilizing tumor cell arrest in the vasculature, activating tumor cell proliferation, boosting tumor cell extravasation, and enhancing tumor cell interaction with the extracellular matrix.Citation25 In contrast, lymphocytopenia can also stimulate the release of suppressive immunological mediators, such as transforming growth factor-β (TGF-β) and IL-10, resulting in immunosuppression and consequently weakening of the lymphocyte functions.Citation26 Our univariate analysis suggested that the pretreatment NLR and PLR were independent risk factors for therapeutic responsiveness and OS in patients with LNM after esophagectomy. Furthermore, the multivariate analysis showed that only NLR < 3.33 had significantly better therapeutic efficacy (HR=2.697, 95% CI: 1.201–7.429, P=0.019) and OS (HR=2.000, 95% CI: 1.127–3.548, P=0.018) than those who had NLR ≥ 3.33
This study has several potential limitations. First, concerning diagnostic criteria of LNM, the selection of enrolled patients mainly depended on positive CT scans instead of pathological biopsy during the observation period, which might have led to an inherent bias. Second, not all hemato-logical markers of inflammation were used in this analysis, because some biomarkers were not routinely examined, such as C-reactive proteinCitation27,Citation28 and fibrinogen.Citation21 Third, it was a single-institution, retrospective study. Finally, 119 patients were enrolled in this study, and the sample size is small and may be insufficient to strengthen our results. Given these limitations, future larger randomized trials are needed to clarify these results.
Conclusion
In summary, this study demonstrated that NLR and VLN were promising as predictive markers for therapeutic effects, and NLR combined with VLN and DLN might be useful biomarkers in predicting outcomes in patients with LNM receiving CRT or single RT after esophagectomy. However, considering the retrospective nature of this study, large-scale prospective trials are still warranted to verify these results.
Acknowledgments
This research was supported by a grant from Suzhou Cancer Clinical Medical Center (grant number Szzx201506).
Disclosure
The authors report no conflicts of interest in this work.
References
- LiuQCaiXWWuBZhuZFChenHQFuXLPatterns of failure after radical surgery among patients with thoracic esophageal squamous cell carcinoma: implications for the clinical target volume design of postoperative radiotherapyPLoS One201495e9722524820177
- NakagawaSKandaTKosugiSOhashiMSuzukiTHatakeyamaKRecurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomyJ Am Coll Surg2004198220521114759776
- ChenGWangZLiuXYLiuFYRecurrence pattern of squamous cell carcinoma in the middle thoracic esophagus after modified Ivor-Lewis esophagectomyWorld J Surg20073151107111417426905
- ChenYZhangZJiangGZhaoKGross tumor volume is the prognostic factor for squamous cell esophageal cancer patients treated with definitive radiotherapyJ Thorac Dis2016861155116127293832
- HolgerssonGSandelinMHoyeESwedish lung cancer radiation study group: the prognostic value of anaemia, thrombocytosis and leukocytosis at time of diagnosis in patients with non-small cell lung cancerMed Oncol20122953176318222565809
- NjolstadTSEngerudHWernerHMSalvesenHBTrovikJPreoperative anemia, leukocytosis and thrombocytosis identify aggressive endometrial carcinomasGynecol Oncol2013131241041524004646
- RoxburghCSMcMillanDCRole of systemic inflammatory response in predicting survival in patients with primary operable cancerFuture Oncol20106114916320021215
- RassouliASalibaJCastanoRHierMZeitouniAGSystemic inflammatory markers as independent prognosticaters of head and neck squamous cell carcinomaHead Neck201537110311024339165
- KozakMMvon EybenRPaiJSThe prognostic significance of pretreatment hematologic parameters in patients undergoing resection for colorectal cancerAm J Clin Oncol201740440541225756348
- YodyingHMatsudaAMiyashitaMPrognostic signifcance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: a systematic review and meta-analysisAnn Surg Oncol201623264665426416715
- AbsengerGSzkanderaJPichlerMA derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patientsBr J Cancer2013109239540023820252
- KohCHBhoo-PathyNNgKLUtility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancerBr J Cancer2015113115015826022929
- XuAMHuangLZhuLWeiZJSignificance of peripheral neutrophil-lymphocyte ratio among gastric cancer patients and construction of a treatment-predictive model: a study based on 1131 casesAm J Cancer Res20144218919524660108
- CannonNAMeyerJIyengarPNeutrophil-lymphocyte and platelet-lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non-small-cell lung cancerJ Thorac Oncol201510228028525299234
- FanWZhangYWangYYaoXYangJLiJNeutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of survival and metastasis for recurrent hepatocellular carcinoma after transarterial chemoembolizationPLoS One2015103e011931225742141
- XieXLuoKJHuYWangJYChenJPrognostic value of preoperative platelet–lymphocyte and neutrophil–lymphocyte ratio in patients undergoing surgery for esophageal squamous cell cancerDis Esophagus2016291798525410116
- FengJFHuangYChenQXPreoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinomaWorld J Surg Oncol2014125824641770
- EisenhauerEATherassePBogaertsJNew response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1)Eur J Cancer200945222824719097774
- HöckelMSchlengerKMitzeMSchäfferUVaupelPHypoxia and radiation response in human tumorsSemin Radiat Oncol1996613910717157
- BalkwillFMantovaniAInflammation and cancer: back to VirchowLancet2001357925553954511229684
- KijimaTArigamiTUchikadoYCombined fibrinogen and neutrophil-lymphocyte ratio as a prognostic marker of advanced esophageal squamous cell carcinomaCancer Sci2017108219319927889946
- ZhuSMiaoCWWangZTPengLLiBWSensitivity value of hematological markers in patients receiving chemoradiotherapy for esophageal squamous cell carcinomaOnco Targets Ther201696187619327789959
- DuanHZhangXWangFXPrognostic role of neutrophil-lymphocyte ratio in operable esophageal squamous cell carcinomaWorld J Gastroenterol201521185591559725987784
- NowarskiRGaglianiNHuberSFlavellRAInnate immune cells in inflammation and cancerCancer Immunol Res201312778424777498
- HonnKVTangDGCrissmanJDPlatelets and cancer metastasis: a causal relationship?Cancer Metastasis Rev1992113–43253511423821
- Salazar-OnfrayFLopezMNMendoza-NaranjoAParadoxical effects of cytokines in tumor immune surveillance and tumor immune escapeCytokine Growth Factor Rev2007181–217118217329145
- ThurnerEMKrenn–PilkoSLangsenlehnerUThe elevated C-reactive protein level is associated with poor prognosis in prostate cancer patients treated with radiotherapyEur J Cancer201551561061925618827
- SzkanderaJStotzMAbsengerGValidation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patientsBr J Cancer2014110118318824201751
