Abstract
Background
Accumulating evidence has suggested a relationship between calcium-sensing receptor (CASR) polymorphisms and cancer risk in different types of cancer; however, the findings from epidemiologic studies have been conflicting. The purpose of this meta-analysis was to assess the clinical susceptibility of CASR polymorphisms in cancer patients.
Materials and methods
This study systematically searched MEDLINE and EMBASE databases for eligible articles through March 2017. The strength of association was expressed as odds ratio and 95% CI. Publication bias, heterogeneity, sensitivity analysis, and subgroup analyses were also examined.
Results
Fourteen related case–control studies were finally identified to be included in the present analysis. The pooled result showed that no significant associations were found among CASR rs1801725, rs1042636, rs12485716, rs4678174, rs1801726, rs17251221, rs10934578, and rs2270916 polymorphisms and cancer risk under all genetic models (P>0.05). The relationship between CASR rs1801725 polymorphism and risk of cancer was consistent in the subgroup analyses, and robust in sensitivity analysis. No publication bias was presented in our pooled-analysis.
Conclusion
The current evidence for our pooled analysis suggests that the CASR polymorphisms are not associated with an increased risk of cancer. Further larger studies are still necessary to warrant and validate the findings in the current meta-analysis.
Introduction
Cancer remains a major public challenge for health care worldwide, with ~12.7 million new cancer cases and 7.6 million cancer-related deaths in 2012.Citation1 Although the exact mechanism of tumor pathogenesis is not clearly exposed, accumulating evidence supported that both environmental and genetic factors contribute to the etiology of cancer.Citation2 The use of single-nucleotide polymorphisms (SNPs) in candidate genes is considered to be an approach to identify novel susceptibility genes for the cancer development. Currently, several epidemiologic studies have indicated that high dietary calcium supplement or high serum calcium may be associated with an increased cancer risk, particularly lethal or advanced disease.Citation3
The calcium-sensing receptor (CASR) belongs to the metabotropic glutamate receptor subclass of G-protein-coupled receptors, and is located on chromosome 3p13.3–21.Citation4 The central role of CASR in calcium homeostasis was through its regulation of parathyroid hormone (PTH) secretion, as well as calcium re-absorption.Citation5 The expression of CASR has previously been reported in bone, parathyroid, and kidney, with clear roles in calcium homeostasis.Citation6 Further research has demonstrated that the functional expression of CASR was also described in other tissues, including prostate, breast, and colon cancer tissue. Loss and gain of function mutations of CASR shows that changes in calcium ion (Ca2+) may mediate by the intracellular signaling. CASR may also influence several tumor-related processes, independent of circulating calcium level regulation.Citation7 Evidence from the epidemiologic studies shows that the SNPs in CASR gene related to serum calcium could function as risk markers, which is of importance for the development of diseases.Citation8 Moreover, genome-wide association studies (GWAS) have also evaluated gene–environment (ie, SNPs in CASR gene and calcium intake) interactions associated with the risk of cancer.Citation9 However, the role of CASR polymorphisms in cancer susceptibility/incidence is not fully understood, due to the relatively small sample sizes and inconclusive results of these studies. We, therefore, performed the present pooled analysis based on all the published data to shed more light on the impact of CASR gene polymorphisms in susceptibility to cancer. To assess the prevalence of these SNPs, the influence of cancer types and ethnicity for cancer risk were also examined in the present analysis. The results of our analysis provide new insight that could be recommended for further investigation.
Materials and methods
Searching strategy
All relevant studies concerning the associations between the CASR polymorphisms and cancer risk, and published from their inception to April 2016, were identified by comprehensive searches of electronic databases, including Pubmed, EMBASE, Web of Science, China National Knowledge Infrastructure (CNKI), SinoMed, and WanFang databases, without any restrictions. The following search key words and medical subject heading terms were used: (“Calcium-Sensing Receptor” OR “CASR”) AND (“cancer” OR “malignancies” OR “neoplasms” OR “tumor” OR “carcinoma”) AND (“polymorphism” OR “variation” OR “variant” OR “single nucleotide polymorphism” OR “mutation” OR “SNP” OR “mutant”). Additionally, reference lists of all the included studies and relevant review articles were also manually searched to identify any eligible studies for inclusion.
Inclusion criteria and exclusion criteria
The included papers should meet the following criteria: 1) assessment of the relationship between cancer risk and the CASR polymorphisms; 2) with a case–control study design; and 3) detailed genotype frequency of cases and controls were provided directly or could be calculated from the article text. Exclusion criteria were: 1) letters, reviews, or case reports; 2) not concerned with cancer risk; 3) overlapping study populations; 4) animal studies; and 5) studies with incomplete data.
Data extraction
For studies that met our inclusion criteria, the following variables were recorded from each eligible study: the first author’s name, year of publication, participant mean age and sex, cancer site, country and ethnicity of the study population, source of control, sample size, number of cases and controls, genotype frequency, and evidence of Hardy–Weinberg equilibrium (HWE) in controls.
Statistical analysis
Crude odds ratios (ORs) with 95% CIs between the CASR polymorphisms and cancer risk, based on genotype frequencies, in cases and controls were pooled to measure the strength of the association. The pooled ORs with 95% CIs were calculated in homozygote model (AA vs BB), heterozygote comparison (AB vs BB), dominant model (AB + AA vs BB), and recessive model (AA vs AB + BB). Statistical heterogeneity across studies was evaluated by using I2 statistics. I2 values of 25%, 50%, and 75% were assigned to have a low, moderate, and high degree of heterogeneity, respectively.Citation10 The chi-square test was conducted to test the distribution of HWE in controls, and P<0.05 indicated disequilibrium of HWE. We then carried out the following subgroup analyses: ethnicity (categorized as North-Americans or Europeans) and cancer types (categorized as colorectal cancer [CRC] or prostate cancer). The subgroup analyses were performed only for the rs1801725 polymorphism because of the small number of studies for other SNPs. To test the robustness of the results, sensitivity analyses were also performed by removing one study at each turn and examining the influence of each individual study on the overall risk estimate. Publication bias of the studies included was examined by using Begg’s funnel plots and Egger’s test.Citation11 A two-tailed P<0.05 was considered statistically significant. All statistical tests were performed using STATA statistical software version 12.0 (StataCorp, College Station, TX, USA).
Results
Identification of eligible studies
shows the study selection process. Our systematic literature search yielded a total of 14 articles investigating the associations between the CASR polymorphisms and cancer risk.Citation12–Citation25 Based on our highly sensitive search strategy, a total of 156 records were sourced from the initial literature search in PubMed, EMBASE, Web of Science, CNKI, SinoMed, and WanFang databases. We excluded 25 records because they were duplicate studies, and a further 104 articles were also excluded for reasons related to the following: review or commentary or letter (n=10), studies in animal or cell lines (n=16), no relevant outcomes (n=15), studies were obviously irrelevant (n=48), not related to CASR gene (n=7), and not related to CASR polymorphism (n=8). After a review of the remaining 27 articles in detail, 14 case–control studies meeting our inclusion criteria were finally selected for the present meta-analysis.
Study characteristics
The characteristics of the included studies for the relationship of CASR polymorphisms with cancer risk are summarized in . The publication years ranged from 2002 to 2015. Of the 14 case–control studies included, an array of cancers, including rectal cancer,Citation12 CRC,Citation13–Citation17,Citation21,Citation23 pancreatic cancer,Citation20 prostate cancer,Citation18,Citation19 breast cancer,Citation22 ovarian cancer,Citation25 and hepatocellular carcinoma were involved.Citation24 Among the included studies, nine studies concerned rs1801725 (A986S),Citation25–Citation32,Citation36 three studies concerned rs1042636 (R990G),Citation14,Citation15,Citation18 three studies concerned rs12485716,Citation15,Citation20,Citation21 three studies concerned rs4678174,Citation15,Citation20,Citation21 three studies concerned rs1801726 (Q1100E),Citation14,Citation15,Citation18 three studies concerned rs17251221,Citation22,Citation24,Citation25 two studies concerned rs10934578,Citation15,Citation21 and two studies concerned rs2270916.Citation15,Citation21 In all of the included studies, genotype distributions of the CASR polymorphisms in controls were in agreement with HWE, except for one study reported by Kim et alCitation21 in rs2270916 polymorphism. A variety of genotyping methods were applied, including polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP), Taqman assay, and iPLEX Gold.
Table 1 Characteristics of all involved studies
Quantitative data synthesis
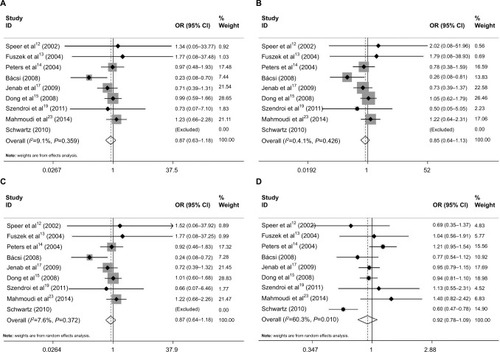
For the CASR A986S polymorphism, nine case–control studies with 4844 cases and 5198 controls were identified. Overall, the aggregated results showed that CASR rs1801725 polymorphism was not significantly associated with cancer risk under the genetic models (the homozygote model: OR=0.87, 95% CI=0.63–1.18, P=0.36, I2=9.1% (); the heterozygote comparison model: OR=0.85, 95% CI=0.64–1.13, P=0.27, I2=0% (); the dominant model: OR=0.87, 95% CI=0.64–1.18, P=0.36, I2=7.6 (); recessive model: OR=0.92, 95% CI=0.78–1.09, P=0.35, I2=60.3% (); respectively).
Figure 2 Forest plot of the association between CASR A986S polymorphism and cancer risk: (A) AA vs SS; (B) AS vs SS; (C) AA+AS vs SS; and (D) AA vs AS+SS.
Notes: The squares and horizontal lines correspond to the study-specific OR and 95% CI. The area of the squares reflects the study-specific weight (inverse of the variance). The diamond represents the pooled OR and 95% CI.
Abbreviations: CASR, calcium-sensing receptor; OR, odds ratio.
Other polymorphisms of CASR, such as rs1042636, rs12485716, rs4678174, rs1801726, rs17251221, rs10934578, and rs2270916, were mentioned in our systematic literature search relationship of CASR polymorphisms with cancer risk. The pooled results revealed that CASR polymorphisms may not increase the risk of cancer under the homozygote model, heterozygote comparison model, dominant model, and recessive model. lists the main results of the meta-analysis of the relations between CASR polymorphisms and cancer susceptibility.
Table 2 Association between SNPs in CASR polymorphism and risk of cancer.
Subgroup analysis
The associations between CASR rs1801725 polymorphism and risk of cancer in a subgroup of articles defined according to ethnicity, source of controls, and cancer type are summarized in . The meta-analysis included five studies in EuropeCitation15,Citation16,Citation19,Citation20,Citation22, and four studies in non-EuropeCitation17,Citation18,Citation21,Citation26. In a subgroup analysis stratified by ethnicity, suggesting that no significant associations were observed between European (homozygote model: OR=0.88, 95% CI=0.60–1.28; heterozygote comparison model: OR=0.91, 95% CI=0.62–1.34; the dominant model: OR=0.88, 95% CI=0.60–1.29; recessive model: OR=0.96, 95% CI=0.86–1.07) and non-European (homozygote model: OR=0.83, 95% CI=0.55–1.24; heterozygote comparison model: OR=0.79, 95% CI=0.51–1.20; dominant model: OR=0.73, 95% CI=0.32–1.63; recessive model: OR=0.87, 95% CI=0.59–1.30) studies. For subgroup analysis of cancer type, we found that no significant associations were observed between the CASR rs1801725 polymorphism and CRC, colon cancer, rectal cancer, colorectal adenoma, and prostate cancer (P>0.05). Subgroup analysis was also carried out with the data segregated by source of controls. The pooled subgroup result showed that no significant associations were observed between the CASR rs1801725 polymorphism and cancer risk in the hospital-based and population-based control groups under all genetic models.
Table 3 Further analyses of CASR rs1801725 polymorphism and risk of cancer.
Publication bias and sensitivity analysis
No evidence for asymmetry was detected in Begg’s funnel plots. The result was also supported by Egger’s tests (homozygote model: P=1.000; heterozygote comparison model: P=0.711; dominant model: P=1.000; recessive model: P=0.754; respectively). To further test the robustness of the results, we conducted a sensitivity analysis by excluding each study at each turn. The statistical significance of the results was not altered when any single study was omitted (data not shown).
Discussion
Cancer is a complex multifactorial disease attributed to a disordered balance between proliferation, differentiation, and apoptosis. Evidence from experimental studies suggests that loss in the function of the CASR could accelerate the progression of neoplastic disease.Citation26 Many in vitro studies have demonstrated disruptions of the growth suppressing effects of elevated extracellular Ca2+ in intestinal epithelial cells, insulinoma cells, gastrinomas, leydig tumor cells, and in colon carcinoma.Citation27 An increasing body of studies indicates the relationship between CASR activation and high expression and secretion of PTH-related peptide, which is the foremost causal factor in hypercalcemia of malignancy, and a contributor to bone metastatic processes.Citation28 Although mutation of the CASR does not appear to be an early event in carcinogenesis, loss or upregulation of normal CASR function can influence several aspects of tumor development. Multiple lines of evidence supported that therapeutic strategies directed at CASR could potentially serve a supportive function in cancer management.
CASR is considered to be an important element of the signaling pathway in mediating the anticancer activities of calcium on cancer development. Previous studies have suggested that calcium may protect against CRC risk through binding to the CASR, or indirectly by binding bile acids and free fatty acids in the colonic lumen, which leads to the induction of tumor cell apoptosis and differentiation.Citation29 Calcium activates cell growth, and differentiation through certain signaling pathways that participate in CASR is considered the likely pathway. It promotes E-cadherin expression and the suppression of β-catenin activation, and possibly modulates the activation of the p38 mitogen-activated protein kinase cascade system.Citation30 Further research has demonstrated that this stimulation is mediated by filamin-A, which can be bound to the A986S mutation site of CASR.Citation31 The abnormal expression of CASR may be associated with differentiation and tumor development.Citation32 The patterns of CASR expression indicate its role in the pathogenesis of CRC; the high expression of CASR is found in normal large intestinal epithelium, yet is lower in well-differentiated CRC tissue, and is decreased in undifferentiated carcinomas.Citation33 Actually, downregulation of the CASR expression in the colonic epithelium is the main element in the pathogenesis of CRC.Citation34 Furthermore, epigenetic inactivation of CASR, such as CASR methylation, has a key role in colorectal carcinogenesis.Citation35 However, the influence of these variants on CASR protein function is not clarified.
Up to now, the reported CASR contains several polymorphisms in cancer risk with unknown clinical significance and values. To provide a comprehensive and reliable conclusion, we performed a comprehensive systematic review and meta-analysis to assess the association between eight extensively studied polymorphisms (rs1801725, rs17251221, rs1042636, rs12485716, rs4678174, rs1801726, rs10934578, and rs2270916) and cancer risk reported until April 2016. The pooled results demonstrated that no significant associations were found between the CASR polymorphisms and cancer risk in any of the genetic models in overall analysis. Our results were very robust, which did not vary materially, in spite of the sensitivity analyses and subgroup analysis performed. Moreover, no publication bias was presented in our present meta-analysis.
A previous meta-analysis based on the same topic has been performed by Jeong et al.Citation36 Our study has several advantages over this previous meta-analysis to make it more conclusive. First, our meta-analysis included only case–control trials, which was highly homogeneous and less selective risk. Second, previously defined key subgroup analyses based on ethnicity, types of cancer, and source of controls were performed to investigate the impact of various parameters on the pooled outcomes, unlike previously. Our meta-analysis, which includes 14 case–control studies, did not reveal any significant associations for all genetic models, even when stratified analysis was conducted according to ethnicity, ethnicity, types of cancer, and sources of controls. Third, the association between the eight polymorphisms (rs1801725, rs1042636, rs12485716, rs4678174, rs1801726, rs17251221, rs10934578, and rs2270916) in the CASR gene and cancer risk under all genetic models have been investigated in our present analysis, which may provide a comprehensive and reliable conclusion. Finally, sensitivity analyses were also performed to assess the stability of the results; namely, a single study in the meta-analysis which was deleted each time to reflect the influence of the individual data set to the pooled OR. All of the results of the subgroup analysis and sensitivity analysis suggest that the data in this meta-analysis are relatively stable and statistically robust.
Several studies quantifying the genetic effect of CASR rs1801725 variant on the susceptibility and prognosis of colorectal, colon or rectal cancer have shown inconsistent results.Citation13–Citation17,Citation21,Citation23 A common alanine (A) to serine (S) polymorphism of amino acid 986 in intracellular C-terminal tail of the CASR revealed that A986S plays an important role in maintaining calcium homeostasis. The “G” or “A” allele was associated with higher circulating PTH and calcium concentrations when compared with the “T” or “S” allele.Citation37 These data support the hypothesis that the mutations in the CASR gene rs1801725 variant are contributed to pathogenesis of CRC. In a subgroup analysis stratified by cancer type (CRC vs prostate cancer), we failed to find any significant association between the CASR rs1801725 polymorphism and the CRC risk. The relationship was consistent in prostate cancer. Future large-scale, well-designed studies are needed to get a deeper insight into the association.
Some potential limitations in our present meta-analysis should be taken into account. First, we pooled the data using unadjusted information, and other factors (ie, age, family history, the interactions among gene–gene, and even gene– environment) may potentially impact on our results and result in heterogeneity. Second, for CASR rs1042636, rs12485716, rs4678174, rs1801726, rs17251221, rs10934578, and rs2270916 polymorphisms and risk of cancer, considering the limited number and sample sizes of the included studies (only two or three studied), additional studies or a database on these topics are needed. Third, because of the limited data of the reviewed studies, we failed to capture the data of serum levels of calcium intake, which could affect our present study. Fourth, accumulating evidence has suggested that the CASR plays a critical role in bone metastasis. Due to the lack of original data of the included studies, we did not evaluate the effects of CASR in bone metastasis in cancer patients; further studies based on this topic are needed. Finally, the analysis was only based on published data, and no unpublished data were included. Hence, some inevitable publication biases might have a potential impact on our results.
Conclusion
In summary, our analysis provides reliable evidence that the CASR polymorphisms may not be associated with overall cancer susceptibility under the current published studies. Our subgroup analysis further indicated that the CASR rs1801725 polymorphism is not associated with an increased risk of CRC and prostate cancer. Large-scale and well methodologically designed studies should be conducted to explore the real relationship between CASR variants and cancer risk.
Acknowledgments
The authors thank Dr Guangzho Chen, Department of Pharmacy, Guangdong Province Agricultural Reclamation Central Hospital, for his data analyses and valuable discussions. Funding was provided by departmental sources.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRMaJZouZJemalACancer statistics, 2014CA Cancer J Clin201464192924399786
- PharoahPDDunningAMPonderBAEastonDFAssociation studies for finding cancer-susceptibility genetic variantsNat Rev Cancer200441185086015516958
- HuncharekMMuscatJKupelnickBColorectal cancer risk and dietary intake of calcium, vitamin D, and dairy products: a meta-analysis of 26,335 cases from 60 observational studiesNutr Cancer2009611476919116875
- CifuentesMRojasCVAntilipolytic effect of calcium-sensing receptor in human adipocytesMol Cell Biochem20083191–2172118622738
- XieRTangBYongXLuoGYangSMRoles of the calcium sensing receptor in digestive physiology and pathophysiology (review)Int J Oncol20144541355136225069966
- AbukawaHManoHArakawaTHakedaYKimuraHKumegawaMTissue specific expression and differential regulation by 1alpha,25-dihydroxyvitamin D3 of the calcium-sensing receptor (CaSR) gene in rat kidney, intestine, and calvariaCytotechnology2001351818619003284
- LiaoJSchneiderADattaNSMcCauleyLKExtracellular calcium as a candidate mediator of prostate cancer skeletal metastasisCancer Res200666189065907316982748
- WardBKMagnoALWalshJPRatajczakTThe role of the calcium-sensing receptor in human diseaseClin Biochem2012451294395322503956
- SkinnerHGSchwartzGGA prospective study of total and ionized serum calcium and fatal prostate cancerCancer Epidemiol Biomarkers Prev200918257557819190170
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- SpeerGCsehKMucsiKCalcium-sensing receptor A986S polymorphism in human rectal cancerInt J Colorectal Dis2002171202412018449
- FuszekPLakatosPTabakARelationship between serum calcium and CA 19-9 levels in colorectal cancerWorld J Gastroenterol200410131890189215222030
- PetersUChatterjeeNYeagerMAssociation of genetic variants in the calcium-sensing receptor with risk of colorectal adenomaCancer Epidemiol Biomarkers Prev200413122181218615598778
- DongLMUlrichCMHsuLGenetic variation in calcium-sensing receptor and risk for colon cancerCancer Epidemiol Biomarkers Prev200817102755276518843020
- BácsiKHitreEKosaJPEffects of the lactase 13910 C/T and calcium-sensor receptor A986S G/T gene polymorphisms on the incidence and recurrence of colorectal cancer in Hungarian populationBMC Cancer2008831718980667
- JenabMMcKayJBueno-de-MesquitaHBVitamin D receptor and calcium sensing receptor polymorphisms and the risk of colorectal cancer in European populationsCancer Epidemiol Biomarkers Prev20091892485249119706842
- SchwartzGGJohnEMRowlandGInglesSAProstate cancer in African-American men and polymorphism in the calcium-sensing receptorCancer Biol Ther201091299499920364112
- SzendroiASpeerGTabakAThe role of vitamin D, estrogen, calcium sensing receptor genotypes and serum calcium in the pathogenesis of prostate cancerCan J Urol20111835710571621703046
- AndersonLNCotterchioMKnightJABorgidaAGallingerSClearySPGenetic variants in vitamin d pathway genes and risk of pancreas cancer; results from a population-based case-control study in Ontario, CanadaPloS One201386e6676823826131
- KimKZShinAKimJAssociation between CASR polymorphisms, calcium intake, and colorectal cancer riskPloS One201383e5962823555732
- LiXKongXJiangLA genetic polymorphism (rs17251221) in the calcium-sensing receptor is associated with breast cancer susceptibility and prognosisCell Physiol Biochem201433116517224481145
- MahmoudiTKarimiKArkaniMParathyroid hormone gene rs6256 and calcium sensing receptor gene rs1801725 variants are not associated with susceptibility to colorectal cancer in IranAsian Pac J Cancer Prev201415156035603925124570
- TangQZhaoYWangYWeiMA genetic variant (rs17251221) in the calcium-sensing receptor relates to hepatocellular carcinoma susceptibility and clinical outcome treated by transcatheter hepatic arterial chemoembolization (TACE) therapyMed Oncol2014311126725270285
- YanSYuanCYangQA genetic polymorphism (rs17251221) in the calcium-sensing receptor is associated with ovarian cancer susceptibilityOncol Rep20153442151215526252839
- RodlandKDThe role of the calcium-sensing receptor in cancerCell Calcium200435329129515200153
- JustinichCJMakNPachecoIThe extracellular calcium-sensing receptor (CaSR) on human esophagus and evidence of expression of the CaSR on the esophageal epithelial cell line (HET-1A)Am J Physiol Gastrointest Liver Physiol20082941G12012917962359
- Tfelt-HansenJBrownEMThe calcium-sensing receptor in normal physiology and pathophysiology: a reviewCrit Rev Clin Lab Sci2005421357015697170
- Van der MeerRKleibeukerJHLapreJACalcium phosphate, bile acids and colorectal cancerEur J Cancer Prev19911Suppl 255621842734
- HobsonSAWrightJLeeFMcNeilSEBilderbackTRodlandKDActivation of the MAP kinase cascade by exogenous calcium-sensing receptorMol Cell Endocrinol20032001–218919812644311
- HjalmGMacLeodRJKiforOChattopadhyayNBrownEMFilamin-A binds to the carboxyl-terminal tail of the calcium-sensing receptor, an interaction that participates in CaR-mediated activation of mitogen-activated protein kinaseJ Biol Chem200127637348803488711390380
- ChakrabartySRadjendiraneVAppelmanHVaraniJExtracellular calcium and calcium sensing receptor function in human colon carcinomas: promotion of E-cadherin expression and suppression of beta-catenin/TCF activationCancer Res2003631677112517779
- SheininYKallayEWrbaFKriwanekSPeterlikMCrossHSImmunocytochemical localization of the extracellular calcium-sensing receptor in normal and malignant human large intestinal mucosaJ Histochem Cytochem200048559560210769043
- RogersACHanlyAMCollinsDBairdAWWinterDCReview article: loss of the calcium-sensing receptor in colonic epithelium is a key event in the pathogenesis of colon cancerClin Colorectal Cancer2012111243021723793
- HizakiKYamamotoHTaniguchiHEpigenetic inactivation of calcium-sensing receptor in colorectal carcinogenesisMod Pathol201124687688421317879
- JeongSKimJHKimMGGenetic polymorphisms of CASR and cancer risk: evidence from meta-analysis and HuGE reviewOnco Targets Ther2016965566926929638
- MarzWSeelhorstUWellnitzBAlanine to serine polymorphism at position 986 of the calcium-sensing receptor associated with coronary heart disease, myocardial infarction, all-cause, and cardiovascular mortalityJ Clin Endocrinol Metab20079262363236917374704