Abstract
Objective
To examine how observed medication nonadherence to 2 second-line, oral anticancer medications (axitinib and everolimus) affects progression-free survival (PFS) among patients with renal cell carcinoma.
Methods
We used an adherence–exposure–outcome model to simulate the impact of adherence on PFS. Using a pharmacokinetic/pharmacodynamic (PK/PD) population model, we simulated drug exposure measured by area under the plasma concentration–time curve (AUC) and minimum blood or trough concentration (Cmin) under 2 scenarios: 1) optimal adherence and 2) real-world adherence. Real-world adherence was measured using the medication possession ratios as calculated from health insurance claims data. A population PK/PD model was simulated on individuals drawn from the Medical Expenditure Panel Survey (MEPS), a large survey broadly representative of the US population. Finally, we used previously published PK/PD models to estimate the effect of drug exposure (i.e., Cmin and AUC) on PFS outcomes under optimal and real-world adherence scenarios.
Results
Average adherence measured using medication possession ratios was 76%. After applying our simulation model to 2164 individuals in MEPS, drug exposure was significantly higher among adherent patients compared with nonadherent patients for axitinib (AUC: 249.5 vs. 159.8 ng×h/mL, P<0.001) and everolimus (AUC: 185.4 vs. 118.0 µg×h/L, P<0.001). Patient nonadherence in the real world decreased the expected PFS from an optimally adherent population by 29% for axitinib (8.4 months with optimal adherence vs. 6.0 months using real-world adherence, P<0.001) and by 5% (5.5 vs. 5.2 months, P<0.001) for everolimus.
Conclusion
Nonadherence by renal cell carcinoma patients to second-line oral therapies significantly decreased the expected PFS.
Introduction
Kidney cancer is among the 10 most common cancers in the USA.Citation1 In 2017, there were an estimated 63,990 news cases -~3.8% of all new oncologic cases - and ~14,400 deaths recorded from kidney and renal pelvis cancer.Citation2 Nine out of every 10 kidney cancers are renal cell carcinomas (RCCs).Citation3 Although 5-year survival for patients with RCC has been increasing, only 23% of patients with stage IV RCC survive to 5 years.Citation4 The majority of RCC treatments (e.g., axitinib, everolimus, pazopanib, sorafenib, sunitinib) are orally administered small molecule drugs, but some new treatments are intravenously administered (e.g., bevacizumab, nivolumab).Citation5
Although some patients prefer convenient oral administration to more invasive intravenous administration, few patients are willing to sacrifice effective treatment response for other considerations if oral medication is not the most effective.Citation6 Nonadherence or poor adherence to oral anticancer therapies has the potential to affect treatment effectiveness and health outcomes.Citation7 Adherence to oral therapies for RCC has ranged from 74% to 95% in studies using claims-based measures such as medication possession ratio (MPR) or proportion of days covered.Citation8,Citation9 Using electronic pill counts, the share of oncology patients who took at least 80% of their prescribed doses of oral therapies for other solid tumor types ranged from 78% to 96%;Citation10–Citation12 adherence estimates using serum samples are lower.Citation13 Further, healthcare providers frequently overestimate oncology patient adherence to self-administered medications.Citation12,Citation14
This study examined the effect of adherence to oral anticancer drugs among adult patients with RCC. We applied the dose adherence–exposure–outcome model to everolimus and axitinib – 2 of the most commonly prescribed second-line RCC treatmentsCitation15 – to estimate how adherence affects drug exposure and patient outcomes. We modeled second-line treatments in RCC that had evidence linking medication exposure to patient outcomes. Building on previously published adherence models,Citation9 we simulated pharmacokinetics/pharmacodynamics (PK/PD), building a model based on inputs from clinical trials, and estimated drug exposure in the real world by simulating different levels of adherence. We then used the modeled exposure to simulate progression-free survival (PFS) outcomes with second-line RCC treatment.
Methods
We used PK/PD modeling to estimate the effect of adherence on drug exposure and downstream outcomes for axitinib and everolimus. In this approach, we constructed a hypothetical cohort of patients that resembled an RCC population as closely as possible, and modeled the relationship between medication dosing and medication concentration in patients’ systems based on previous clinical trials. To test the impact of adherence levels on outcomes, we then varied the assumed adherence rate of our hypothetical cohort, which ultimately impacted the level of drug that was absorbed into the patient’s system. Based on the relationship between medication exposure and patient outcomes in clinical trials, our model finally simulated the effect of nonadherence on PFS for these hypothetical patients based on different assumed adherence rates.
Our framework relied on a simulation model that comprised 4 modules.Citation7 In the first module, we used axitinib- and everolimus-recommended dosing from US Food and Drug Administration (FDA)-approved labeling.Citation16,Citation17 In the second module, we adapted adherence rates from published studies using claims data analyses.Citation9 In our targeted literature reviews, we were unable to identify adherence studies specific to axitinib and everolimus in RCC patients, so instead we adapted adherence rates for modeling purposes from a real-world study of adherence rates in RCC patients. In the third module, we measured exposure by modeling PK/PD: first, by matching existing models to clinical trials assuming optimal adherence, and then incorporating measures of real-world medication adherence.Citation18–Citation21 In the fourth module, we translated the exposure measured with imperfect adherence rates into changes in PFS as our final outcome.Citation20
Simulated population
Previously published exposure modeling studies did not specify correlations for the independent variables used in their models.Citation18,Citation20 Generating a synthetic population based purely on the summary statistics included in the literature would ignore correlations that we know to exist across race, sex, smoking, and body weight. To include reasonable correlations across the independent variables of interest, we generated a population for this study based upon patients diagnosed with any cancer in the 2012 Medical Expenditure Panel Survey (MEPS).Citation22 There were 2164 patients in the MEPS cohort with observed sex, age, body mass index, smoking status, and ethnicity. Because MEPS reports body mass index, not height and weight, some assumptions were required to obtain height and weight (Supplementary material). The simulation population was bootstrapped from the MEPS population of 2164 patients (weighted to be nationally representative) to achieve an unweighted sample of 21,640 individual records.
Dose and adherence
The recommended doses for axitinib and everolimus were identified from their respective FDA labels. Axitinib (5 mg) is taken orally every 12 h,Citation17 whereas everolimus (10 mg) is taken orally once every 24 h.Citation16
Two separate dosing schedules were derived for each patient in the simulation: optimal adherence and real-world adherence. In the optimal adherence schedule, patients took the medication exactly as prescribed. In the real-world adherence schedule, previously published adherence rates were used. The adherence rate for everolimus was reported previously and used for both everolimus and axitinib, as the latter had not been reported in the literature (Supplementary material).Citation9 Nonadherence was assumed to occur along 2 dimensions: taking the medication at a different time of day than prescribed or not taking the medication (Supplementary material). Failure to take the medication was measured using the MPR, defined as the total days of supply during the treatment period until the date of the last prescription divided by the total treatment period until the date of the last prescription administration claim.
Exposure and outcomes
Dosing regimen was converted into measures of drug exposure using previously published PK models for axitinib and everolimus. The key exposure metrics used in the model were area under the plasma concentration–time curve (AUC) and minimum blood or trough concentration (Cmin). Mean and median AUC and Cmin levels were extracted, in addition to the PK diagnostic plots for visual predictive checks (Supplementary material). The identified population PK models for axitinib and everolimus used 2 compartments with the first-order lag time.Citation18,Citation20,Citation21,Citation23 Exposure was measured with AUC for axitinib (mean: 375 ng×h/mL)Citation20 and everolimus (mean: 120 µg×h/L).Citation18 Exposure for everolimus was also measured with Cmin (mean: 12.1 µg/L).Citation18 The Pmetrics v1.5.0 software package for RCitation19 was used to simulate patient drug exposure based on optimal and real-world adherence. The model fit was compared versus average and median AUC and Cmin levels, and the blood concentration diagnostic plots with the corresponding figures in the published literature ( and ).
Using the relationship between blood concentration and PFS in published studies, we modeled the effect of changes in AUC on PFS for axitinib and changes in Cmin on PFS for everolimus. Different exposure units were used because studies estimated the effect of drug exposure on PFS (i.e., PD) using different exposure metrics (namely, AUC for axitinib and Cmin for everolimus). For axitinib, the PFS hazard ratio was 0.909, indicating that a 100-unit increase in AUC decreased the PFS hazard by 9.1%.Citation20 For everolimus, the effect of exposure on PFS was stratified into 3 exposure groups by Cmin: 4.47 months for Cmin <10 ng/mL, 5.52 months for Cmin between 10 and 30 ng/mL, and 5.36 months for Cmin >30 ng/mL.Citation24 PFS was computed for each patient in the simulation, across 10 replications, under both real-world and optimal adherence; the difference in outcomes was the impact of adherence on PFS.
Results
Our literature review identified sufficient parameters to conduct the simulation exercise for all 4 phases of the model. describes the key parameters of interest. Axitinib and everolimus dosing was obtained from their FDA labels. Mean MPR was 0.76, and increased drug exposure increased median PFS for both axitinib and everolimus.
Table 1 Model parameters used in the simulation
Over 60% of our patient population was older than 60 years of age and the mean weight waŝ81 kg ().
Table 2 Summary statistics of population
Each patient was assigned an overall adherence rate drawn from the full distribution of MPR as reported previously.Citation9 Mean MPR was 0.76 for everolimus; only 25.3% of patients had optimal adherence (MPR=1). Due to the small sample size of axitinib patients in the cited analysis, we assumed that MPRs for everolimus and axitinib were identical.Citation9 Overall, 46.4% of the simulated population was adherent (MPR≥0.80), 41.2% were moderately adherent (0.50≤MPR<0.80), and 12.4% of patients had poor adherence (MPR<0.50).
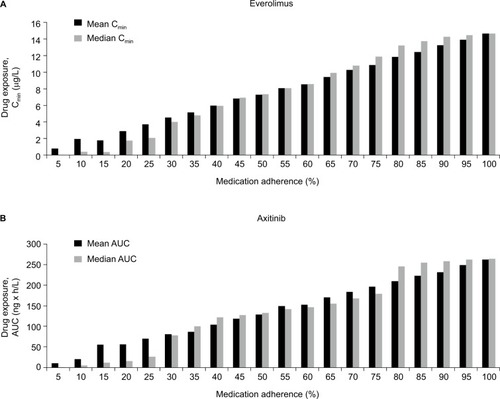
After incorporating patient nonadherence, average drug exposure declined significantly. Patients treated with everolimus with MPR≥0.80 adherence had an average Cmin of 14.0 versus 8.9 µg/L among patients with MPR <0.80. For axitinib, the corresponding Cmin values were 3.1 and 1.8 ng/mL, respectively. Lower adherence rates also led to lower drug exposure when exposure was measured using AUC. For everolimus, AUC was 185.4 µg×h/L among patients with MPR ≥0.80, but only 118.0 µg×h/L among patients with MPR <0.80. The corresponding AUCs for patients using axitinib were 249.5 and 159.8 ng×h/mL for adherent and nonadherent patients, respectively. Both measures of drug exposure (AUC and Cmin) were significantly reduced due to nonadherence (P<0.001) for both medications versus optimal adherence ().
Figure 1 Mean drug exposure by patient adherence group for patients treated with everolimus (A) and with axitinib (B).
In our simulation model, real-world PFS for patients with RCC using oral treatments was significantly lower than PFS in clinical trials. We compared the predicted PFS based on exposure levels derived from real-world adherence levels versus PFS levels with optimal adherence. As shown in for everolimus, the average patient with optimal adherence can expect to have a PFS of 5.5 months, whereas it is 5.2 months for patients based on real-world adherence, a difference of 0.3 months (10 days) or a 5% decline (P<0.001). For axitinib, the average patient with optimal adherence can expect to have a PFS of 8.4 months, whereas it is 6.0 months for patients based on real-world adherence, a difference of 2.4 months (73 days) or a 29% decline (P<0.001).
Figure 2 Progression-free survival with optimal and real-world adherence (A) and in adherent and nonadherent patients (B) treated with axitinib and everolimus.
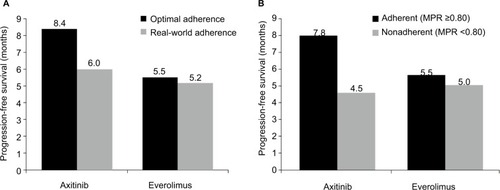
This decrease in real-world PFS was driven by a differences in PFS between adherent and nonadherent patients (). For everolimus, adherent patients (MPR≥0.80) could expect a PFS of 5.5 months, whereas it is 5.0 months for nonadherent patients (MPR<0.80), a difference of 0.5 months (16 days) or a 9% decline (P<0.001). For axitinib, adherent patients had an expected PFS of 7.8 months, whereas it is 4.5 months for nonadherent patients. This difference represents a decrease of 3.3 months (101 days) or a 42% (P<0.001) decrease in PFS between nonadherent versus adherent patients.
Discussion
In our simulation model, patient nonadherence was associated with decreased real-world PFS compared with clinical trial PFS. Our modeling closely resembled published studies for PK exposure outcomes.Citation20,Citation24 Patient nonadherence decreased levels of drug exposure as measured by the blood concentration of the drug. As a direct result of adherence-related decreases in drug exposure, PFS decreased by 29% for patients with RCC taking axitinib and by 5% for patients with RCC taking everolimus.
These findings indicate that physicians selecting treatments for patients with RCC should consider how real-world effectiveness differs from efficacy measured in clinical trials, in part because of patient nonadherence. One key component affecting adherence is probably whether a treatment is administered orally or through an infusion at physicians’ offices. One review studyCitation25 of adherence to oral anticancer medications found that 1 in 5 patients was nonadherent to oral RCC treatments; similar findings have been identified in other cancers.Citation26 Although most patients with RCC currently receive oral anticancer treatments, injectable drugs – for which adherence is typically higherCitation27 – are available (e.g., nivolumab, temsirolimus). In addition to considerations of treatment efficacy, safety, and cost, physicians should also examine whether a treatment’s mode of administration is likely to affect real-world effectiveness due to patient nonadherence.
To our knowledge, this study is the first to measure the impact of patient nonadherence in second-line RCC treatments using a simulation model. Our finding that oral medications decrease real-world treatment effectiveness has been echoed by prior studies using similar methods for the treatment of other diseases. For instance, a previous report estimated the impact of adherence on renal transplant patients and found that patients who frequently missed both required doses had a higher proportion of days below the target range of cyclosporine exposure.Citation7 Nonadherence had a greater impact on exposure in renal transplant patients than in the treatment of patients with RCC in our study, as nonadherence to cyclosporine occurred more frequently than nonadherence to axitinib and everolimus in our study.
There were several limitations to our study. First, we were unable to identify adherence estimates specific to axitinib or everolimus. Instead, we assumed the same adherence between both treatments, based on claims data for adult patients, which may overestimate actual adherence using prescription fills instead of doses taken or underestimate actual adherence if patients self-pay for medication. Second, we are not able to identify the source of nonadherence, which could be related to patient preference, forgetfulness, or dose delays due to toxicity. Third, these results should not be extrapolated to other tumor types or diseases. The relationship between adherence and exposure, and between exposure and outcomes, can vary by tumor and disease type. Fourth, to model survival, we used AUC for axitinib and Cmin for everolimus, due to the availability of existing models in the literature. However, AUC may be a more sensitive exposure measure to changes in adherence compared with Cmin. Specifically, while there is a direct correlation between PFS and AUC for axitinib in the literature, for everolimus previously published research only captured Cmin bands, where small decreases in adherence and exposure may not change modeled PFS if an exposure–efficacy band was not crossed. We also considered modeling the effect of adherence on PFS for other RCC treatments, but we could not find literature measuring the relationship between exposure and PFS for other RCC agents. Fifth, our estimates of the relationship between medication exposure and PFS come from clinical trials. In this study, we assumed that this relationship would translate to the real world, but the external validity of this assumption is uncertain. Finally, our study evaluates the utility of different treatments based solely on PFS, but other factors matter to patients. For instance, we do not incorporate patient disutility from intravenous infusions, the cost to patients for clinic visits to receive intravenous infusions (e.g., costs of transportation, child care, time off from work), or the importance of other efficacy end points (e.g., overall survival rather than PFS). On the other hand, we do not capture potential clinical disadvantages from using oral medications due to less frequent clinical surveillance, as the patient does not need to visit medical facilities as frequently as in the case of using intravenous infusions.
Conclusion
This study found that patient nonadherence to oral anticancer medications decreases their real-world effectiveness as measured by PFS. When prescribing anticancer medication for patients with RCC, physicians should take into account a patient’s prior treatment adherence behavior and how this may impact real-world treatment effectiveness.
Supplementary materials
Table S1 Exposure results summary
Methods
Body mass index calculations
Because the Medical Expenditures Panel Survey (MEPS) reports body mass index (BMI) instead of individual height and weight, some assumptions were required to obtain patient weight and ideal weight. To compute the patient weight for the axitinib analysis, average adult height was used (stratified into men and women) to convert the BMI into weight in kilograms. The resulting weight was constrained to be within the range observed in the trial (37–130 kg). To compute the ideal weight for the everolimus analysis, height was first approximated using the MEPS BMI and the mean total body weight as reported previously.Citation1 Then, the ideal weight for men was computed according to the Devine formula and for women using the Robinson formula.Citation2,Citation3
Adherence rates for everolimus and axitinib
We conducted a literature review to identify adherence rates for axitinib and everolimus. PubMed, EBSCO, and Google Scholar research databases were queried using search strings built on key terms including the generic drug names, renal cell carcinoma, adherence, MEMSCaps, and other measurements of adherence. All studies measuring adherence in patients receiving treatment for renal cell carcinoma (RCC) were screened. The search yielded one RCC adherence study,Citation4 in which patients with two or more doses/administrations of RCC treatment initiated therapy with sunitinib, sorafenib, pazopanib, axitinib, temsirolimus, or everolimus. This study used the medication possession ratio to estimate RCC patient adherence to everolimus across all lines of therapy at 76% through claims data. Real-world adherence rates were not available for axitinib, so the everolimus adherence rate was applied to axitinib, assuming similar patient populations and therefore adherence behaviors.
Incorporating real-world adherence
For the first component of real-world adherence, we estimated the inter-dose interval to allow for variability in the time of day the patient took the medication. We applied a normal distribution centered on the recommended time and calibrated the standard deviation to match an estimate from the literature that 55% of people took their medication within 2 hours of the scheduled time.Citation5 Once the dosing schedule was simulated, we used a second simulation that randomly removed doses based on the adherence rate. For instance, if the adherence rate was 50%, then we assigned a random number to each scheduled dose and included the dose if the random number was <50% and included the dose if the random number was ≥50%.
Visual predictive check results
We conducted a visual predictive check to compare the results of our exposure model against previously published models. In addition, displays key descriptive metrics from our exposure model as compared to the relevant published models.
Everolimus dose–exposure modelling
The everolimus dose–exposure model produced results that were similar but not identical to those found in the literature.
shows the reproduced and shows the original blood concentration of everolimus based on published results.Citation1 The original mean and median area under the plasma concentration–time curves (AUCs) were 130 and 127 µg×h/L, and the reproduced were 138 and 131 µg×h/L, respectively. The published results had a sample size of 53 patients, while our simulated sample contained more than 20,000 observations (2164 RCC patients across 10 replications), so comparing the statistical distributions is not meaningful. The five curves displayed in the study results panel for captured different percentiles of drug exposure for the population. As expected, higher percentiles of exposure resulted in greater concentrations of AUC.
Axitinib dose–exposure modelling
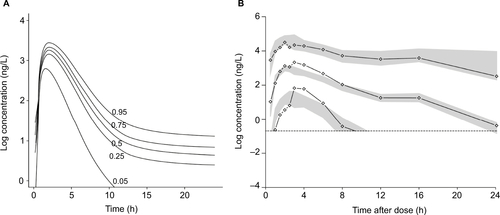
As was the case with everolimus, the axitinib dose–exposure model reflects but is not identical to the exposure model in the literature. shows the reproduced and shows the original log concentration plots for axitinib based on the published model.Citation6 The median (range) of the published results was substantially higher than that observed in our simulation: 375 ng×h/mL (32.8–1,728) versus 264 ng×h/mL (61.0–430), respectively. Only the median and range of AUC were available in the published model, so a statistical comparison of the two distributions was not feasible, but the match was not as good as with everolimus based on visual inspection and summary statistics. Similar to the everolimus figure, the five curves for the study results panel modeled different percentiles of drug exposure, with higher percentiles of exposure resulting in higher concentrations of AUC.
Figure S1 Everolimus dose–exposure study results (A) compared with clinical trial results (B).
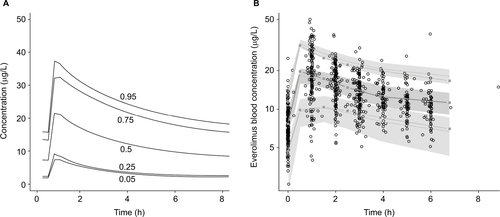
Figure S2 Axitinib dose–exposure study results (A) compared with clinical trial results (B).
References
- MoesDJAPressRRden HartighJvan der StraatenTde FijterJWGuchelaarHJPopulation pharmacokinetics and pharmacogenetics of everolimus in renal transplant patientsClin Pharmacokinet201251746748022624503
- RobinsonJLupkiewiczSPalenikLLopezLArietMDetermination of ideal body weight for drug dosage calculationsAm J Health Syst Pharm198340610161019
- DevineBJGentamicin therapyDrug Intell Clin Pharm1974811650655
- MargolisJPrincicNDoanJLenhartGMotzerRAnalysis of real world treatment compliance in a cohort of 2,395 patients with metastatic renal cell carcinoma (mRCC)ASCO Annu Meet Proc20153315 Suppl4546
- ThivatEVan PraaghIBelliereAAdherence with oral oncologic treatment in cancer patients: interest of an adherence score of all dosing errorsOncology2013842677423128040
- RiniBIMelicharBUedaTAxitinib with or without dose titration for first-line metastatic renal-cell carcinoma: a randomised double-blind phase 2 trialLancet Oncol201314121233124224140184
Acknowledgments
This research was sponsored by Bristol-Myers Squibb. The authors acknowledge Alison Silverstein and Taylor Watson for administrative and technical support. Professional editorial assistance was provided by Juan Sanchez-Cortes, PhD, of PPSI, funded by Bristol-Myers Squibb.
Disclosure
Jason Shafrin, Jeffrey Sullivan, Jacquelyn W Chou and J Ross Maclean are employees of Precision Health Economics, a consulting firm for the life sciences industry. Justin F Doan is an employee of and holds stock in Bristol-Myers Squibb. Michael N Neely reports no conflicts of interest related to this work.
References
- National Cancer InstituteCommon cancer types Available from: https://www.cancer.gov/types/common-cancersAccessed November 6, 2017
- National Cancer InstituteCancer stat facts: kidney and renal pelvis cancer Available from: https://seer.cancer.gov/statfacts/html/kidrp.htmlAccessed November, 6, 2017
- American Cancer SocietyWhat is kidney cancer? Available from: https://www.cancer.org/cancer/kidney-cancer/about/what-is-kidney-cancer.htmlAccessed November 6, 2017
- ChittoriaNRiniBIRenal cell carcinoma Available from: www.cleve-landclinicmeded.com/medicalpubs/diseasemanagement/nephrology/renal-cell-carcinoma/Accessed November 6, 2017
- MinguetJSmithKHBramlageCPBramlagePTargeted therapies for treatment of renal cell carcinoma: recent advances and future perspectivesCancer Chemother Pharmacol201576221923325963382
- LiuGFranssenEFitchMIWarnerEPatient preferences for oral versus intravenous palliative chemotherapyJ Clin Oncol19971511101158996131
- MacleanJRPfisterMZhouZRoyATuomariVAHeifetsMQuantifying the impact of nonadherence patterns on exposure to oral immunosuppressantsTher Clin Risk Manag2011714915621691585
- ByfieldSAMcPheetersJTBurtonTMNagarSPHackshawMDPersistence and compliance among US patients receiving pazopanib or sunitinib as first-line therapy for advanced renal cell carcinoma: a retrospective claims analysisJ Manag Care Spec Pharm201521651552226011553
- MargolisJPrincicNDoanJLenhartGMotzerRAnalysis of real world treatment compliance in a cohort of 2,395 patients with metastatic renal cell carcinoma (mRCC)ASCO Annu Meet Proc20153315 Suppl4546
- de Figueiredo JuniorAGForonesNMStudy on adherence to capecitabine among patients with colorectal cancer and metastatic breast cancerArq Gastroenterol201451318619125296077
- PartridgeAHArcherLKornblithABAdherence and persistence with oral adjuvant chemotherapy in older women with early-stage breast cancer in CALGB 49907: adherence companion study 60104J Clin Oncol201028142418242220368559
- WaterhouseDMCalzoneKAMeleCBrennerDEAdherence to oral tamoxifen: a comparison of patient self-report, pill counts, and microelectronic monitoringJ Clin Oncol1993116118911978501505
- LevineAMRichardsonJLMarksGCompliance with oral drug therapy in patients with hematologic malignancyJ Clin Oncol198759146914763625261
- PartridgeAHAvornJWangPSWinerEPAdherence to therapy with oral antineoplastic agentsJ Natl Cancer Inst200294965266111983753
- SunMLarcherAKarakiewiczPIOptimal first-line and second-line treatments for metastatic renal cell carcinoma: current evidenceInt J Nephrol Renovasc Dis2014740140725378943
- Afinitor (everolimus) [prescribing information]East Hanover, NJNovartis Pharmaceuticals Corporation2010
- Inlyta (axitinib) tablets [prescribing information]New York, NYPfizer Inc2012
- MoesDJPressRRden HartighJvan der StraatenTde FijterJWGuchelaarHJPopulation pharmacokinetics and pharmacogenet-ics of everolimus in renal transplant patientsClin Pharmacokinet201251746748022624503
- NeelyMNvan GuilderMGYamadaWMSchumitzkyAJelliffeRWAccurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for RTher Drug Monit201234446747622722776
- RiniBIMelicharBUedaTAxitinib with or without dose titration for first-line metastatic renal-cell carcinoma: a randomised double-blind phase 2 trialLancet Oncol201314121233124224140184
- TortoriciMACohenEEPithavalaYKPharmacokinetics of single-agent axitinib across multiple solid tumor typesCancer Chemother Pharmacol20147461279128925336084
- Agency for Healthcare Research and QualityMedical Expenditure Panel Survey (MEPS)Rockville, MDU.S. Department of Health and Human Services2012
- GarrettMPolandBBrennanMHeeBPithavalaYKAmanteaMAPopulation pharmacokinetic analysis of axitinib in healthy volunteersBr J Clin Pharmacol201477348049223834452
- RavaudAUrvaSRGroschKCheungWKAnakOSellamiDBRelationship between everolimus exposure and safety and efficacy: meta-analysis of clinical trials in oncologyEur J Cancer201450348649524332451
- GeynismanDMWickershamKEAdherence to targeted oral anticancer medicationsDiscov Med2013158323124123636140
- FoulonVSchöffskiPWolterPPatient adherence to oral anticancer drugs: an emerging issue in modern oncologyActa Clin Belg2011662859621630604
- DanesiRBoniJPRavaudAOral and intravenously administered mTOR inhibitors for metastatic renal cell carcinoma: pharmacokinetic considerations and clinical implicationsCancer Treat Rev201339778479223375248
