Abstract
Background
Thymic atrophy was discovered in tumor-bearing mice in recent years.
Methods
Flow cytometry was carried out including Annexin V-FITC/PI double staining, PI staining, Terminal dUTP nick-end labeling, CD3-FITC/CD19-PE and CD8-FITC/CD4-PE double staining. Enzyme-linked immunosorbent assay and polymerase chain reaction were also investigated.
Results
According to our experiments, we demonstrated that no signs of apoptosis in thymocytes were found in H22-bearing mice, while the proportions of CD4+ T cells and CD8+ T cells in thymuses were remarkably increased, the opposite tendency was found in peripheral bloods, and only CD3+CD8+ T cells were discovered in H22 solid tumors. We further discovered that the level of thymosin alpha 1 (Tα1) and the expression of Wnt4 in thymus of H22-bearing mice were significantly improved than control, which indicated the active proliferation and differentiation of thymocytes. Our study revealed that CD8+ T cells could not effectively eliminate H22 cells independently when CD4+ T cells were suppressed by tumors, while the body would only enhance the differentiation and maturation of T cells in thymuses and release them to solid tumor to reinforce antitumor immunocompetence, leading to a vicious cycle which finally led to thymic atrophy.
Conclusion
Our data propose a novel mechanism of tumor-induced thymic atrophy regulated by abnormal immunoreaction and may provide new ideas for the immunotherapy of tumors.
Introduction
As a primary lymphoid organ, the thymus provides a highly specialized microenvironment for the differentiation and maturation of immunocompetent T lymphocytes, which consists of an outer cortex and inner medulla.Citation1–Citation5 Thymosin alpha 1 (Tα1), a biologically active peptide mainly existing in thymic epithelial cells, can enhance cellular immune responses, modulate secretion of cytokines, and block steroid-induced apoptosis of thymocytes.Citation6–Citation8 Wnt4 has recently been shown to regulate multiple developmental processes reflected by accumulating the downstream molecule β-catenin.Citation9
T lymphocytes including CD8+ T cells and CD4+ T cells have been considered as the main protagonists in orchestrating the anti-tumor response.Citation10–Citation13 CD8+ T cells impart cytolytic activity and cytokine expression in anti-tumor immunity,Citation14 and previous studies have shown that patients bearing tumors would universally have better outcomes when treated with massive infiltration of CD8+ T cells.Citation15 CD4+ T cells play an important role in modulating anti-tumor immune response via activating and expanding CD8+ T cells, recruiting and activating natural killer cells and macrophages, and secreting IFN-γ.Citation16–Citation18
Cancer has become a major concern for human health due to the low cure rate and high mortality.Citation19,Citation20 Hepatocellular carcinoma, the fifth most common cancer and third leading cause of cancer death worldwide, is mainly caused by the hepatitis B virus.Citation21–Citation24 Thymic atrophy is accompanied with tumorigenesis which finally leads to the perturbed output of mature T cells in hosts,Citation25–Citation29 which suggests that the tumor is one of the major factors resulting in thymic involution,Citation30 and tumor-induced immunosuppression is responsible for the failure of cancer therapy.Citation31
Given the immune function of the thymus and T cells, it is of great value to investigate the possible mechanism of tumor-induced thymic atrophy. It has been reported that apoptosis and block in T cell maturation are responsible for thymic atrophy in tumor-bearing hosts,Citation32 while in our study, we focused on the population and cellular condition of T cells in H22-bearing mice and demonstrated another possible mechanism of thymic atrophy.
Materials and methods Materials
Tα1 was synthesized by Beijing Protein Innovation Co. (Beijing, China) (purity of >99% based on HPLC). Fetal calf serum was obtained from Hangzhou Sijiqing Co (Zhejiang, China). CD19-PE, CD3-FITC, CD4-PE, and CD8-FITC anti-mouse antibodies were from Tianjin Sungene Biotech Co., Ltd (Tianjin, China). One Step TUNEL Apoptosis Assay Kit and Annexin V-FITC Apoptosis Detection Kit were purchased from Beyotime Biotechnology Co., (Shanghai, China). Propidium iodide (PI) was provided by Sigma-Aldrich Co. (St Louis, MO, USA). All other chemicals and agents were of analytical grades.
Animals and cells
Kunming SPF mice (female, 20–22 g, 8-week-old) were purchased from the Department of Experimental Animals, Academy of Military Medical Science, Beijing, and acclimatized to the facility for 1 week prior to experiments. These animals were housed under pathogen-free conditions at a relative humidity (50±5%) and controlled temperature (20°C-25°C), and they were allowed free access to tap water and fed with a standard pellet diet on a 12 h light/dark cycle. All experimental procedures following the National Institutes of Health guidelines for the care and use of laboratory animals were approved by the Local Ethics Committee for Animal Care and took place at Tianjin University of Science and Technology. H22 hepatoma cells were purchased from Shanghai Institute for Biological Sciences (Chinese Academy of Sciences, Shanghai, China) and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum at 37°C in a humidified incubator containing 5% CO2.
All mice were randomly divided into two groups. Mice in the model group were implanted with H22 hepatoma cells in the right forelimb armpit with 0.2 mL of 5×106 cells under sterile conditions, and empty treatments were done in the control group. Control and model groups received saline solution orally throughout the experimental period and mice were sacrificed to provide experiment samples for further research after 3 weeks’ administration.
Thymus index (%) = average weight of thymus / (average body weight) × 100
H&E staining
Thymuses of mice in each group were embedded with paraffin and stained with H&E using standard procedures for the structure observation. Thymocytes from different groups were obtained through a steel mesh under aseptic conditions, fixed with paraformaldehyde, and stained with H&E for morphologic examination of individual cells. The staining results were observed using microscopy.
Annexin V-FITC/PI double staining
The apoptosis in mice thymocytes of each group was examined using a double staining method with Annexin V-FITC/PI. According to the manufacturer’s instructions, thymic cell suspension was prepared and washed with cold PBS, Annexin V-FITC was added and incubated for 10 min at room temperature away from light after which PI was added. Stained cells were analyzed by FACScalibur flow cytometry (BD Biosciences, San Jose, CA, USA).
Analysis of cell cycle distribution
The cell cycle distribution was analyzed through the flow cytometry detection method. Thymic cell suspensions in different groups were prepared and fixed in 75% ethanol at 4°C overnight. After washing with PBS, the cells were stained with PI working solutions (0.1% TritonX-100, 100 mg/mL PI, 0.01 mg/mL RNase) for 30 min in the dark. Twenty thousand events of each sample were acquired by CellQuest Pro and analyzed by Modifit, and cell cycle distribution was assessed by DNA content.
TUNEL assay
TUNEL assays were performed to detect apoptotic cells in thymuses. Thymocytes were washed twice before being suspended in permeabilization solution (10 mg sodium citrate and 10 μL Triton X-100 in 10 mL sterile water) for 2 min on ice. After permeabilization, samples were washed with 1 mL PBS and stained with TUNEL Reaction Mixture for 1 h protected from light. Positive control samples were treated with 10 μL DNase I before staining. After staining, samples were simultaneously assessed using a flow cytometer.
Detection of T lymphocyte subpopulation
Lymphocyte subpopulations in peripheral blood and H22 tumors were tested with CD3-FITC/CD19-PE and CD8-FITC/CD4-PE reagents. Peripheral blood of each group was collected from the orbital venous plexus, and solid tumors were obtained from H22-bearing mice. The tubes containing 100 μL samples (1×107 cells/mL) were mixed with antibodies, followed by 15 min away from light. Then red blood cells were removed by adding RBC lysis buffer, and finally these samples were resuspended in 1×PBS and evaluated by flow cytometer.
CD4+ T cell and CD8+ T cell population in thymus was tested with CD8-FITC and CD4-PE reagents. Thymocyte suspensions were produced by forcing the thymus through a wire-mesh screen and diluting with PBS. Following centrifugation (1,500 rpm, 5 min), lymphocytes were collected and then incubated with the antibodies for 15 min in the dark. Thymocytes were analyzed after PBS washing on a flow cytometer and analyzed with CellQuest software.
Detection of Tα1 levels
The Tα1 levels in serum and thymuses of mice were analyzed through ELISA method. The water-soluble protein extracts of thymuses and serum with coating buffer were inoculated in a 96-well Elisa plate at 4°C overnight, the mixtures were transferred and the wells were incubated with blocking buffer for 1 h at 37°C. After washing with PBST, levels of Tα1 in thymuses and serum of mice were detected by adding rabbit anti-Tα1 antibody (Bioss Biotechnology Co., Beijing, China; 1:200) and goat anti-rabbit IgG antibody (Bioss Biotechnology Co.; 1:200) respectively. The chemosynthetic Tα1 was used as standard. The absorbance was read at 450 nm with a reference of 630 nm with BioRad Model 680 Microplate Reader. (Bio-Rad Laboratories Inc., Hercules, CA, USA)
Expression of Wnt4 in thymocytes
The primer pairs used are shown in in . RNA was isolated from thymocytes using Trizol reagents (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. A total of 1 μg of RNA was transcribed into cDNA using a PrimeScript RT reagents kit (Takara Biomedical Technology Co., Beijing, China). Quantitative real-time PCR was performed by Q-PCR tower 2.0 system (Analytik Jena AG, Jena, Germany) with the following conditions: 95°C for 2 min, 40 cycles of 95°C for 10 s, 60°C for 32 s, 72°C for 30 s.
Table 1 Primer sequences used in RT-PCR
Statistical analysis
All values from the present study are expressed as mean±SD from at least three independent experiments, and each one was performed in triplicate. Statistical analysis was carried out using one-way ANOVA test followed by Student’s t-test (SPSS 19.0; IBM Corporation, Armonk, NY, USA). The level of significance was set at p<0.01.
Results
Results of thymus weights in mice
As shown in , the body weights of tumor-bearing mice showed no significant difference from the control group, while the thymus weights and thymus indices were obviously decreased, which proved that the solid tumors in vivo could induce thymic atrophy of the host.
Table 2 Effects of solid tumors on thymuses in mice
Results of H&E staining
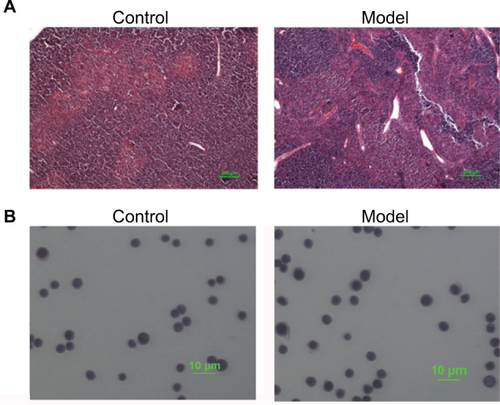
Pathological changes of mice thymuses were observed by H&E staining. As shown in , thymuses of normal mice were well structured with obvious boundary of the cortex and medulla, whereas the epithelial networks of thymuses in H22-bearing mice were damaged and discontinued, and the extracellular matrix was significantly increased. The staining results of thymocytes are shown in , thymocytes of tumor-bearing mice remained integral and formed round, and showed no obvious difference compared to the control group.
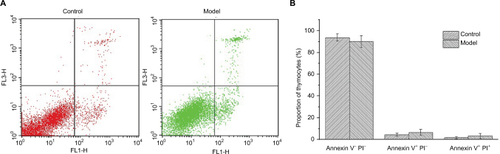
Apoptosis cells tested by Annexin V/PI assay
Annexin V-FITC/PI staining was conducted on thymocytes to determine the cellular state, permeability change, and disruption of cell membrane in early apoptosis would lead phospholipidine serine (PS) exposed to the external surface, which makes it easy to combine with annexin V bearing FITC, PI has poor membrane permeability and only apoptotic cells in late stage or necrotic cells can be stained. As shown in , there was almost 90% of thymocytes of mice in both control and model groups in quadrant 3, indicating that T cells in mice thymuses of these two groups were normal cells and the nearly 10% T cells were found to be apoptotic mainly due to the process of grinding for preparation of the thymocytes’ suspension.
Figure 2 Annexin V-FITC/PI staining results of T cells in thymuses.
Notes: (A) Annexin V–PI– (quadrant 3), Annexin V+PI– (quadrant 4), and Annexin V+PI+ (quadrant 1) represent normal T cells, cells in early apoptosis, and late apoptosis. (B) Proportion of thymocytes in different stages.
Abbreviation: PI, propidium iodide.
Cell cycle distribution of thymocytes
PI could bind to DNA and RNA in cells, and only DNA would be stained after RNA removal by RNase. The distribution of cell cycle could be reflected by fluorescence intensity through flow cytometry. As shown in , there was no significant difference found in cell cycle distribution of thymocytes in tumor-bearing mice compared to the control group, which indicated the good condition of cell shape and growth.
Table 3 Distribution of cell cycle and apoptosis rate of thymocytes
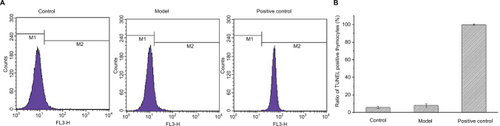
Thymocyte apoptosis detection by TUNEL staining
Cell apoptosis activated DNA endonuclease activity by cutting the genomic DNA, and the exposed 3′-OH was combined with dUTP marked by FITC with the TdT catalyst, and cells were tested by flow cytometry. As shown in , there were no TUNEL positive thymocytes found in mice of both control and model groups, which indicated that tumors did not result in DNA degradation of thymocytes.
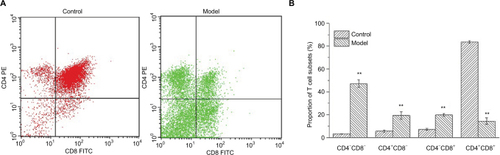
The differentiation of CD4+ and CD8+ T cells in thymuses
CD4+ and CD8+ T cells are of critical importance in the execution of immune response on hepatocarcinogenesis. As shown in , thymuses of H22-bearing mice showed lower proportions of CD4+ CD8+ T cells and higher proportions of CD4+CD8− and CD4−CD8+ T cells (p<0.01), which indicated that H22 solid tumors could accelerate the development, differentiation, and maturation of T cells in the thymus.
Figure 4 Changes in the proportions of lymphocyte subsets.
Notes: (A) Upper left (quadrant 2) means the thymocytes combined with PE−CD4+ antibodies, Upper right (quadrant 1) means thymocytes which can be combined with both antibodies, Lower left (quadrant 3) means the cells combined with no antibodies, Lower right (quadrant 4) means the cells combined with FITC−CD8+ antibodies. (B) Proportions of T lymphocyte subsets in normal and H22-bearing mice. **p<0.01 vs control group.
Proportions of lymphocyte subsets in peripheral blood and solid tumor
As shown in , the proportions of T cell subsets in peripheral blood of H22-bearing mice were significantly decreased (p<0.01) compared to the control group, and large amounts of tumor-infiltrating CD3+CD8+ T cells were discovered in H22 solid tumors, suggesting that H22 cells’ proliferation in vivo would induce T cells’ aggregation in solid tumor with lower immunocompetence. While the noticeable increase (p<0.01) in the proportion of CD19+ B cells in peripheral blood might indicate that some distinct tumor-related antibodies are existed in tumor hosts.
Table 4 Percentages of lymphocyte subsets in peripheral blood and tumors in different groups (mean ± standard deviation, n=10)
Levels of Tα1 in serum and thymuses of mice
As shown in , the levels of Tα1 in serum and thymuses of H22-bearing mice were both significantly increased (p<0.01) compared to the control group, which indicated that H22 solid tumors could promote the secretion of Tα1 from thymuses, and enhance the vitality of thymic cells, finally resulting in the over-differentiation of T cells, which would contribute to thymic atrophy in the host.
Table 5 Levels of Tα1 in serum and thymuses of mice in each group (mean±SD, n=10)
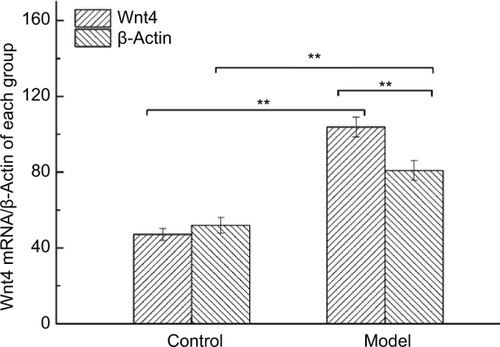
Expression of Wnt4 in thymocytes of H22-bearing mice
As shown in , mRNA levels of Wnt4 in thymocytes of H22-bearing mice were significantly increased (p<0.01) compared to the control group, and β-actin which was used as loading control also showed higher values in the model group, suggesting that thymocytes in tumor-bearing mice were proliferous and active.
Discussion
Cancer occurrence depends on a complex process of multiple factors and stages. The inhibition effects of tumor cells on immune reactivity were responsible for the disorder of the immune system in the tumor host, which would lead to the lower cure rate.Citation33 Large studies have suggested that organisms could resist the malignant tumor spontaneously with mostly cellular immunity mediated by T lymphocytes.Citation34,Citation35 T cell differentiation status and the metabolic properties of T cells may play an important role in regulating their anti-tumor functionality.Citation36
The thymus, as the dominant source of cellular immunity, provides a suitable microenvironment for T cells’ differentiation and maturation. Thymic atrophy would seriously impact the body’s immunity and disturb homeostasis, and generally occurs among the elderly.Citation37 While recent studies have shown that the presence of cancer could also accelerate the involution of thymus in tumor-bearing host, some scholars believe that some factors or antigens released from malignant tumors would have toxic or stimulating effects on the thymus and induce the apoptosis of thymocytes, thus leading to thymic atrophy.Citation38–Citation41 The immunodepression of anti-tumor activity caused by thymic atrophy would be beneficial to immunoevasion of tumor cells.Citation42,Citation43
Our previous studies showed that the indexes of thymuses in H22-bearing mice were significantly decreased in contrast to normal mice. Thus, we propose a hypothesis that the mechanism of thymic atrophy in tumor-bearing mice is over- differentiation of thymocytes as a way of initiating immune response. T cells in the thymus would continuously differentiate and mature to protect the body during tumorigenesis, while even mature T cells without activation could not effectively induce tumor cell apoptosis, resulting in a higher differentiation and maturation rate than proliferation rate of thymocytes, and eventually leading to thymic atrophy.
Therefore, we established an H22-bearing mice model in the present experiment and used the thymuses to investigate the mechanisms of thymic atrophy caused by H22 solid tumors. In order to detect the condition of thymocytes in H22-bearing mice, we employed H&E staining, Annexin V/FITC and PI staining, cell cycle detection, and TUNEL staining assays. Results showed that there were no signs of apoptosis in thymocytes of H22-bearing mice (, –, ), while the structure of the thymus was significantly destroyed due to the thymic atrophy (). These results suggested a distinct direction on the mechanism of thymic atrophy in tumor-bearing mice, which guided us to further determine the cellular conditions and subpopulations of thymocytes to study this novel mechanism.
The proportions of T lymphocyte subsets in thymuses, peripheral blood, and solid tumors were detected to further research the possible mechanism of thymic atrophy in H22-bearing mice. Results showed that the proportions of CD4+ T cells and CD8+ T cells in thymuses of H22-bearing mice were markedly increased compared to controls (), while the opposite tendencies were found in peripheral blood, and only CD3+CD8+ T cells were discovered in solid tumors (), consistent with Kuss et al’s and Saito et al’s research on patients suffering from different types of cancer.Citation44,Citation45 As we know, CD8+ T cells play an important role in recognizing tumor epitopes and eliciting tumor rejection,Citation46 while CD4+ T cells would assist the cytotoxic activity of CD8+ T cells in limiting tumor cell growth and the generation of memory CD8+ T cells for preventing tumor recurrences.Citation47 Our results indicated that CD4+ T cells might be suppressed by H22 cells, and CD8+ T cells could not effectively eliminate tumors without the help of CD4+ T cells, which resulted in the growth of tumors with CD8+ T cells gathered around, while the body would only enhance the differentiation and maturation of T cells in thymuses, finally leading to thymic atrophy.
Tα1, a biological response modifier, could enhance the immune system through promoting differentiation and maturation of T lymphocytes.Citation48,Citation49 Wnt4 has been proven to be required for the development of the thymus, as it controls mesenchymal-to-epithelial transition and epithelial cell proliferation in the thymus.Citation50 β-actin, an important cytoskeletal protein, seems to be overexpressed in actively moving cancer cells.Citation51 In our experiments, we found that the three indicators were all significantly increased in tumor-bearing mice compared to normal mice (, ), which revealed that the existence of tumors could enhance the immune function and accelerate the proliferation and differentiation of thymocytes, and when the differentiation rate was higher than proliferation rate, thymic atrophy occurred.
In conclusion, H22 tumor cells would encourage the differentiation and maturation of thymocytes to enhance immune function, while unactivated T cells could not effectively inhibit the H22 solid tumors’ growth or induce their apoptosis, then more unactivated mature T cells were produced and released from the thymus, causing a vicious cycle which finally led to thymic atrophy.
Acknowledgments
The study was supported by the Natural Science Foundation of Agriculture Department (number 201303082-3) and National Natural Science Foundation of China (number 31271975).
Disclosure
The authors report no conflicts of interest in this work.
References
- GeQZhaoYEvolution of thymus organogenesisDev Comp Immunol2013391–2859022266420
- FrancelinCPaulinoLCGameiroJVerinaudLEffects of Plasmodium berghei on thymus: high levels of apoptosis and premature egress of CD4(+)CD8(+) thymocytes in experimentally infected miceImmunobiology2011216101148115421601941
- LimaACFrancelinCFerrucciDLStach-MachadoDRVerinaudLThymic alterations induced by Plasmodium berghei: expression of matrix metalloproteinases and their tissue inhibitorsCell Immunol20122791535923089194
- RadojevicKRakinAPilipovicIEffects of catecholamines on thymocyte apoptosis and proliferation depend on thymocyte microenvironmentJ Neuroimmunol20142721–2162824837703
- RiemannMAndreasNFedoseevaMCentral immune tolerance depends on crosstalk between the classical and alternative NF-kappaB pathways in medullary thymic epithelial cellsJ Autoimmun2017815467
- LiJLiuCHWangFSThymosin alpha 1: biological activities, applications and genetic engineering productionPeptides201031112151215820699109
- GoldsteinALGoldsteinALFrom lab to bedside: emerging clinical applications of thymosin α1Expert Opin Biol Ther20099559360819392576
- NaylorPHQuadriniKGaraciERasiGHaddenJWImmunopharmacology of thymosin alpha1 and cytokine synergyAnn N Y Acad Sci2007111223524417567942
- LiWZhangYZhangMHuangGZhangQWnt4 is overexpressed in human pituitary adenomas and is associated with tumor invasionJ Clin Neurosci201421113714124200887
- LeeJLimKTSJSZ glycoprotein (38kDa) prevents thymus atrophy and enhances expression of IL-2 and IL-12 in diethylnitrosamine-induced hepatocarcinogenesisInt Immunopharmacol201213336236922569343
- NakayamaEShiratsuchiYKobayashiYNagataKThe importance of infiltrating neutrophils in SDF-1 production leading to regeneration of the thymus after whole-body X-irradiationCell Immunol20112681242821320703
- GodetYFabreEDossetMAnalysis of spontaneous tumor-specific CD4 T-cell immunity in lung cancer using promiscuous HLA-DR telomerase-derived epitopes: potential synergistic effect with chemotherapy responseClin Cancer Res201218102943295322407833
- ZhangXYYangBYWangJYMoXZhangJHuaZCFADD is essential for glucose uptake and survival of thymocytesBiochem Biophys Res Commun2014451220220725078620
- GaoKLiXZhangLTransgenic expression of IL-33 activates CD8 +, T cells and NK cells and inhibits tumor growth and metastasis in miceCancer Lett2013335246347123499895
- HiraokaNTumor-infiltrating lymphocytes and hepatocellular carcinoma: molecular biologyInt J Clin Oncol201015654455120924634
- GhoshSSarkarMGhoshTAbsence of CD4(+) T cell help generates corrupt CD8(+) effector T cells in sarcoma-bearing Swiss mice treated with NLGP vaccineImmunol Lett2016175313927178306
- KnockeSFleischmann-MundtBSaborowskiMMannsMPKühnelFWirthTCWollerNTailored tumor immunogenicity reveals regulation of CD4 and CD8 T Cell responses against cancerCell Rep20161792234224627880900
- WangMWangHTangYEffective inhibition of a Strongylocentrotus nudus eggs polysaccharide against hepatocellular carcinoma is mediated via immunoregulation in vivoImmunol Lett20111411748221893096
- DuLYangYHWangYMXueCHKuriharaHTakahashiKAntitumour activity of EPA-enriched phospholipids liposomes against S180 ascitic tumour-bearing miceJ Funct Foods201519970982
- MoLChenYLiWGuoSWangXAnHZhanYAnti-tumor effects of (1→3)-beta-d-glucan from Saccharomyces cerevisiae in S180 tumor-bearing miceInt J Biol Macromol20179538539227838421
- XiSPengYMinukGYThe combination effects of Shen-Ling-Bai-Zhu on promoting apoptosis of transplanted H22 hepatocellular carcinoma in mice receiving chemotherapyJ Ethnopharmacol201619011227235019
- ChenFSunYZhengSLAntitumor and immunomodulatory effects of ginsenoside Rh2 and its octyl ester derivative in H22 tumor-bearing miceJ Funct Foods201732382390
- LingNZhouXJiYLiWJiCQiZImmunomodulatory and cellular antioxidant activities of kappa-selenocarrageenan in combination with Epirubicin in H22 hepatoma-bearing miceBiomed Pharmacother20179113213728448867
- YingSWangJWangYA novel mutant 10Ala/Arg together with mutant 144Ser/Arg of hepatitis B virus X protein involved in hepatitis B virus-related hepatocarcinogenesis in HepG2 cell linesCancer Lett2016371228529126706415
- ZhangGLJiangLYanQLiuRHZhangLAnti-tumor effect of matrine combined with cisplatin on rat models of cervical cancerAsian Pac J Trop Med20158121055105926706679
- LiuDYangPZhangYQWater-soluble extract of Saxifraga stolonifera has anti-tumor effects on Lewis lung carcinoma-bearing miceBioorg Med Chem Lett201626194671467827575479
- HuBWangJGuoYPre-clinical toxicity and immunogenicity evaluation of a MUC1-MBP/BCG anti-tumor vaccineInt Immunopharmacol20163310811826896668
- IwaiHInabaMFetal thymus graft prevents age-related hearing loss and up regulation of the IL-1 receptor type II gene in CD4(+) T cellsJ Neuroimmunol20122501–21822652460
- ValanciuteAMozuraiteRBalnyteIDidziapetrieneJMatuseviciusPStakisaitisDSodium valproate effect on the structure of rat glandule thymus: Gender-related differencesExp Toxicol Pathol2015677–839940625937562
- KaiserlianDSavinoWHassidJDardenneMStudies of the thymus in mice bearing the Lewis lung carcinoma. III. Possible mechanisms of tumor-induced thymic atrophyClin Immunol Immunopathol19843233163256331932
- MandalDBhattacharyyaALahiryLChoudhuriTSaGDasTFailure in peripheral immuno-surveillance due to thymic atrophy: importance of thymocyte maturation and apoptosis in adult tumor-bearerLife Sci200577212703271616019036
- MandalDLahiryLBhattacharyyaABhattacharyyaSSaGDasTTumor-induced thymic involution via inhibition of IL-7Rα and its JAK-STAT signaling pathway: protection by black teaInt Immunopharmacol20066343344416428079
- YoungMRIRedirecting the focus of cancer immunotherapy to premalignant conditionsCancer Lett2017391838828130162
- KarayannopoulouMAnagnostouTMargaritiAKostakisCKritsepi-KonstantinouMPsallaDSavvasIEvaluation of blood T-lymphocyte subpopulations involved in host cellular immunity in dogs with mammary cancerVet Immunol Immunopathol2017186455028413049
- KavanaghMEConroyMJClarkeNEImpact of the inflammatory microenvironment on T-cell phenotype in the progression from reflux oesophagitis to Barrett oesophagus and oesophageal adenocarcinomaCancer Lett2016370111712426519754
- SukumarMKishtonRJRestifoNPMetabolic reprograming of anti-tumor immunityCurr Opin Immunol201746142228412583
- LustigAWeeraratnaATWoodWW3rdTranscriptome analysis of age-, gender- and diet-associated changes in murine thymusCell Immunol20072451426117499630
- MastinoAPiacentiniMGrelliSInduction of apoptosis in thymocytes by prostaglandin E2 in vivoDev Immunol1992242632711364176
- LopezDMLopez-CeperoMWatsonGAGanjuASotomayorEFuXXModulation of the immune system by mammary tumor-derived factorsCancer Invest1991966436531684133
- PrinsRMGrafMRMerchantREBlackKLWheelerCJThymic function and output of recent thymic emigrant T cells during intracranial glioma progressionJ Neurooncol2003641–2455412952285
- ShankerASinghSMSodhiAAscitic growth of a spontaneous transplantable T cell lymphoma induces thymic involution. 1. Alterations in the CD4/CD8 distribution in thymocytesTumor Biol2000215288298
- HaddenJWImmunodeficiency and cancer: prospects for correctionInt Immunopharmacol2003381061107112860163
- ThomasESmithDCLeeMYRosseCInduction of granulocytic hyperplasia, thymic atrophy, and hypercalcemia by a selected subpopulation of a murine mammary adenocarcinomaCancer Res19854511 Pt 2584058444053054
- KussIHathawayBFerrisRLGoodingWWhitesideTLDecreased absolute counts of t lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neckClin Cancer Res200410113755376215173082
- SaitoTDworackiGGoodingWLotzeMTWhitesideTLSpontaneous apoptosis of CD8+ T lymphocytes in peripheral blood of patients with advanced melanomaClin Cancer Res2000641351136410778963
- XuLLinYYaoDTumor antigen-specific CD8+, T cells are negatively regulated by PD-1 and Tim-3 in human gastric cancerCell Immunol2017313435128110884
- FesenkovaVSultanHCelisECD4 T Cells in antitumor immunityEncyclopedia of Immunobiology20164441450
- YuanCZhengYZhangBThymosin alpha1 promotes the activation of myeloid-derived suppressor cells in a Lewis lung cancer model by upregulating Arginase 1Biochem Biophys Res Commun2015464124925526111447
- LiuDYuZYinJEffect of ulinastatin combined with thymosin alpha1 on sepsis: a systematic review and meta-analysis of Chinese and Indian patientsJ Crit Care20173925926628069319
- CaprioliAVillasenorAWylieLAWnt4 is essential to normal mammalian lung developmentDev Biol2015406222223426321050
- SimiczyjewAMazur AntoninaJAmpeCMalicka-BłaszkiewiczMvan TroysMNowakDActive invadopodia of mesenchymally migrating cancer cells contain both β and γ cytoplasmic actin isoformsExp Cell Res2015339220621926548725