Abstract
After introducing the new molecules for the treatment of patients with tumoral pathology, the therapeutical decision will be taken depending on the molecular profile performed upon the harvested tissues. This major modification makes the molecular and morphological analysis an essential part in the clinical management of patients and the pathologist plays an important role in this process. The quality and reproducibility of the results are imperative today and they depend on both the reliability of the molecular techniques and the quality of the tissue we use in the process. Also, the genomics and proteomics techniques, used increasingly often, require high-quality tissues, and pathology laboratories play a very significant role in the management of all phases of this process. In this paper the parameters which must be followed in order to obtain optimal results within the techniques which analyze nucleic acids and proteins were reviewed.
Introduction
The recent progress made in the detection of molecular events with a key role in the development of tumors, as well as the advent of new drugs capable of blocking molecular targets, has led to the mandatory increase in standardization and quality control for the molecular biology techniques employed. The same applies to the tissue-processing stages that precede these assays.Citation1–Citation3 Nowadays, the detection of mutated or amplified genes such as HER2, KRAS, BRAF, and C-KIT makes personalized treatment possible, and in addition, the evaluation of estrogen (E) and progesterone (P) expression, for example, has led to the improvement of the clinical condition of patients.Citation4–Citation7 Furthermore, the genomics and proteomics techniques that are increasingly being used often require high-quality tissues; therefore, the pathology laboratories play a very significant role in the management of all phases of this process.Citation8 In recent years, efforts made precisely in this regard have remarkably improved the techniques, the reagents, and the equipment in terms of standardization and quality control methods. Consequently, the errors that occurred during the analytical phase have been reduced by ~10 times. Therefore, it could be argued that these methods have reached an acceptable level of reliability.Citation9 In addition, attention is now being paid to the preanalytical phase, as it was somewhat overlooked in the past; however, it is becoming more important with the advent of molecular diagnostics and targeted therapies.Citation10
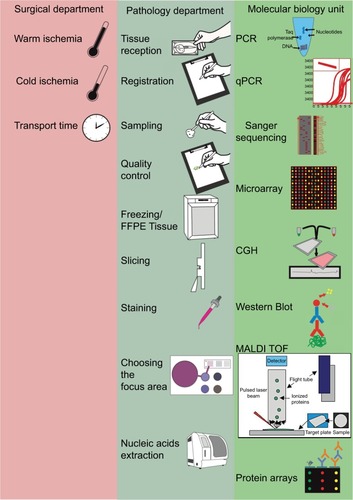
The preanalytical phase includes all procedures performed upon a tissue fragment, from harvesting to the molecular analysis. The main departments involved in this process are surgery and especially pathology.Citation11 The reception of tissues, their fixation, freezing or embedding in paraffin, the diagnostic, and the evaluation of tissue quality are all essential stages of the preanalytical phase and are the exclusive domains of the pathologist.Citation12 The archives of the pathology departments are, at present, an important source of tissues where molecular biology techniques can be applied in order to identify new markers, which link to the evolution of patients and their response to the treatment. Both classic techniques, such as immunohistochemistry (IHC), and the recent ones need the standardization of the preanalytical stages. In this paper we focused on parameters which can be followed in order to obtain high-quality tissues for molecular testing and also the limitations of applying the new techniques on formalin-fixed paraffin-embedded tissues (FFPE) ().
Figure 1 Overview of the general responsibilities of surgical, pathology, and molecular medicine departments in the tissue analysis flow.
Note: The pathology departments are at the core of tissue analysis, thus ensuring the quality control for tissue processing in all of the departments involved in biomedical research and in state-of-the-art molecular analyses.
Abbreviations: CGH, chorionic gonadotrophin; FFPE, formalin-fixed paraffin-embedded; MALDI TOF, mass spectrometry; PCR, polymerase chain reaction; qPCR, quantitative PCR.
Protein evaluation
At present, a series of molecular biomarkers are used in the clinical management of patients, and they need to be precisely assessed. This is why each pathology laboratory that assesses these biomarkers must provide accurate and reproducible results. This requires a standardization of the methods employed in tissue processing.Citation13
IHC
Generally, fixation must be made within a timeframe of up to 12 hours. During this period, the storage of tissues at 4°C or at room temperature does not influence the quality of the immunomarking. The effects seen after >12 hours seem to be antigen specific.Citation14–Citation16
The fixative solution on the other hand is influenced by the concentration, the pH, and the presence of the buffer as these factors contribute toward optimal immunomarking. The best formula is the 10% neutral-buffered formalin (NBF) with a pH between 5 and 7, although it may differ from one antigen to another. The ratio between the volume of the tissue and that of the fixative solution may vary considerably. It is generally accepted that the optimum ratio for a good fixation is 1:10; however, the ratio has been reported to vary from 1:10 to 1:20.Citation17
Differences may also occur between various areas of the tissue sample, such as between the periphery and center of a sample as this affects the intensity and the number of positive cells. The penetration of tissues takes place at different speeds, depending on its depth; in order to double the depth, the time must be increased by four times.Citation18 Of note is the fact that formalin ensures the fast penetration of tissues but remains slow in terms of fixation. For a better quality of the tissues used for immunochemistry purposes, fixation is more important than penetration time. Experiments performed on mouse liver showed a plateau after ~24 hours. The temperature at which fixation takes place is also very important, as it is faster at 37°C than at 25°C. The pH also influences fixation time, which is shorter at 7.0 than at 4.0. The rinsing of tissues fixed for a long period of time shows that fixation is a reversible process.Citation19
The extended ischemia due to late fixation may decrease the expression of estrogen receptor (ER) and progesterone receptor (PR) in breast cancer. The ER expression begins to drop 2 hours after sampling, and the PR expression drops after 1 hour. After 8 hours, the expression becomes completely negative. Therefore, fixation should be made within a maximum of 1 hour.Citation20 A minimum fixation time of 6–8 hours is required for the optimal expression of receptors, while the maximum fixation time that may be reached without a change in the ER, PR, or HER2 expression is 72 hours.Citation21–Citation23
In order to show the importance of standardizing the immunohistochemical expression of ER and PR in breast cancer, a Guideline Recommendation was published at the initiative of the American Society of Clinical Oncology and the College of American Pathologists. In these guidelines, it was noted that ~20% of the IHC determinations are inaccurate, mostly due to a “variation in preanalytic variables”. According to these guidelines, the requirement for optimal tissue handling is that the time between the reception of the tissue and fixation should be as short as possible. The tissue fragments must be cut into slices that are ~5 mm thick, fixed only in 10% NBF, in quantities suitable for a good penetration of the tissue (10-fold greater than the volume of the specimen). A higher or lower concentration of NBF will not be accepted.Citation24 The fixation time should be between 6 and 72 hours. Also, the cold ischemia time, the type of fixator, and the fixating time should be recorded. Each fragment should be accompanied by a sheet indicating the warm and cold ischemia times (the surgeon’s duty) and also the fixation time, the type of fixator, and the fixation duration (the pathologist’s duty). The time between tumor harvesting and fixation should be kept at <1 hour. The pathologist must keep track of these parameters and notify them to the team performing the study.
With respect to the expression of HER-2, slides must be stored for no more than 6 weeks prior to the evaluation. Just like in the case of the receptors, the time until fixation should be as short as possible. The fixation time should be longer than 6 hours; however, it should not exceed 48 hours. Fixation should be made in a sufficient volume of 10% NBF.Citation25
In proteomics, certain signal transduction pathway phosphoproteins, known sensitive indicators of tissue state, may suffer changes in expression levels that mask the levels at the time of excision, depending on tissue handling and cold ischemia delay. Due to factors such as postexcision hypoxia and stress-response signals, certain kinase proteins may suffer expression level alterations during the cold ischemia delay. As a solution to this unwanted variance, Espina et alCitation26 have developed guidelines based on studies regarding postexcision phosphoprotein expression fluctuations in different organs. To further compensate for rapid enzyme function alteration, procedures such as thermal or pressure inactivation of enzymes involved in cell signaling (protein kinases, phosphatases, or even RNAses) have been developed.Citation27 However, such methods lead to the loss of tissue morphology preservation. In any case, protein phosphorylation changes significantly after 10–15 minutes of cold ischemia. For example, it has been noted that phosphorylated mTOR levels greatly increase after 45 minutes.Citation28 Based on a range of evidence, the TuBaFrost consortium has suggested a maximum time of 30 minutes to flash-freezing, based on mass spectrometry-based evidence.Citation29 As general recommendations for the reduction of preanalytical variability, Espina et alCitation26 indicate that elapsed time between tissue extraction and stabilization should not reach 20 minutes and that additional variables, such as sample excision and collection time, elapsed time to preservation or stabilization, and length of fixation time, should be recorded to provide a measure of sample quality. Additional proteome integrity assays should be performed, and these include Western blot (WB) for the evaluation of marker protein proteolysis, effects of preservation, and posttranslational modifications; evaluation of proteolysis of abundant proteins over time by 2-dimensional (2D)-gel electrophoresis (GE), and evaluation of tissue morphology and immunoreactivity through microscopic evaluation.Citation30
After samples have been received in the pathology laboratory, tissues may undergo formalin fixation, which leads to protein–protein cross-linking and requires digestion with proteinases. This may affect protein assay results, as shown in studies comparing some commonly used preservation methods, including formalin fixation. A first study noticed that, in contrast to fresh frozen samples, FFPE tissues failed to yield identifiable target proteins through 2D GE, and another team subsequently reported similar results through the use of chromatographic methods,Citation31,Citation32 supposedly because of the protein mesh that forms after protein cross-linking. These difficulties were later resolved by the use of new protocols.Citation33 However, other studies reported minimal modification of proteins after formalin fixation.Citation34 To circumvent possible formalin-induced protein modifications, some studies have suggested the use of alternative fixatives, such as FineFix, RCL2, and HOPE.Citation35,Citation36
In order to optimize protein extraction from FFPE tissues, Shi et al have used a method widely applied in IHC. FFPE were boiled in an antigen retrieval solution of Tris-HCL (2% sodium dodecyl sulfate [SDS]), followed by incubation. Fresh tissue from the same sample was processed to compare the efficiency of protein extraction. Evaluation of the extraction quality done by GE and mass spectrometry showed better results for high temperature-heated FFPE tissues.Citation33
In the process of tissue protein separation for gel-based assays, enzymatic digestion and application of increased detergent concentrations such as SDS can produce peptides, which can be further modified by tissue formalin increasing the risk of interference with mass spectrometric analysis methods.Citation37,Citation38 Protein extraction methods may use different homogenization techniques, which may damage proteins by exposure to tissue proteases that can cause protein degradation, especially in frozen tissues. Consequently, some studies recommend the use of quick, low-temperature protein extraction methods and the use of protease enzymatic inhibitors.Citation39 To avoid the risk of protease-mediated protein degradation altogether, other more integrated proteomic-inclined approaches may employ heat, instead of freezing stabilization methods, as they have been shown to have superior results when compared to a combination of flash-freezing and enzymatic inhibitors.Citation40 Protein extraction yield as well as quality measured by WB assays has also been shown to be enhanced through the use of an established (EBX) and commercial buffer (EBX Plus) when used on a variety of cancerous and noncancerous tissues that have undergone formalin fixation even for extended periods of time (144 hours).Citation41
Recently, FFPE tissues have been regarded as a possible source of material for WB assays. Results from a study on renal cancer showed that, compared to frozen tissue extracts, FFPE tissues lead to similar results through WB, although membrane protein expression was reported to have a greater variability.Citation42 In addition, RP protein array analysis showed similar results in the level of intact protein extraction rate, with a greater sensitivity attributed to the RP protein array method, in contrast to WB assays ().Citation43
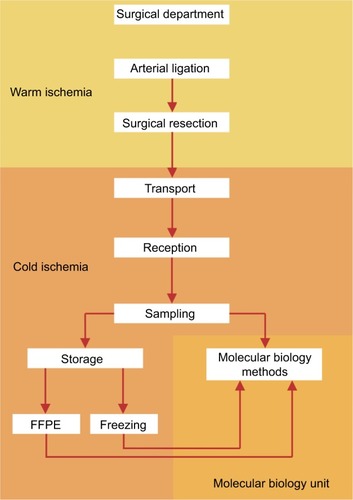
Figure 2 The steps of the preanalytical phase and their matching with the times of warm/cold ischemia.
Note: Most steps are possible during cold ischemia, thus reducing as much as possible processing time and ensuring a good tissue quality for molecular testing.
Abbreviation: FFPE, formalin-fixed paraffin-embedded.
Nucleic acids
The various procedures that tissues undergo either in surgery or in the pathology laboratory have different effects in terms of biomolecule stability. Among the most sensitive of molecules, RNA expression may vary even with little exposure to the environment. For instance, warm ischemia has been proven to affect RNA quality and level detection, the degradation of which may be lowered through tissue cooling.Citation44 On the other hand, some studies have shown that cold ischemia duration decreases RNA integrity levels only to a modest degree, affecting both RNA integrity number and yielded quantity levels,Citation45,Citation46 while in other studies such effects of cold ischemia were not observed.Citation47,Citation48 The American Society of Clinical Oncology and College of American Pathologists have produced guidelines regarding the cold ischemia interval, which should remain below 2 hours.Citation25 Others propose a gold standard of 30 minutes, although some evidence exists that even with such a small delay, effects on tissues such as gene expression may be significant.Citation49,Citation50 Concurrent measurement of the reduction of housekeeping gene expression levels may provide information regarding the levels of RNA quality degradation.Citation51 The fixation process has great influence on RNA quality because it causes strand breakage and cross-linking with other biomolecules. RNA from FFPE samples was found to be heavily degraded and fragmented so that only short sequences, ~100–200 nucleotides long, could be recognized and amplified through polymerase chain reaction (PCR).Citation52,Citation15 Small tissue fragments and short fixation times – 8 hours in formalin – should provide the best nucleic acid conservation and best tissue morphology preservation.Citation53 The quality of these parameters may also be preserved through a variant of the formaldehyde fixation process, which lowers the process temperature at 4°C.Citation54 As the level of RNA integrity conservation in FFPE specimens is low, alternative solutions, such as RNAlater tissue freezing, may be employed with significant results. Frozen tissues are known to provide a greater quantity of high-quality RNA and DNA than FFPE tissues for assays involving nucleic acids.Citation55,Citation45 By causing precipitation of tissue RNA-ses, it has been shown that RNAlater increases both RNA integrity number values and also can cause a threefold increase in RNA-yielded quantity results that are similar to or even greater than those obtained through the flash freeze technique.Citation56 Other studies, however, suggest that freezing still may be preferred over RNAlater. Consequently, The Tumor Analysis Best Practices Group, a group of investigators employing a commercial microarray platform widely used in clinical trials, has the following recommendations: all tissue samples should be flash frozen within minutes of surgery and stored at ≤–80°C. Samples should also be kept in small, airtight containers and kept from drying out during frozen storage by placing fragments of ice in with the sample.Citation57
In addition to mRNA, the recently discovered microRNA is a potential biomarker for many diseases that impose surgical treatment.Citation58 Unlike the former, however, microRNA seems to have greater postexcision stability than mRNA, possibly because of its stable association with proteins in the Argonaute 2 complex.Citation59 Many studies have shown that expression profiles of microRNAs generally remain comparable to those derived from frozen tissues, independently of the use of widely used fixatives or storage time or even the employed microRNA profile assay method.Citation60 Also, microRNA expression might not be influenced by the use of methods such as formalin fixation methods or immunocytochemical methods.Citation61 Finally, studies that assess the effect of warm or cold ischemia time intervals on microRNA profiles are still missing.
As we have mentioned, nucleic acids are more susceptible to degradation through the use of fixation techniques. Consequently, fresh-frozen tissue specimens are the preferred source for nucleic acid-based assays. However, because of the limited availability of such tissues, attention has been given to FFPE tissues as an alternative source of genetic material. After surgery, it is known that a longer prefixation time interval may cause DNA integrity level change.Citation62 A possible solution is the use of commercial formulations capable of denaturing nucleases, such as the Whatman FTA paper, which even allows the storage of tissues at room temperature while preventing DNA damage and increasing DNA assay results after tissue processing shown to be excellent sources of DNA; FFPE tissues create additional problems in terms of material extraction and analysis.Citation63–Citation65 In addition, it has been observed that DNA isolated from FFPE tissue contains more PCR inhibitors than DNA isolated from nonfixed specimens.Citation66 However, new studies that compare modern DNA isolation methods may provide recommendations for the selection of protocols that yield optimal results with nucleic acid assays such as PCR.Citation67 Other nucleic assays may be deemed impractical on FFPE tissues. Some sequencing studies have shown through sequencing methods that as much as one artificial mutation per 500 bases may result from the formalin fixation process.Citation68 However, next-generation sequencing (NGS) has yielded excellent results even with single-nucleotide variant calls or interpretation of insertions and deletions, which are some of the issues arising when analyzing FFPE tissues.Citation69 Furthermore, a new study evaluating the lung cancer KRAS, BRAF, and EGFR gene mutations has compared both the NGS and real time-PCR assays and proved that while the results obtained by both methods were similar, NGS was able to detect both single-nucleotide and insertion-deletion variations. In addition, a new study by Wagle et alCitation70 provides a method combining hybridization-capture and deep sequencing, which maximize sequence yield and lower overall assay costs. Regarding a-chorionic gonadotrophin, early studies suggested that DNA samples obtained from FFPE tissues were uninterpretable because of various errors such as oversaturation and nonuniformity in replicates.Citation71 Recent advances have surmounted these impediments and even compared these new methods to suggest optimal a-chorionic gonadotrophin platforms.Citation72
If both the warm and hot ischemia times are optimal, fixation using paraformaldehyde does not produce any alterations of the nucleic acids. Considering that formaldehyde acts mainly on the protein level by forming –CH3 chemical connections, it has a lower effect on the quality and preservation of both DNA and RNA. Fragmentation of the nucleic acids is mainly found in paraffin-embedded tissues. Still, new assays for molecular analysis such as next-generation sequencing or methylation arrays are associated with limited fragmentation of the nucleic acids and the results of the analysis are not influenced by the degree of alteration in paraffin-embedded tissues. Thus, even if the experience of various pathology laboratories is limited in this regard, molecular analyses should use paraffin-embedded tissues that are not older than 10 years, as according to the guidelines.Citation73–Citation75
In the case of protein decalcification, the process may influence molecular testing, considering that one of the most common types of biopsies that are decalcified are the bone marrow biopsies. Thus, the International Council for Standardization in Hematology (ICSH) has set up guidelines that aim to bring together the various protocols for tissue decalcification and still maintain an optimum quality of the biopsy material for the IHC staining.Citation76 The best protocol of decalcification for IHC staining is ethylenediaminetetraacetic acid based, lasting between 16 and 24 hours, and the strong acids used have allowed the keeping of the various differentiation markers on the cell surface. Decalcification should be followed by a careful rinsing for about 10 minutes in order to properly remove the decalcification agent. Combining the protocols is not recommended, with heating and steering improving decalcification, and should always be considered.Citation77 Even if protein degradation is quite different from the degradation of nucleic acids, current international pathology practice protocols require that the used protocols for tissue processing take into consideration DNA and/or RNA degradation. Thus, in order to also achieve a good tissue quality for molecular testing, the decalcification time is between 16 and 24 hours and the used substance is 14% ethylenediaminetetraacetic acid. Shortening this time is only acceptable for morphology assessment. In case the laboratory also requires molecular, cytogenetic, or flow cytometry assays, a longer time is required, as shown in .
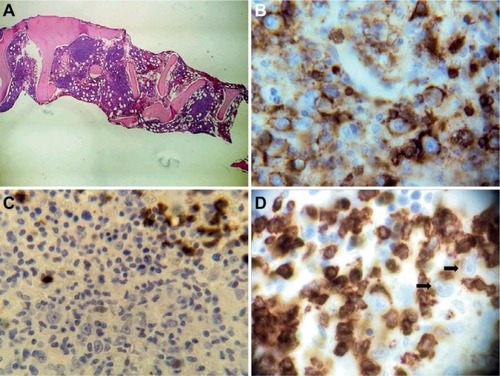
Figure 3 Pathology diagnosis in hematological malignancies.
Notes: (A) Bone marrow biopsy of a T-cell-rich/diffuse large B-cell lymphoma. (B) CD45+ cells in a bone marrow biopsy of a T-cell-rich/diffuse large B-cell lymphoma. (C) CD15− cells in a bone marrow biopsy of a T-cell-rich/diffuse large B-cell lymphoma. (D) CD3 negative staining in the large B cells but positive in the small T cells in a bone marrow biopsy of a T-cell-rich/diffuse large B-cell lymphoma (the black arrows show positive CD3+ cells).
Conclusion
Molecular diagnostics have become a reality. In the coming years, they will account for an increasingly significant fraction of the activity of pathology laboratories. In response to the new requirements, more and more laboratories will have to adapt their activities and implement, control, and standardize the procedures associated with the preanalytical phase. Each laboratory should create a dedicated unit, with specific equipment and specialist staff. The stages mentioned in the present article indicate that the pathologist is central in the processes required for molecular testing. The physicians working in pathology laboratories therefore will have to quickly familiarize themselves with the principles and techniques of molecular biology, thus acquiring dual competences, as morphological and also molecular pathologists.
Acknowledgments
Ravnit Grewal and Ciprian Tomuleasa contributed equally to the current paper and are considered senior authors. Sergiu Susman received funding from a PN-III-P2-2.1-BG-2016-0117 grant awarded by the Romanian Government, as well as an internal grant (code 4944/4/08.03/2016) of the Iuliu Hatieganu University of Medicine and Pharmacy, Cluj Napoca. Ioana Berindan-Neagoe and Sergiu Susman were financed by the POSCCE 709/2010 grant with title: “Clinical and economical impact of proteome and transcriptome molecular profiling in neoadjuvant therapy of triple negative breast cancer (BREASTIMPACT)”. Ciprian Tomuleasa received funding from the Romanian Research Ministry, contracts PN-II-RU-TE-2014-4-1783 (awarded to Young Research Teams) and CNFIS-FDI-2017-1350 (awarded to Institutional Development Funds), as well as from an international collaboration grant between Romania and the People’s Republic of China, contract 57 BM/2016.
Disclosure
The authors report no conflicts of interest in this work.
References
- KiernanUBiomarker rediscovery in diagnosticsExpert Opin Med Diagn20082121391140023496785
- PattersonSDuBoseRThe role of biomarkers in the future of drug developmentExpert Opin Drug Discov20061319920423495841
- LeeJWFigeysDVasilescuJBiomarker assay translation from discovery to clinical studies in cancer drug development: quantification of emerging protein biomarkersAdv Cancer Res20079626929817161683
- StopeckABrown-GlabermanUWongHThe role of targeted therapy and biomarkers in breast cancer treatmentClin Exp Metastasis201229780781922692561
- KelleyRKVan BebberSLPhillipsKAVenookAPPersonalized medicine and oncology practice guidelines: a case study of contemporary biomarkers in colorectal cancerJ Natl Compr Canc Netw201191132521233242
- CorlessCLBarnettCMHeinrichMCGastrointestinal stromal tumours: origin and molecular oncologyNat Rev Cancer2011112865878
- BeelenKZwartWLinnSCCan predictive biomarkers in breast cancer guide adjuvant endocrine therapy?Nat Rev Clin Oncol20129952954122825374
- MaesEBroeckxVMertensIAnalysis of the formalin-fixed paraffin-embedded tissue proteome: pitfalls, challenges, and future prospectivesAmino Acids201345220521823592010
- LippiGBecan-McBrideKBehúlováDPreanalytical quality improvement: in quality we trustClin Chem Lab Med201351122924123072858
- CeriottiFCappellettiPCaputoMA risk-analysis approach to the evaluation of analytical qualityClin Chem Lab Med201150677121958343
- DimensteinIBGrossing biopsies: an introduction to general principles and techniquesAnn Diagn Pathol200913210611319302959
- NovisDADetecting and preventing the occurrence of errors in the practices of laboratory medicine and anatomic pathology: 15 years’ experience with the College of American Pathologists’ Q-PROBES and Q-TRACKS programsClin Lab Med200424496597815555751
- van KriekenJNormannoNBlackhallFGuideline on the requirements of external quality assessment programs in molecular pathologyVirchows Archiv20124621273723250354
- WolffACHammondMESchwartzJNAmerican Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancerArch Pathol Lab Med20071311184319548375
- van MaldegemFde WitMMorsinkFMuslerAWeegenaarJvan NoeselCEffects of processing delay, formalin fixation, and immunohis-tochemistry on RNA recovery from formalin-fixed paraffin-embedded tissue sectionsDiagn Mol Pathol200817515818303406
- Yildiz-AktasIZDabbsDJBhargavaRThe effect of cold ischemic time on the immunohistochemical evaluation of estrogen receptor, progesterone receptor, and HER2 expression in invasive breast carcinomaMod Pathol20122581098110522460807
- BuesaRJPeshkovMVHow much formalin is enough to fix tissues?Ann Diagn Pathol201216320220922483550
- BerodAHartmanBKPujolJFImportance of fixation in immunohistochemistry: use of formaldehyde solutions at variable pH for the localization of tyrosine hydroxylaseJ Histochem Cytochem19812978448506167611
- ZouNLiangQHeHUltrasound-facilitated formalin fixation of biological specimensBiotech Histochem201086641342020854223
- FoxCHJohnsonFBWhitingJRollerPPFormaldehyde fixationJ Histochem Cytochem19853388458533894502
- KhouryTSaitSHwangHDelay to formalin fixation effect on breast biomarkersMod Pathol200922111457146719734848
- GoldsteinNSFerkowiczMOdishEManiAHastahFMinimum formalin fixation time for consistent estrogen receptor immunohistochemical staining of invasive breast carcinomaAm J Clin Pathol20031201869212866377
- TongLCNelsonNTsourigiannisJMulliganAMThe effect of prolonged fixation on the immunohistochemical evaluation of estrogen receptor, progesterone receptor, and HER2 expression in invasive breast cancer: a prospective studyAm J Surg Pathol201135454555221358301
- HammondMEHayesDFDowsettMAmerican Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancerArch Pathol Lab Med2010134690792220524868
- WolffACHammondMESchwartzJNAmerican Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancerJ Clin Oncol200725111814517159189
- EspinaVEdmistonKHHeibyMA portrait of tissue phospho-protein stability in the clinical tissue procurement processMol Cell Proteomics20087101998201818667411
- SvenssonMSkoldKNilssonANeuropeptidomics: MS applied to the discovery of novel peptides from the brainAnal Chem2007791151617262921
- JuhlHPreanalytical aspects: a neglected issueScand J Clin Lab Invest Suppl201070suppl 2426365
- MorenteMMagerRAlonsoSTuBaFrost 2: standardising tissue collection and quality control procedures for a European virtual frozen tissue bank networkEur J Cancer200642162684269117027255
- EricssonCFranzénBNistérMFrozen tissue biobanks. Tissue handling, cryopreservation, extraction, and use for proteomic analysisActa Oncol200645664366116938807
- AhramMFlaigMJGillespieJWEvaluation of ethanol-fixed, paraffin-embedded tissues for proteomic applicationsProteomics20033441342112687609
- HoodBLConradsTPVeenstraTDMass spectrometric analysis of formalin-fixed paraffin-embedded tissue: unlocking the proteome withinProteomics20066144106411416800036
- ShiSLiuCBalgleyBMLeeCTaylorCRProtein extraction from formalin-fixed, paraffin-embedded tissue sections: quality evaluation by mass spectrometryJ Histochem Cytochem200654673974316399996
- SprungRBrockJTanksleyJEquivalence of protein inventories obtained from formalin-fixed paraffin-embedded and frozen tissue in multidimensional liquid chromatography-tandem mass spectrometry shotgun proteomic analysisMol Cell Proteomics2009881988199819467989
- StantaGMucelliSPPetreraFBoninSBussolatiGA novel fixative improves opportunities of nucleic acids and proteomic analysis in human archive’s tissuesDiagn Mol Pathol200615211512316778593
- MangeAChaurandPPerrochiaHRogerPCaprioliRMSolassolJLiquid chromatography-tandem and MALDI imaging mass spectrometry analyses of RCL2/CS100-fixed, paraffin embedded tissues: proteomics evaluation of an alternate fixative for biomarker discoveryJ Proteome Res20098125619562819856998
- HoodBLDarflerMMGuielTGProteomic analysis of formalin-fixed prostate cancer tissueMol Cell Proteomics20054111741175316091476
- NirmalanNJHarndenPSelbyPJBanksREMining the archival formalin-fixed paraffin-embedded tissue proteome: opportunities and challengesMol Biosyst2008471272018563244
- OlivieriEHerbertBRighettiPGThe effect of protease inhibitors on the two-dimensional electrophoresis pattern of red blood cell membranesElectrophoresis200122356056511258769
- SvenssonMBorenMSköldKHeat stabilization of the tissue proteome: a new technology for improved proteomicsJ Proteome Res20098297498119159280
- WolffCSchottCPorschewskiPReischauerBBeckerKSuccessful protein extraction from over-fixed and long-term stored formalin-fixed tissuesPLoS One201161e1635321305021
- NishimuraTNomuraMTojoHProteomic analysis of laser-microdissected paraffin-embedded tissues: (2) MRM assay for stage-related proteins upon non-metastatic lung adenocarcinomaJ Proteomics20107361100111019944198
- ChungJLeeSKrisYBraunschweigTTraicoffJHewittSA well-based reverse-phase protein array applicable to extracts from formalin-fixed paraffin-embedded tissueProteomics2008210–111539154721136801
- ChungJYBraunschweigTWilliamsRFactors in tissue handling and processing that impact RNA obtained from formalin-fixed, paraffin-embedded tissueJ Histochem Cytochem200856111033104218711211
- HatzisCSunHYaoHEffects of tissue handling on RNA integrity and microarray measurements from resected breast cancersJ Natl Cancer Inst2011103241871188322034635
- FlorellSCoffinCHoldenJPreservation of RNA for functional genomic studies: a multidisciplinary tumor bank protocolMod Pathol200114211612811235903
- BaoWGZhangXZhangJGBiobanking of fresh-frozen human colon tissues: impact of tissue ex-vivo ischemia times and storage periods on RNA qualityAnn Surg Oncol20122051737174422711177
- MickePOhshimaMTahmasebpoorSBiobanking of fresh frozen tissue: RNA is stable in nonfixed surgical specimensLab Invest200686220221116402036
- FriedeLGrossmanRHuntRMSternSNational Biospecimens Network BlueprintDurham, NCCostella Group Inc2003
- FreidinMBBhudiaNLimENicholsonAGCooksonWOMoffattMFImpact of collection and storage of lung tumor tissue on whole genome expression profilingJ Mol Diagn201214214014822240448
- CroninMPhoMDuttaDMeasurement of gene expression in archival paraffin-embedded tissues: development and performance of a 92-gene reverse transcriptase-polymerase chain reaction assayAm J Pathol20041641354214695316
- DottiIBoninSBasiliGEffects of formalin, methacarn, and fineFIX fixatives on RNA preservationDiagn Mol Pathol201019211212220502189
- BussolatiGAnnaratoneLMedicoED’ArmentoGSapinoAFormalin fixation at low temperature better preserves nucleic acid integrityPLoS One201166e2104321698245
- SrinivasanMSedmakDJewellSEffect of fixatives and tissue processing on the content and integrity of nucleic acidsAm J Pathol200216161961197112466110
- ScicchitanoMDalmasDBertiauxMPreliminary comparison of quantity, quality, and microarray performance of RNA extracted from formalin-fixed, paraffin-embedded, and unfixed frozen tissue samplesJ Histochem Cytochem200654111229123716864893
- ChowdaryDLathropJSkeltonJPrognostic gene expression signatures can be measured in tissues collected in RNAlater preservativeJ Mol Diagn200681313916436632
- HoffmanEPGuidelines: expression profiling-best practices for data generation and interpretation in clinical trialsNat Rev Genet20045322923714970825
- VoliniaSCalinGALiuCGA microRNA expression signature of human solid tumors defines cancer gene targetsProc Natl Acad Sci U S A200610372257226116461460
- TurchinovichAWeizLLangheinzABurwinkelBCharacterization of extracellular circulating microRNANucleic Acids Res201139167223723321609964
- BovellLShanmugamCKatkooriVRmiRNAs are stable in colorectal cancer archival tissue blocksFront Biosci2012E411937
- SchusterCBudcziesJFaberCKirchnerTHlubekFMicroRNA expression profiling of specific cells in complex archival tissue stained by immunohistochemistryLab Invest201191115716520661226
- JewellSSrinivasanMMcCartLAnalysis of the molecular quality of human tissuesAm J Clin Pathol2002118573374112428794
- PetrasMLLeffertsJAWardBPSuriawinataAATsongalisGJKRAS detection in colonic tumors by DNA extraction from FTA paper: the molecular touch-prepDiagn Mol Pathol201120418919322089345
- FerrerIArmstrongJCapellariSEffects of formalin fixation, paraffin embedding, and time of storage on DNA preservation in brain tissue: a BrainNet Europe StudyBrain Pathol200717329730317465988
- DuvalKAubinRElliottJOptimized manual and automated recovery of amplifiable DNA from tissues preserved in buffered formalin and alcohol-based fixativeForensic Sci Int2010428088
- HennigGGehrmannMStroppUAutomated extraction of DNA and RNA from a single formalin-fixed paraffin-embedded tissue section for analysis of both single-nucleotide polymorphisms and mRNA expressionClin Chem201056121845185320947696
- SamSSLebelKABissaillonCLTafeLJTsongalisGJLeffertsJAAutomation of genomic DNA isolation from formalin-fixed, paraffin-embedded tissuesPathol Res Pract20122081270570723057998
- WilliamsCPonténFMobergCA high frequency of sequence alterations is due to formalin fixation of archival specimensAm J Pathol199915551467147110550302
- KerickMIsauMTimmermannBTargeted high throughput sequencing in clinical cancer settings: formaldehyde fixed-paraffin embedded (FFPE) tumor tissues, input amount and tumor heterogeneityBMC Med Genomics201146821958464
- WagleNBergerMFDavisMJHigh-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencingCancer Discov201221829322585170
- NassiriMGugicDOlczykJRamosSVincekVPreservation of skin DNA for oligonucleotide array CGH studies: a feasibility studyArch Dermatol Res2007299735335717665208
- KrijgsmanOIsraeliDHaanJCCGH arrays compared for DNA isolated from formalin-fixed, paraffin-embedded materialGenes Chromosomes Cancer201251434435222162309
- IonelALucaciuOTăbăranFHistopathological and clinical expression of periodontal disease related to the systemic inflammatory responseHistol Histopathol201732437938427440198
- CiobanuLTantauMValeanSRifaximin modulates 5-fluoroura-cil-induced gastrointestinal mucositis in ratsEur Rev Med Pharmacol Sci201620234993500127981532
- LucanCPopLAFlorianAHLA genotyping using next generation sequencingRom J Intern Med20165429810427352438
- TorlakovicEEBrynesRKHyjekEICSH guidelines for the standardization of bone marrow immunohistochemistryInt J Lab Hematol201537443144925977137
- DimensteinIBBone grossing techniques: helpful hints and proceduresAnn Diagn Pathol200812319119818486895
