Abstract
Objective
Increased expression of programmed death-ligand 1 (PD-L1) on tumor cells can be found in various malignancies; however, very limited information is known about its role in anal squamous cell carcinoma (ASCC). This study explored PD-L1 expression in ASCC patients and its association with patients’ clinicopathological features, CD8+ T cell infiltration, and prognosis.
Methods
Formalin-fixed paraffin-embedded tumor samples from 26 patients with ASCC were retrieved. The levels of PD-L1 expression on the membrane of both tumor cells and tumor-infiltrating mononuclear cells (TIMCs) were evaluated by immunohistochemistry. CD8+ T cell densities, both within tumors and at the tumor–stromal interface, were also analyzed. Baseline clinicopathological characteristics, human papilloma virus (HPV) status, and outcome data correlated with PD-L1-positive staining.
Results
PD-L1 expression on tumor cells and TIMCs was observed in 46% and 50% of patients, respectively. Nineteen patients (73%) were HPV positive, with 7 showing PD-L1-positive staining on tumor cells and 9 showing PD-L1-positive staining on TIMCs. Increasing CD8+ density within tumors, but not immune stroma, was significantly associated with decreased PD-L1 expression by both tumor cells and TIMCs (P=0.0043 and P=0.0007). Patients with negative PD-L1 expression had significantly better progression-free survival (P=0.038 and P=0.0443) and a non-statistically significant trend toward longer overall survival (P=0.0882 and P=0.1222) compared with patients with positive PD-L1 expression.
Conclusion
PD-L1 is widely expressed on the membrane of tumor cells and TIMCs in ASCCs. Its negative impact on prognosis may be due to the diminished CD8+ T cell infiltration within tumors.
Introduction
Anal squamous cell carcinoma (ASCC), most of which arise from infection with human papilloma virus (HPV), is the most common histological type of the anal canal malignant disease around the world.Citation1 The major initial treatment for ASCC is chemoradiotherapy (CRT), which consists of radiotherapy combined with 5-fluoro-uracil and mitomycin C.Citation2 This method could generally achieve good local control and thus avoid radical surgery;Citation3,Citation4 however, early and late toxicities induced by CRT remain considerable.Citation5,Citation6 Furthermore, 10–20% of patients are not sensitive to CRT or relapse early after treatment. Once relapse occurs, only 40% of patients can be salvaged by abdominoperineal resection,Citation7 but patients with locally advanced or metastatic disease who receive palliative chemotherapy have 5-year survival rates of 15%. Thus, effective therapeutic methods need to be developed for these ASCC patients.Citation8
Recent advances in immunotherapy offer promising new strategies for treatment of some solid tumors.Citation9 The programmed death-1/programmed death-ligand 1 (PD-1/PD-L1) immune checkpoint inhibitor, for example, is a representative of the novel immunotherapeutic strategies. PD-1 is a transmembrane receptor that is expressed by activated T cells and B cells, whereas PD-L1 is constitutively expressed on subsets of macrophages and dendritic cells, and its expression may be further upregulated by some inflammatory cytokines such as IFN-γ.Citation10,Citation11 The interaction between PD-L1 and PD-1 results in the suppression of T cell activation and proliferation, and thereby, the dampening of the host antitumor immune response. Various kinds of tumors can express PD-L1, including breast, thymic, gastric, penile, and renal cell carcinomas.Citation12–Citation16 PD-L1 aberrant expression on tumor cells is believed to result in the suppression of local immune responses, and thus evade T cell-mediated killing and increase the risk of cancer progression.
ASCC, similar to other virus-associated malignancies, is highly immunogenic. ASCC patients whose tumors harbor high numbers of CD8+ tumor-infiltrating lymphocytes (TILs) demonstrate improved overall survival (OS).Citation17 However, the immune system often ultimately fails to control their growth. Therefore, the immune resistance established in the tumor microenvironment should be broken. Given the promising results of PD-1/PD-L1-based immunotherapies in other virus-associated malignancies including Merkel cell carcinoma and hepatocellular carcinoma,Citation18,Citation19 we sought to assess PD-L1 expression in ASCC. In the current study, PD-L1 expression in both tumor cells and tumor-immune infiltrating cells was determined, and its association with clinicopathological characteristics and survival was investigated. We also determined whether PD-L1 expression correlates with intratumoral and stromal CD8+ T cell density.
Materials and methods
Study patients
Twenty-six patients with histologically diagnosed ASCC were consecutively selected from Sun Yat-sen University Cancer Center and the Sixth Affiliated Hospital of Sun Yat-sen University between 2003 and 2015. Patients were treated for ASCC with radical CRT with or without surgery. Paraffin-embedded tumor tissues from all patients were obtained through a colonoscopy biopsy before treatment, and sufficient follow-up clinical data were available. Patients with concurrent other malignancies or HIV infection were excluded. Clinicopathological features including patients general characteristics, tumor characteristics, and the time of diagnosis, relapse, and last follow-up were all recorded. Complete stage information (TNM) at presentation was documented. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This retrospective study was approved by the Ethics Committee of Sun Yat-sen University Cancer Center and the Sixth Affiliated Hospital of Sun Yat-sen University, and written informed consent was obtained from all patients.
Immunohistochemistry
PD-L1, CD8, and p16 (as a surrogate of HPV infection)Citation20,Citation21 were stained using a standard protocol as previously described.Citation22 Briefly, freshly cut formalin-fixed paraffin-embedded specimens were dewaxed in xylene, hydrated in graded alcohol, and washed in phosphate-buffered saline; after neutralizing endogenous peroxidase (0.3% H2O2 for 10 min), the microwave antigen retrieval method was applied using Tris–EDTA (pH 9.0). Subsequently, the slides were preincubated with blocking serum and then were incubated overnight at 4°C with each primary antibody: p16 (1:100, ZM0205; ZSGB-BIO, Beijing, People’s Republic of China), CD8 (1:100, ZA0508, ZSGB-BIO), and PD-L1 (1:100, #13684; Cell Signaling Technology, Shanghai, People’s Republic of China). Once the above procedure was completed, the sections were serially rinsed, incubated with secondary antibodies, visualized using diaminobenzidine, and counterstained with hematoxylin. Tonsil tissues were used as positive controls for PD-L1 and CD8, and cervical squamous cell carcinoma specimens were used as positive controls for p16 (). Specimens of identical tissues stained without primary antibody were used as negative controls.
Staining quantification
The immunohistochemically stained tissue sections were evaluated separately by 2 pathologists who had no prior knowledge of the clinicopathological features.
PD-L1 expression levels on tumor cells or tumor- infiltrating mononuclear cells (TIMCs) were scored separately as reported in recent trials and other studies.Citation16–Citation23 Tumor and immune cells demonstrating at least moderate 5% or greater PD-L1 staining on the cell surface (membranous) were considered positive.
Slides stained for CD8 were annotated for areas of intratumoral (within tumor nest) and tumor stroma surrounding the epithelial component of the tumor.Citation17 In each location, using a high-power microscopic field (×400; 0.0625 mm2), 4 independent areas with the most abundant CD8+ T cell infiltration were selected and the numbers of CD8+ T cells were counted manually from the digital images. The average positive-staining counts were used for statistical analyses.
Positive p16 status was defined as strong and diffuse nuclear and cytoplasmic staining in ≥70% of the tumor cells ().Citation20
Statistical analysis
The Chi-squared test or Fisher’s exact test was used to analyze the correlations between PD-L1 expression and patient characteristics for categorical variables. Pearson product-moment correlation coefficients were used to determine the correlations between the intratumoral and stromal numbers of CD8+ T cells. We evaluated whether PD-L1 expression was associated with the prognosis of ASCC patients. PFS was defined as the time from the date of initial diagnosis to the date of disease progression or death due to any cause. OS was defined from the initial diagnosis until the date of death or last follow-up. The Kaplan–Meier method was used to evaluate the patients’ survival curves, and the log-rank test was used to assess the significance of differences between PD-L1-positive and PD-L1-negative groups. All statistical analyses were performed using SPSS 20.0 statistical software. P-values declared in this study were all based on 2-sided tests, and those less than 0.05 were interpreted as statistically significant.
Results
Clinical characteristics of patients with ASCC
The baseline clinicopathological features of the 26 patients are shown in . The median age of the patients at diagnosis was 52.5 years, ranging from 27 to 76 years. The 26 cases included 4 males (15%) and 22 females (85%). The histological grade was defined as well- (n=9), moderate-(n=13), and poorly differentiated (n=4) carcinoma. Fifteen cases (58%) were found to be node positive by cytological confirmation. The median follow-up period was 40.9 months (range 1.3–126.4). At the end of the follow-up period, 5 patients had died, 5 had disease progression but were alive, and 16 had no evidence of disease.
Table 1 Association of PD-L1 expression status and clinicopathological features in 26 ASCC patients
PD-L1 expression on the membrane of tumor cells and TIMCs
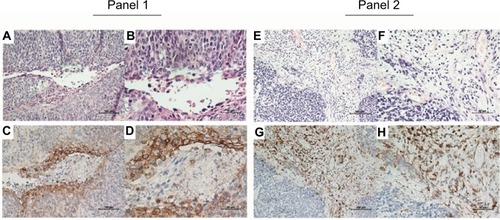
Of the 26 primary ASCC tumors examined, PD-L1 expression on neoplastic cells was observed in 12 (46%) cases. Clusters of PD-L1-positive TIMCs in tumor stroma were identified in 13 (50%) tumors. Essentially, all positive samples showed moderate-to-strong membranous staining (). We classified all samples into 4 categories according to the PD-L1 expression on neoplastic cells and TIMCs (). Of the 13 patients with PD-L1-positive TIMCs, 9 had PD-L1-positive neoplastic cells. Of the 13 patients with PD-L1-negative TIMCs, 10 had PD-L1-negative neoplastic cells. The proportion of PD-L1(+/+) or PD-L1(−/−) patients was significantly higher than the proportion of PD-L1(+/−) or PD-L1(−/+) patients (P=0.047). Of the 19 (73%) HPV-positive tumors, 7 showed PD-L1 staining on tumor cells and 9 showed PD-L1 staining within the immune stroma.
Figure 1 Representative immunohistochemical staining of PD-L1 in ASCC.
Notes: H&E (A, B, E, and F) and PD-L1-positive (C, D, G, and H) staining on tumor cells (Panel 1) and TIMCs (Panel 2). Original magnification: ×200 (A, C, E, and G) and ×400 (B, D, F, and H).
Abbreviations: PD-L1, programmed death-ligand 1; ASCC, anal squamous cell carcinoma; H&E, hematoxylin and eosin; TIMCs, tumor-infiltrating mononuclear cells.
Relationship between PD-L1 positivity and clinicopathological features
depicts the distributions of clinicopathological characteristics according to positivity for PD-L1. In detail, PD-L1 expression on the membrane of tumor cells and TIMCs did not correlate with age (P=1.000 and P=1.000), gender (P=0.598 and P=0.593), histological grade (P=0.306 and P=0.096), T stage (P=0.701 and P=0.226), lymph node status (P=0.692 and P=1.000), Union International Cancer Center stage (P=0.462 and P=1.000), or p16 infection (P=0.190 and P=1.000) ().
PD-L1 expression and clinical outcome
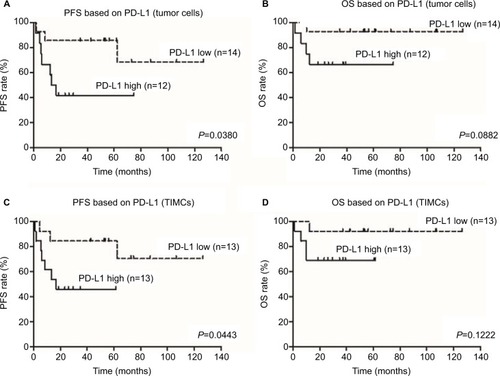
Of the 26 patients with primary ASCC tumors examined in this study, 10 experienced disease progression and 5 died from disease. On Kaplan–Meier analysis, PD-L1 expression on tumor cells and in the immune stroma was associated with shorter PFS (P=0.0380 and P=0.0443) and OS (P=0.0882 and P=0.1222), but only the association with PFS reached the level of statistical significance ().
Figure 2 Correlation of PD-L1 expression with PFS and OS in 26 patients with ASCC.
Notes: Kaplan–Meier survival curve showing worse progression-free survival for ASCC patients whose tumor cells or TIMCs demonstrated membranous PD-L1 expression (A and C). High PD-L1 expression on tumor cells or TIMCs showed a trend toward poorer prognosis than low PD-L1 expression (B and D).
Abbreviations: PD-L1, programmed death-ligand 1; PFS, progression-free survival; OS, overall survival; ASCC, anal squamous cell carcinoma; TIMCs, tumor-infiltrating mononuclear cells.
Correlation between PD-L1 expression and CD8+ T cell infiltration
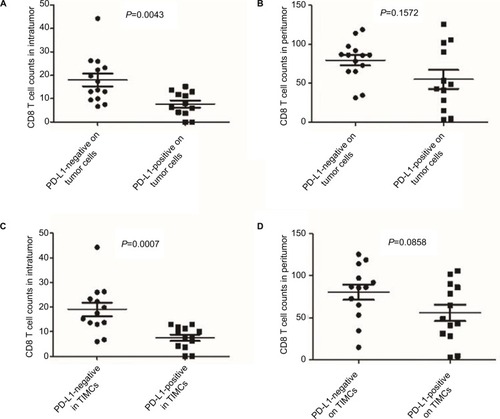
As CD8+ T cells are central to the adaptive immune response to ASCC, we quantified CD8+ infiltration in tumor and associated stroma by manual cell counting from the digital images, and compared the average CD8+ counts in each location with the expression of PD-L1 on tumor cells or immune stroma. Representative images of CD8 assay results are shown in . We found that the numbers of CD8+ T cells in the intratumoral and stromal areas had a significant positive correlation (). Further analyses revealed that the increased CD8+ T cell counts of the intratumoral location were associated with decreased PD-L1 expression by both tumor cells and immune stroma (P=0.0043 and P=0.0007); moreover, CD8+ T cells in the stromal location were also associated with reduced PD-L1 expression on tumor cells and TIMCs, but this relationship was not statistically significant (P=0.1572 and P=0.0858) ().
Figure 3 Strong correlation between the numbers of CD8+ T cells in the intratumoral location and stroma.
Abbreviation: HPF, high-power field.
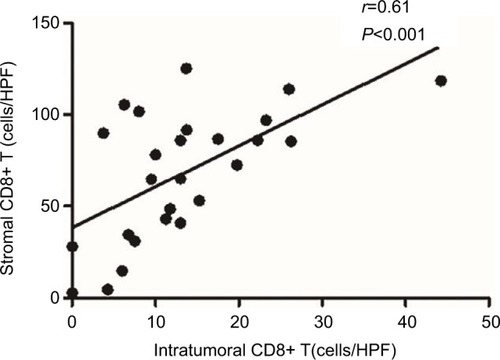
Figure 4 CD8+ T cell counts in each location according to different PD-L1 status.
Notes: Increasing CD8+ T cell counts of the intratumoral location were associated with decreased PD-L1 expression by both tumor cells and immune stroma (A and C). CD8+ T cells of the stromal location were also associated with reduced PD-L1 expression on tumor cells and TIMCs but were not statistically significant (B and D).
Abbreviations: PD-L1, programmed death-ligand 1; TIMCs, tumor-infiltrating mononuclear cells.
Taken together, these findings are compatible with a role for CD8+ T cells in responding to PD-L1 expression in the ASCC microenvironment and are consistent with some previous findings.Citation24,Citation25
Discussion
Increased expression of PD-L1 on tumor cells can be found in various malignancies, including head and neck, gastric, colorectal, prostate, pancreatic, esophageal, breast, renal, and non-small-cell lung cancers, melanoma, and ASCC.Citation26–Citation35 PD-L1 expression by tumor cells has been correlated with both favorable and unfavorable prognoses in several malignancies.Citation35–Citation39 To our knowledge, this is the first study to investigate PD-L1 expression on TIMCs and its association with clinical outcomes of patients with ASCC. We found that PD-L1 is widely expressed on the membrane of tumor cells and TIMCs. PD-L1 expression on tumor cells and in the immune stroma was associated with shorter PFS and OS rates. Furthermore, we also demonstrated that PD-L1 expression on tumor cells and on TIMCs correlated with reduced numbers of CD8+ T cells in tumors. These results imply that PD-L1-positive tumors may define a subset of immune-tolerant ASCC and provide a rational basis for subsequent investigation of PD-1/PD-L1-targeted immunotherapy.
Thus far, few studies have explored the PD-L1 expression in ASCC. Govindarajan et al were the first to describe PD-L1 expression in ASCC, suggesting that PD-L1 positivity was associated with worse crude recurrence rates and crude event rates, and marginally worse crude mortality rates.Citation36 However, Balermpas et al recently showed that high PD-L1 expression on tumor cells correlated with better local control.Citation35 In our study, patients with negative PD-L1 expression had significantly better PFS and a non-statistically significant trend toward longer OS compared with patients with positive PD-L1 expression. This discrepancy may be attributable to differences in patient selection. The anti-PD-L1 antibody we used in our study was validated in a recent report presented as an ASCO poster (http://meetinglibrary.asco.org/content/132010-144), and this antibody was also used in some other studies.Citation22,Citation40,Citation41
The previous study evaluated HIV-positive and HIV- negative patients collectively, whereas cases with HIV infection were excluded from the current study. As an HIV-associated cancer, the standardized incidence ratio of anal cancer was much higher in people with HIV/AIDS than in transplant recipients.Citation42 One concern is that the antitumor immunity in immunodeficient patients was potentially compromised compared with immunocompetent groups. Another concern is that antiretroviral treatment could alter the immune response. A third concern is that the impact of HIV itself on patients’ outcome would not be negligible. Moreover, in order to exclude the influence of CRT on local immune status and even PD-L1 expression, paraffin-embedded tumor tissues were obtained through colonoscopy biopsies from all patients before treatment in the current study.
Recently, preliminary results from a Phase II trial evaluating the efficacy of Nivolumab, an anti-PD-1 mAb, in patients with refractory metastatic ASCC were presented.Citation43 The overall response rate in those patients was 21%; the median PFS was 4.1 months. However, the types of tumors that are good candidates for this type of treatment remain unknown. Accumulating data suggest that PD-L1-positive tumors have higher response rates to blockade of the PD-1/PD-L1 pathway, although response to treatment for PD-L1-negative patients was also observed.Citation44,Citation45 This indicates that the PD-L1 expression level may be a potential predictive biomarker for therapies targeting the PD-1/PD-L1 pathway. Of note, a previous biomarker analysis with immune checkpoint inhibitors focused on PD-L1 expression on tumor cells rather than tumor-infiltrating immune cells. Based on a recent study showing a potential predictive role for PD-L1 expression on immune cells in patients with metastatic bladder cancer receiving checkpoint inhibitors, the importance of PD-L1 expression in stromal immune cells should also be further studied.Citation45 In our study, we found a comparable PD-L1-positive expression rate between TIMCs in tumor stroma and tumor cells (50% vs 46%). Interestingly, patients with PD-L1 expression on the tumor cell membrane were more likely to have elevated PD-L1 expression on TIMCs (). Thus, further prospective studies are warranted to uncover whether PD-L1 status impacts the prognoses of patients receiving immunotherapy for ASCC and whether PD-L1 expression on tumor cells or TIMCs has differing predictive value.
Tumors upregulate PD-L1 expression either via activated oncogenic signaling (innate resistance) or in response to IFN-γ released by TILs (adaptive resistance). On the basis of their PD-L1 status and the presence or absence of TILs, tumors can be classified into 4 groups.Citation46 Malignant melanoma has been extensively studied, and a high proportion of type I (PD-L1 positive with TILs driving adaptive immune resistance) and type II (PD-L1 negative with no TILs indicating immune tolerance) microenvironments are seen while the proportion of type III (PD-L1 positive with no TILs indicating intrinsic induction) and type IV (PD-L1 negative with TILs indicating the role of other suppressor[s] in promoting immune tolerance) is very low. In contrast in ASCCs, we frequently found that PD-L1-positive ASCC patients tended to have less CD8+ T cell infiltration in intratumoral as well as stromal locations compared with those who were PD-L1-negative, indicating intrinsic induction may happen. This highlights the unique characteristic of the relationship between CD8+ T cells and PD-L1 expression in ASCC. The oncogene drivers and genetic aberrations of the cancer as well as the tissues they arise from likely determine the proportions of various human tumors that fit into each of these 4 groups. However, the early events leading to the aberrant expression of PD-1/PD-L1 by tumor cells and/or stroma cells in ASCC is still unclear.
ASCC is often associated with infectious agents, most notably high-risk oncogenic HPV which has been linked with strong immune responses because of the presence of viral proteins.Citation35 Some reports have shown that tumor inflammation and PD-L1 expression are present to a higher degree in HPV-positive tumors.Citation47,Citation48 However, it is unclear whether the PD-1/PD-L1 axis plays a greater role in HPV-positive ASCCs compared to HPV-negative ASCCs. In our cohort, most subjects were HPV positive (73.1%). Interestingly, the tendency that PD-L1 expression on tumor cells and TIMCs was higher in HPV-negative patients might, at least in part, explain the well-known favorable prognosis observed in patients with HPV-positive ASCCs. Our results are consistent with the result from penile and oropharyngeal squamous cell carcinoma.Citation49,Citation50 However, the use of p16 immunohistochemistry as a surrogate for HPV infection and the small sample sizes may interfere with the analysis results in the current study.
In recent years, PD-1/PD-L1-targeted immunotherapy has been shown to be clinically effective and thus represents a promising therapeutic strategy in select cancer patients.Citation51 Such efficacy is also expected for locally advanced and/or metastatic ASCCs, which respond poorly to various chemotherapeutic agents. Some important findings are presented in our study. We found that PD-L1 expression was not different between age, gender, or tumor stage at diagnosis, indicating that checkpoint inhibition may have a similar effect in various ASCC patients. Interestingly, we did not find a difference in PD-L1 expression between p16-positive and p16-negative patients. However, there was a tendency toward PD-L1 expression on tumor cells and TIMCs among p16-negative ASCC patients. Whether the effect of a PD-1/PD-L1 checkpoint inhibitor is different between HPV-positive and HPV-negative ASCC patients should be explored.
There are several limitations to our findings. First, our study included a relatively small sample size, and thus, additional studies with larger samples are needed to confirm the results from our study. Second, PD-L1 expression was evaluated from small samples obtained by colonoscopy biopsies conducted in our study, which could affect the accuracy of immunological evaluation. Third, our findings may have some selection bias because we made a retrospective analysis. Therefore, objective prospective studies are needed to confirm our results.
In conclusion, the majority of ASCC samples showed membranous PD-L1 expression. PD-L1 positivity on TIMCs and tumor cells was significantly associated with diminished CD8+ T cell infiltration and predicted poor prognosis among ASCC patients. Overall, our findings support continued exploration of PD-1/PD-L1 checkpoint inhibitors for patients with ASCC.
Conclusion
We found that PD-L1 is widely expressed on the membrane of tumor cells and TIMCs. PD-L1 expression on both tumor cells and TIMCs was associated with shorter PFS and OS. Furthermore, we also demonstrated that PD-L1 expression on tumor cells and on TIMCs was correlated with reduced numbers of CD8+ T cells. These results imply that PD-L1-positive tumors may define a subset of immune-tolerant ASCC and provide a rational basis for the exploration of PD-1/PD-L1 checkpoint inhibitors for patients with ASCCs.
Acknowledgments
This study was funded by a grant from Industry-University-Research Collaboration of Guangdong province (KY035541). The authors thank professors Peirong Ding, Zhenhai Lu, Gong Chen, and Liren Li for assisting in selection of study patients and offering insightful guidance for this study. The authors owe thanks to pathologist Qiuliang Wu for his great help in the evaluation of immunohistochemical staining. They also gratefully acknowledge the assistance of the colleagues at State Key Laboratory of Oncology in South China and the Department of Pathology in Sun Yat-sen University Cancer Center.
Supplementary materials
Figure S1 The tonsil tissue specimens were used as positive controls for CD8 (A and D) as well as PD-L1 (B and E) staining. The cervical squamous cell carcinoma specimens were used as positive controls for p16 staining (C and F).
Note: Original magnification: ×200 (A, B and F), ×100 (C), and ×400 (D and E).
Abbreviation: PD-L1, programmed death-ligand 1.
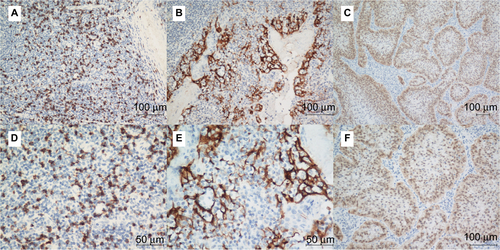
Figure S2 Representative immunohistochemical staining of p16 in ASCC patient samples.
Note: Original magnification: ×100 (A) and ×200 (B).
Abbreviation: ASCC, anal squamous cell carcinoma.
Figure S3 Correlation of PD-L1 expression between tumor cells and TIMCs (P=0.047).
Note: Patients with PD-L1-positive TIMCs were more likely to have higher PD-L1 expression on the tumor cell membrane.
Abbreviations: PD-L1, programmed death-ligand 1; TIMCs, tumor-infiltrating mononuclear cells.
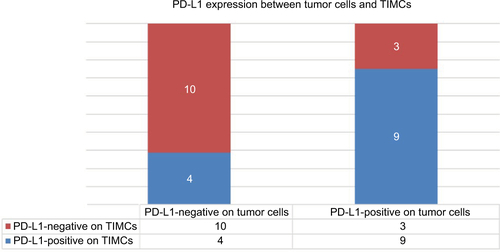
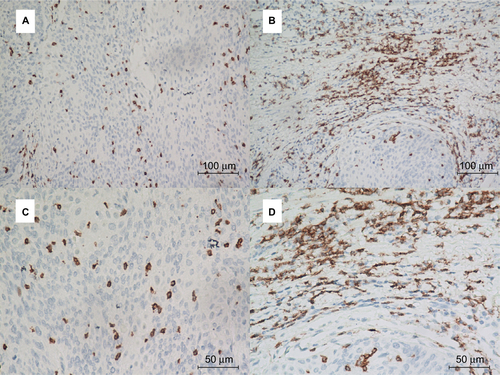
Figure S4 Representative immunohistochemical staining of CD8+ T cells in ASCC.
Notes: PD-L1 staining is analyzed at the intratumoral (A and C) and peritumoral locations (B and D). Top row: ×200 original magnification; bottom row: ×400 original magnification.
Abbreviations: ASCC, anal squamous cell carcinoma; PD-L1, programmed death-ligand 1.
Disclosure
The authors report no conflicts of interest in this work.
References
- BernardiMPNganSYMichaelMMolecular biology of anal squamous cell carcinoma: implications for future research and clinical interventionLancet Oncol20151616e611e62126678214
- JamesRDGlynne-JonesRMeadowsHMMitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 × 2 factorial trialLancet Oncol201314651652423578724
- RenehanAGSaundersMPSchofieldPFO’DwyerSTPatterns of local disease failure and outcome after salvage surgery in patients with anal cancerBr J Surg200592560561415739215
- Glynne-JonesRKadalayilLMeadowsHMACT II Study GroupTumour- and treatment-related colostomy rates following mitomycin C or cisplatin chemoradiation with or without maintenance chemotherapy in squamous cell carcinoma of the anus in the ACT II trialAnn Oncol20142581616162224827136
- Glynne-JonesRRenehanACurrent treatment of anal squamous cell carcinomaHematol Oncol Clin North Am20122661315135023116582
- GilbertDCGlynne-JonesRIntensity-modulated radiotherapy in anal cancer: where do we go from here?Clin Oncol (R Coll Radiol)201325315315422921809
- SchillerDECummingsBJRaiSOutcomes of salvage surgery for squamous cell carcinoma of the anal canalAnn Surg Oncol200714102780278917638059
- GilbertDCWilliamsAAllanKp16INK4A, p53, EGFR expression and KRAS mutation status in squamous cell cancers of the anus: correlation with outcomes following chemo-radiotherapyRadiother Oncol2013109114615124021343
- BordonYImmunotherapy: checkpoint parleyNat Rev Cancer2015151325503072
- KeirMEButteMJFreemanGJSharpeAHPD-1 and its ligands in tolerance and immunityAnnu Rev Immunol20082667770418173375
- ChangCHQiuJO’SullivanDMetabolic competition in the tumor microenvironment is a driver of cancer progressionCell201516261229124126321679
- BaptistaMZSarianLODerchainSFPintoGAVassalloJPrognostic significance of PD-L1 and PD-L2 in breast cancerHum Pathol2016471788426541326
- YokoyamaSMiyoshiHNakashimaKPrognostic value of programmed death ligand 1 and programmed death 1 expression in thymic carcinomaClin Cancer Res201622184727473427166394
- ThompsonEDZahurakMMurphyAPatterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stromaGut201766579480126801886
- UdagerAMLiuTYSkalaSLFrequent PD-L1 expression in primary and metastatic penile squamous cell carcinoma: potential opportunities for immunotherapeutic approachesAnn Oncol20162791706171227217541
- ChoueiriTKFayAPGrayKPPD-L1 expression in nonclear-cell renal cell carcinomaAnn Oncol201425112178218425193987
- HuWHMiyaiKCajas-MonsonLCLuoLLiuLRamamoorthySLTumor-infiltrating CD8(+) T lymphocytes associated with clinical outcome in anal squamous cell carcinomaJ Surg Oncol2015112442142626287957
- NghiemPTBhatiaSLipsonEJPD-1 blockade with pembrolizumab in advanced Merkel-cell carcinomaN Engl J Med2016374262542255227093365
- KudoMImmune checkpoint inhibition in hepatocellular carcinoma: basics and ongoing clinical trialsOncology201792Suppl 1506228147363
- Serup-HansenELinnemannDSkovrider-RuminskiWHøgdallEGeertsenPFHavsteenHHuman papillomavirus genotyping and p16 expression as prognostic factors for patients with American Joint Committee on Cancer stages I to III carcinoma of the anal canalJ Clin Oncol201432171812181724821878
- SchlechtNFBrandwein-GenslerMNuovoGJA comparison of clinically utilized human papillomavirus detection methods in head and neck cancerMod Pathol201124101295130521572401
- XieQKZhaoYJPanTProgrammed death ligand 1 as an indicator of pre-existing adaptive immune responses in human hepatocellular carcinomaOncoimmunology201657e118125227622038
- ZouMXPengABLvGHExpression of programmed death-1 ligand (PD-L1) in tumor-infiltrating lymphocytes is associated with favorable spinal chordoma prognosisAm J Transl Res2016873274328727508049
- TsutsumiSSaekiHNakashimaYProgrammed death-ligand 1 expression at tumor invasive front is associated with epithelial-mesenchymal transition and poor prognosis in esophageal squamous cell carcinomaCancer Sci201710861119112728294486
- SaigusaSToiyamaYTanakaKImplication of programmed cell death ligand 1 expression in tumor recurrence and prognosis in rectal cancer with neoadjuvant chemoradiotherapyInt J Clin Oncol201621594695226919982
- WuCZhuYJiangJZhaoJZhangXGXuNImmunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significanceActa Histochem20061081192416530813
- NomiTShoMAkahoriTClinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancerClin Cancer Res20071372151215717404099
- DroeserRAHirtCViehlCTClinical impact of programmed cell death ligand 1 expression in colorectal cancerEur J Cancer20134992233224223478000
- OhigashiYShoMYamadaYClinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancerClin Cancer Res20051182947295315837746
- MuenstSSoysalSDGaoFObermannECOertliDGillandersWEThe presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancerBreast Cancer Res Treat2013139366767623756627
- ThompsonRHDongHLohseCMPD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinomaClin Cancer Res20071361757176117363529
- GevenslebenHDietrichDGolletzCThe immune checkpoint regulator PD-L1 is highly expressed in aggressive primary prostate cancerClin Cancer Res20162281969197726573597
- TaubeJMAndersRAYoungGDColocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escapeSci Transl Med20124127127ra37
- SchmidtLHKümmelAGörlichDPD-1 and PD-L1 expression in NSCLC indicate a favorable prognosis in defined subgroupsPLoS One2015108e013602326313362
- BalermpasPMartinDWielandUHuman papilloma virus load and PD-1/PD-L1, CD8+ and FOXP3 in anal cancer patients treated with chemoradiotherapy: rationale for immunotherapyOncoimmunology201763e128833128405521
- GovindarajanRGujjaSSiegelERProgrammed cell death-ligand 1 (PD-L1) expression in anal cancerAm J Clin Oncol Epub20161115
- ZhangYHuangSGongDQinYShenQProgrammed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancerCell Mol Immunol20107538939520514052
- HinoRKabashimaKKatoYTumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanomaCancer201011671757176620143437
- SchalperKAVelchetiVCarvajalDIn situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomasClin Cancer Res201420102773278224647569
- Twyman-Saint VictorCRechAJMaityARadiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancerNature2015520754737337725754329
- SchultheisAMScheelAHOzretićLPD-L1 expression in small cell neuroendocrine carcinomasEur J Cancer201551342142625582496
- GrulichAEvan LeeuwenMTFalsterMOVajdicCMIncidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysisLancet20073709581596717617273
- MorrisVKSalemMENimeiriHNivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicentre, single-arm, phase 2 studyLancet Oncol201718444645328223062
- TopalianSLHodiFSBrahmerJRSafety, activity, and immune correlates of anti-PD-1 antibody in cancerN Engl J Med2012366262443245422658127
- PowlesTEderJPFineGDMPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancerNature2014515752855856225428503
- TengMWNgiowSFRibasASmythMJClassifying cancers based on T-cell infiltration and PD-L1Cancer Res201575112139214525977340
- BadoualCHansSMerillonNPD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancerCancer Res201373112813823135914
- Lyford-PikeSPengSYoungGDEvidence for a role of the PD-1: PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinomaCancer Res20137361733174123288508
- OttenhofSRDjajadiningratRSde JongJThygesenHHHorenblasSJordanovaESExpression of programmed death ligand 1 in penile cancer is of prognostic value and associated with HPV statusJ Urol20171973 Pt 169069727697578
- KimHSLeeJYLimSHAssociation between PD-L1 and HPV status and the prognostic value of PD-L1 in oropharyngeal squamous cell carcinomaCancer Res Treat201648252753626511814
- SharonEStreicherHGoncalvesPChenHXImmune checkpoint inhibitors in clinical trialsChin J Cancer201433943444425189716
