Abstract
Background
Mutations of BRAFV600E and TERT promoters are associated with thyroid cancer development. This study further investigated association of these mutations with clinicopathological characteristics from patients with papillary thyroid carcinoma (PTC).
Methods
Tumor tissues from 342 PTC patients were obtained for DNA extraction and polymerase chain reaction amplification to detect the BRAFV600E mutation using amplification-refractory mutation system-polymerase chain reaction. TERT promoter mutations were assessed using Sanger DNA sequencing. The association of these gene mutations with clinicopathological characteristics was then statistically analyzed.
Results
Two hundred and seventy of 342 (78.9%) PTC patients harbored the BRAFV600E mutation, which was associated with older age male patients. Moreover, TERT promoter mutations occurred in 12 of 342 (3.5 %) PTC patients, all of whom also had the BRAF mutation. One hundred thirty-three patients with papillary thyroid microcarcinoma (PTMC) had no TERT mutations. Statistically, the coexistence of BRAF and TERT promoter mutations were significantly associated with older age, larger tumor size, extrathyroidal extension, and advanced tumor stage, but not with central lymph node metastasis, lateral lymph node metastasis, numbers of lymph node metastasis >5, and numbers of involved/harvested lymph nodes (No. of LNs involved or harvested). The multivariate analyses showed older age (odds ratio [OR], 2.194; 95% CI: 1.117–4.311; p=0.023), larger tumor size (OR, 4.100; 95% CI: 2.257–7.450; p<0.001), and multiplicity (OR, 2.240; 95% CI: 1.309–3.831; p=0.003) were all independent predictors for high prevalence of extrathyroidal extension. However, there was no statistical association with any clinicopathological characteristics except for Hashimoto thyroiditis in PTMC.
Conclusion
The current study demonstrated that the coexistence of BRAF and TERT promoter mutations were associated with the PTC aggressiveness, although these mutations were not associated with PTC lymph node metastasis or with PTMC.
Introduction
Thyroid cancer is the most frequently occurring endocrine malignancy, with an increasing rate of incidence over the last 3 decades.Citation1 Histological classification of thyroid cancer mainly includes papillary, follicular, Hürthle cell, medullary, and anaplastic carcinomas. Papillary, follicular, and Hürthle cell carcinomas are derived from the follicular epithelium and produce thyroglobulin, and are considered as well-differentiated thyroid carcinomas.Citation2 Papillary thyroid carcinoma (PTC) accounts for up to 85% of all thyroid cancers.Citation3 Generally, PTC has an excellent prognosis with a relatively low mortality rate, but a small portion of PTC patients suffers from an aggressive form of the disease with tumor invasion and metastasis.Citation4 Accurate identification of this group of PTC patients is of crucial importance to optimizing individualized PTC treatment. However, current PTC risk stratification is based on conventional clinicopathological factors, which are often insufficient to accurately identify the high-risk PTC patients at an early stage. Thus, better biomarkers could help identify patients with aggressive PTC for more aggressive treatment options.
Toward this end, accumulating evidence indicates that gene mutations are involved in the development and progression of PTC.Citation5,Citation6 Mutation of the proto-oncogene BRAF at V600E, is the most prevalent mutation in PTC, being present in ~29%–83% of PTC patients.Citation5 BRAF protein is a member of the rapidly accelerated fibrosarcoma kinase family of growth signal transduction protein kinases and plays a role in regulating activity of the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase signaling pathway to promote cell growth, differentiation, and anti-apoptosis. Thus, the BRAFV600E mutation was shown to be a potent MAPK activator and occurs in many human cancers, including thyroid cancer.Citation7 Previous studies showed that the BRAFV600E mutation was associated with 1 or more aggressive PTC clinical characteristics, such as older age, extrathyroidal extension (ETE), lymph node metastasis, aggressive histological subtypes, impaired iodine uptake, or tumor recurrence.Citation8–Citation10 However, other studies reported no statistical association of the BRAFV600E mutation with these high-risk clinicopathological characteristics.Citation11–Citation13
Additionally, TERT is the catalytic subunit of the enzyme telomerase, which maintains telomere ends by addition of the telomere repeat TTAGGG. TERT plays a role in cell senescence, aberrant expression of which is associated with human cancer development.Citation14 Mutations of the TERT promoter arise in certain types of human cancer, including melanoma, bladder cancer, glioblastoma, and thyroid cancer, and frequently occur as 2 recurrent somatic mutation hotspots, that is, chr5:1,295,228 C > T (C228T) and chr5:1,295,250 C > T (C250T).Citation15–Citation18 Mutations of the TERT promoter were shown to be a promising indicator of PTC aggressiveness and poor prognosis, and were consistently validated in several previous studies.Citation15,Citation19 In addition, Liu et al first reported a link between mutations of the TERT promoter and BRAFV600E in PTC, and subsequent studies further demonstrated the coexistence of TERT promoter and BRAFV600E mutations in thyroid cancers and their association with the most aggressive PTC clinical factors and worse prognosis.Citation16,Citation20–Citation23
In this study, we further investigated the frequency of BRAFV600E and TERT promoter mutations for association with clinicopathological characteristics in PTC patients. We expected to provide more insightful information for association of BRAF and TERT promoter mutations with the most aggressive PTC phenotypes.
Materials and methods
Patients and tissue samples
This study included 342 consecutive patients who underwent surgical PTC resection between August 2016 and August 2017 at The Department of Endocrine and Breast Surgery, The First Affiliated Hospital, Chongqing Medical University (Chongqing, China). However, patients who refused to be included for genetic testing or for whom there was a lack of clinical and pathological data were excluded from the study. Among these 342 patients, 251 were clinical lymph node-negative (cN0) PTC patients and 91 were clinical lymph node-positive (cN1) patients, confirmed by fine-needle aspiration and neck ultrasound. Two hundred ninety patients (84.8%) received a total thyroidectomy. The remaining patients underwent lobectomy with isthmectomy. All patients also received central lymph node dissections (CLND), while 322 patients (94.1%) also received lateral lymph node dissections (LLND), including therapeutic (91 of 322) and prophylactic (231 of 322) dissections. Patients were histologically diagnosed with PTC according to the World Health Organization for the diagnosis of PTC.Citation2 However, the PTC subtypes were not routinely identified in our institution. The tumor/node/metastasis (TNM) staging was defined according to the 8th edition of the American Joint Committee on Cancer.Citation24 The fresh tissue samples were collected during surgery, snap-frozen in liquid nitrogen, and stored at −80°C.
In this study, paraffin-embedded tissue sections were obtained and re-evaluated to confirm histological diagnosis by 2 pathologists. Their clinicopathological information, including age, sex, tumor size, multifocality, Hashimoto thyroiditis (HT), ETE, central lymph node metastasis (CLNM), lateral lymph node metastasis (LLNM), numbers of lymph node metastasis (LNMN) >5, numbers of involved and harvested lymph nodes (No. of LNs involved or harvested), and TNM stage were collected for data analyses.
Ethics approval
This study was approved by the ethics committee of The First Affiliated Hospital, Chongqing Medical University. The written informed consent, including genetic test and medical record reviews, was obtained from each patient or their next of kin. The data were anonymized for analysis to protect patients’ confidentiality.
DNA extraction and mutation analysis
Tumor tissue samples from all PTC patients underwent extraction of genomic DNA using a kit from Amoy Diagnostics Co. Ltd. (Catalog #ADx-TI01, Xiamen, China), according to the manufacturer’s protocol. The DNA samples were diluted in dithioerythritol solution and the concentration of the DNA samples was measured using a Nano-100 spectrophotometer (Allsheng Co. Ltd, Hangzhou, China). The final concentration waŝ0.4–1.0 ng/μL.
To assess gene mutations in tumor samples of these patients, we first used a human BRAFV600E amplification-refractory mutation system-polymerase chain reaction (PCR) kit (Amoy Diagnostics Co. Ltd.). In brief, we amplified the genomic DNA using PCR, conditions of which included 3 phases, that is, 95°C for 5 min, 15 cycles of 95°C for 25 s, 64°C for 20 s, 72°C for 20 s, and then 31 cycles of 93°C for 25 s, 60°C for 35 s, 72°C for 20 s. The 5-carboxyfuorescein (FAM) and 5-hexachloro-fuorescein (HEX) signals were collected at 60°C and the cycle threshold (Ct) values (the number of cycles in each reaction tube when the fluorescent signal reaches the set threshold) of FAM and HEX signals were reached to determine the mutation spot according to the Ct value. The Ct value of the sample >28 was considered as negative, whereas the Ct value of the sample <28 was positive. Furthermore, the TERT promoter known as C228T and C250T was also examined by using PCR followed by the direct DNA sequencing. The TERT promoter region was amplified with primer pairs according to a previous study.Citation25 The PCR products were then separated by gel electrophoresis and a targeting DNA band was sequenced using the Big Dye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, Waltham, MA, USA) and analyzed using the ABI-Prism 3500DX genetic analyzer (Thermo Fisher Scientific).
Statistical analysis
The data for the continuous variables were summarized as mean ± SD, while the categorical data were summarized with frequencies and percentage. We performed the χ2-test or independent t-test to compare the clinicopathological characteristics of PTC patients with tumor DNA mutations and Pearson χ2-test with the Bonferroni correction or the one-way analysis of variance with least significant difference post hoc test to analyze continuous variables or categorical data in multiple comparisons, respectively. Univariate and multivariate analyses were performed to calculate the odds ratios for ETE and variables. All statistical analyses were assessed using SPSS software version 18.0.0.0 for IBM computers (SPSS Inc., Chicago, IL, USA), and P-values <0.05 were considered statistically significant.
Results
Characteristics of PTC patients and BRAFV600E and TERT promoter mutations
A total of 342 PTC patients were enrolled in this study, which included 99 males and 243 females with the mean age ± SD of 42.4±13.2 years (ranged between 13 and 81 years old). The mean tumor size was 14.8±9.2 mm (ranged between 2.0 and 55.0 mm), resulting in 133 patients (38.8%) being diagnosed with microcarcinoma (tumor maximum diameter ≤10 mm) according to the World Health Organization criteria.Citation2 All tumor samples from these patients were successfully genotyped for BRAFV600E and TERT promoter mutations (). The data showed that 270 (78.9%) and 12 (3.5%) patients carried the BRAFV600E and TERT promoter mutations, respectively, while 72 (21.0%) cases had no mutations for either gene. We also found that the TERT C228T mutation (10 of 12) was more prevalent than C250T mutation (2 of 12), and that these mutations were mutually exclusive. Patients carrying TERT promoter mutations also had the BRAFV600E mutation.
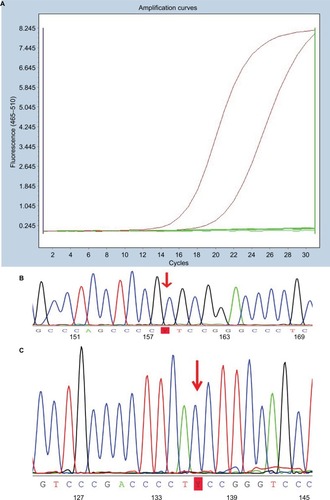
Figure 1 Illustration of polymerase chain reaction data on mutations of BRAFV600E and TERT promoters.
Notes: (A) Amplification plot of PTC with BRAFV600E mutation. (B) TERT promoter C228T mutation identified in a case of PTC. (C) TERT promoter C250T mutation identified in a case of PTC.
Abbreviation: PTC, papillary thyroid carcinoma.
Association of BRAFV600E or TERT promoter mutations with clinicopathological data from PTC patients
We then associated BRAFV600E and TERT promoter mutations with clinical features from PTC patients and found that the presence of the BRAFV600E mutation was associated with older patient age (p=0.018), male patients (p=0.010), and HT (p=0.002; ). However, we did not observe an association with tumor size, ETE, CLNM, LLNM, No. of LNs involved and harvested, and TNM stages (). The presence of TERT promoter mutations were associated with older patient age (p<0.001), larger tumor size (p<0.001), ETE (p<0.001), and advanced disease stages (p<0.001), but not associated with CLNM, LLNM, LNMN >5, or No. of LNs involved and harvested ().
Table 1 Association of BRAFV600E or the TERT promoter mutations with clinicopathological characteristics of PTC patients
Association of both BRAFV600E and TERT promoter mutations with clinical data from PTC patients
Since all TERT mutation-positive patients also had the BRAF mutation, we divided the 342 PTC patients into 3 groups: BRAF/TERT− (no any BRAF or TERT promoter mutations), BRAF+/TERT− (positive BRAF mutation, but negative for TERT promoter mutations), and BRAF+/TERT+ (positive for both BRAF and TERT promoter mutations). As shown in , male patients without HT were more common in the BRAF+/TERT− group compared with the BRAF−/TERT− group, while the coexistence of BRAFV600E and TERT promoter mutations were associated with older patient age (p<0.001), larger tumor size (p<0.001), ETE (p<0.001), and advanced disease stage (p<0.001), compared with the other 2 groups. However, no statistically significant difference was found in lymph node metastasis among the 3 groups, including CLNM, LLNM, LNMN >5, or No. of LNs involved and harvested. Interestingly, we found that the BRAF+/TERT+ group had a significant association with LLNM (p=0.031) compared with the BRAF+/TERT− group, although this factor was nonsignificant in the corrected analyses for multiple comparisons.
Table 2 Association of BRAFV600E/TERT promoter mutations with clinical characteristics of PTC patients
Association between ETE and clinicopathological characteristics and BRAFV600E/TERT promoter mutations in PTC
As shown in , our univariate analyses showed that the presence of ETE was significantly associated with older age, larger tumor size, multifocality, and BRAF+/TERT+, while our multivariate analysis revealed that older age (odds ratio [OR], 2.194; 95% CI: 1.117–4.311; p=0.023), larger tumor size (OR, 4.100; 95% CI: 2.257–7.450; p<0.001), and multiplicity (OR, 2.240; 95% CI: 1.309–3.831; p=0.003) were all independent predictors for high prevalence of ETE. Compared with BRAF−/TERT− group, the patients harboring both BRAF and TERT promoter mutations tended to have a higher ETE risk, although it was not statistically significant (p=0.069).
Table 3 Univariate and multivariate analysis of the clinical characteristics and gene mutations that could be associated with ETE
Association of BRAFV600E/TERT promoter mutations with clinical data from papillary thyroid microcarcinoma (PTMC)
As shown in , there were 108 of 133 (81.2%) cases of PTMC carrying the BRAFV600E mutation, corresponding to 78.9 % of the overall cohort. There was no statistical association with any clinicopathological characteristics except HT. Similarly, in contrast to patients without HT, the prevalence of the BRAF mutation in PTMC was significantly lower in patients with HT.
Table 4 Association of the BRAFV600E mutation with clinical characteristics of patients with papillary thyroid microcarcinoma
Discussion
In the current study, we confirmed that the BRAFV600E mutation was associated with older age male PTC patients. Furthermore, the coexistence of BRAF and TERT promoter mutations were significantly associated with high-risk clinicopathological characteristics from PTC patients. In particular, the prevalence of the BRAFV600E mutation occurred in 78.9% of the 345 patients, which was relatively higher than that of patients from Western countries, but similar to that of Asian patients, including Japanese, Korean, and Chinese.Citation8,Citation22,Citation26,Citation27 Our data further showed that the BRAFV600E mutation was not associated with other aggressive clinicopathological features. To the best of our knowledge, several previous studies suggested that BRAF mutations were associated with certain clinicopathological features, such as ETE,Citation28,Citation29 older age,Citation25 and tumor size,Citation26 whereas other studies have shown that presence of the BRAF mutation was not associated with any clinicopathological features.Citation17,Citation18,Citation30 These inconsistent data indicate that additional research on the BRAF mutation in combination with other gene mutations could be needed to predict aggressive PTC phenotypes. For example, the finding of intra-tumor heterogeneity of the BRAF mutation and percentage of mutant BRAF alleles in PTC might partially explain this inconsistency.Citation31 In our current study, we found that the presence of the BRAF mutation was highly associated with a low rate of the concurrent HT, which is consistent with previous studies.Citation32,Citation33 The concurrent HT could antagonize PTC progression.Citation34,Citation35 Kim et al also showed HT as an independent predictor for less aggressive BRAF-negative and BRAF-positive PTCs,Citation36 although the underlying mechanism remains to be elucidated.
Furthermore, our current study showed that the overall prevalence of TERT promoter mutations was 3.5% in these PTC patients, which was lower than the average range between 4.2% and 25%.Citation37 Two previous Chinese and Korean studies showed a remarkably lower mutation rate of the TERT promoter than that of studies from Western countries.Citation25,Citation26 This difference in prevalence might be due to a larger size of the PTC lesion, and the relatively smaller tumor size might result in the low frequency of the TERT promoter mutations in our current study, although exceptions do occur. For instance, Lee et al reported a noticeably higher rate (14.5%) of TERT promoter mutation in 207 Korean PTC patients.Citation20 However, the patients from that study and our current one that used the same methodology to detect the TERT promoter mutations did have similar age and tumor size, which did not support the association of the TERT promoter mutation prevalence with tumor size. One possible reason is because TERT promoter mutation rate is different in different subtypes of PTC. Specifically, the prevalence of TERT promoter mutations was highest in tall-cell PTC compared with that of conventional PTC and follicular variant PTC.Citation23 In our current study, we did not have data on the PTC variants, which could be one of the limitations to our current work. Our current data did show that PTC patients with TERT promoter mutations were significantly associated with aggressive clinical features and advanced TNM stage, which is consistent with previous studies.Citation15,Citation16,Citation18 In addition, we did not detect any BRAF−/TERT+ PTCs. Such a combination of gene mutations is rare in PTC patients.Citation25,Citation27,Citation38 Previous studies, including a number of meta-analyses, demonstrated that concurrent BRAF and TERT promoter mutations enhanced PTC aggressiveness.Citation16,Citation20–Citation22,Citation25,Citation26,Citation38,Citation39 Our current work also showed a similar result, which confirmed a strong incremental effect of the combined BRAF and TERT promoter mutations on promotion of PTC progression. Indeed, activation of the TERT promoter can generate the binding motifs for the E-twenty six (ETS) transcription factors, which could, in turn, activate the MAPK signaling pathway.Citation40 The MAPK pathway activated by BRAFV600E was able to upregulate the ETS and led to TERT overexpression.Citation41 These studies could help us to explain why mutations of both genes had synergistic or additive effects on PTCs, although further study is needed to elucidate this.
In the current study, we performed more extensive lymph node dissection in most patients compared with previous studies to further assess whether the association of these 2 gene mutations with PTC lymph node metastasis.Citation16,Citation22,Citation25,Citation27 This is because occult lymph node metastasis to the central or lateral neck compartment occurs in 42.9%–55% of cN0 PTC patients. Thus, the extent of lymph node resection is critical to discover such a metastasis.Citation42,Citation43 Xing et al observed that BRAFV600E and the TERT promoter mutations were both significantly associated with PTC lymph node metastasis with therapeutic CLND and LLND.Citation16 In contrast, other studies have failed to reproduce such data, although these data were used for prophylactic CLND and therapeutic LLND.Citation22,Citation30 The most important value of our current study is that 92.0% (231 of 251) of cN0 patients received prophylactic CLND + LLND, and as many subclinical metastatic lymph nodes as possible were dissected to accurately assess the relationship between lymph node metastasis and genetic mutations. Although there was a higher incidence of lymph node metastasis in BRAF+/TERT+ group, there was no association between gene mutations and lymph node metastasis. Thus, the coexistence of BRAF and TERT promoter mutations cannot be used as a useful predictor of PTC lymph node metastasis. Nevertheless, a larger sample size is needed to verify our current finding due to the low prevalence of TERT promoter mutations.
The definition of ETE refers to tumor extension beyond the thyroid capsule into the adjacent tissues, which is an important negative prognostic factor and closely associated with tumor recurrence and patients’ mortality. Previous studies reported that BRAF+/TERT+ PTC patients had a higher prevalence of ETE, but none of these studies conducted multivariate analysis.Citation16,Citation21,Citation25 Interestingly, our current study showed that BRAF+/TERT+ was not an independent influencing factor for ETE, and the possible reason may be because association of BRAF+/TERT+ with older age and larger tumor size weaken the influence of gene mutation on ETE in multivariate analysis. In addition, we analyzed the association of the BRAF and TERT promoter mutations with PTMCs, which was prevalent according to a previous study.Citation44 We found that the presence of the BRAFV600E mutation was not associated with any high-risk PTMC clinicopathological features. However, we did not detect any TERT promoter mutations in PTMC, which was different from the previous study in a European cohort (4.7%).Citation45 The reason for this discrepancy is unknown, but may be due to differences in ethnicity, region, and methodologies used to detect the TERT promoter mutations. The previous studies and our current one did show that mutations of these genes were not associated with any aggressive PTMC behaviors.
Our current study does have some limitations. For example, we do not have follow-up data on these patients, since all patients were recently admitted to our hospital. Moreover, our current study was unable to provide data on association of more extensive surgical approaches (e.g., prophylactic lymph node dissection) with the traditional surgical approaches (e.g., therapeutic lymph node dissection), or prognosis of patients stratified by these 2 gene mutations. In addition, due to the smaller PTMC sample size, such a conclusion may lack the credibility. There is a selection bias due to not all cN0 patients undergoing prophylactic LLND, which might neglect occult lymph node metastasis.
Conclusion
In summary, we demonstrated the coexistence of BRAFV600E and TERT promoter mutations were associated with PTC clinicopathological aggressiveness, but did not associate with lymph node metastasis, and were not useful to identify the aggressive PTMCs. These results provide evidence to settle the dispute over the roles of BRAFV600E and TERT promoter mutations in lymph node metastasis of PTC and tailor the individual treatment of PTC patients.
Acknowledgments
The authors would like to thank Huili Bai and Yangli Zhang of Clinical Molecular Medicine Assessment Center, The First Affiliated Hospital, Chongqing Medical University (Chongqing, China), for technical support and Medjaden Bioscience Limited (Hong Kong, China) for editing the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- PellegritiGFrascaFRegalbutoCSquatritoSVigneriRWorldwide increasing incidence of thyroid cancer: update on epidemiology and risk factorsJ Cancer Epidemiol2013201396521223737785
- LloydRVORKlöppelGRosaiJWHO Classification of Tumours of Endocrine Organs4th edLyon, FranceIARC Press2017
- DaviesLWelchHGIncreasing incidence of thyroid cancer in the United States, 1973–2002JAMA2006295182164216716684987
- ChoSWChoiHSYeomGJLong-term prognosis of differentiated thyroid cancer with lung metastasis in Korea and its prognostic factorsThyroid201424227728623758653
- XingMBRAF mutation in thyroid cancerEndocr Relat Cancer200512224526215947100
- Cancer Genome Atlas Research NIntegrated genomic characterization of papillary thyroid carcinomaCell2014159367669025417114
- DaviesHBignellGRCoxCMutations of the BRAF gene in human cancerNature2002417689294995412068308
- XingMAlzahraniASCarsonKAAssociation between BRAF V600E mutation and mortality in patients with papillary thyroid cancerJAMA2013309141493150123571588
- XingMBRAF Mutation and Thyroid Cancer RecurrenceJ Clin Oncol201533222482248326124474
- TufanoRPTeixeiraGVBishopJCarsonKAXingMBRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: a systematic review and meta-analysisMedicine201291527428622932786
- TroviscoVSoaresPPretoAType and prevalence of BRAF mutations are closely associated with papillary thyroid carcinoma histotype and patients’ age but not with tumour aggressivenessVirchows Arch2005446658959515902486
- FugazzolaLPuxedduEAveniaNCorrelation between B-RAFV600E mutation and clinico-pathologic parameters in papillary thyroid carcinoma: data from a multicentric Italian study and review of the literatureEndocr Relat Cancer200613245546416728573
- NamJKJungCKSongBJIs the BRAF(V600E) mutation useful as a predictor of preoperative risk in papillary thyroid cancer?Am J Surg2012203443644121803329
- KirkpatrickKLMokbelKThe significance of human telomerase reverse transcriptase (hTERT) in cancerEur J Surg Oncol200127875476011735173
- MeloMda RochaAGVinagreJTERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomasJ Clin Endocrinol Metab2014995E754E76524476079
- XingMLiuRLiuXBRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrenceJ Clin Oncol201432252718272625024077
- GeorgeJRHendersonYCWilliamsMDAssociation of TERT promoter mutation, but not BRAF mutation, with increased mortality in PTCJ Clin Endocrinol Metab201510012E1550E155926461266
- NasirdenASaitoTFukumuraYIn Japanese patients with papillary thyroid carcinoma, TERT promoter mutation is associated with poor prognosis, in contrast to BRAF V600E mutationVirchows Arch2016469668769627718012
- LiuTWangNCaoJThe age- and shorter telomere-dependent TERT promoter mutation in follicular thyroid cell-derived carcinomasOncogene201433424978498424141777
- LeeSEHwangTSChoiYLPrognostic significance of TERT promoter mutations in papillary thyroid carcinomas in a BRAF(V600E) mutation-prevalent populationThyroid201626790191027184112
- LiuXQuSLiuRTERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancerJ Clin Endocrinol Metab2014996E1130E113624617711
- JinLBRAF and TERT promoter mutations in the aggressiveness ofpapillary thyroid carcinoma: a study of 653 patientsOncotarget2016714183461835526943032
- LiuXBishopJShanYHighly prevalent TERT promoter mutations in aggressive thyroid cancersEndocr Relat Cancer201320460361023766237
- AminMBESGreeneFLByrdDRBrooklandRKWashingtonMKAJCC Cancer Staging Manual8th edNew YorkSpringer International2017
- AsmannYWSunJZhangJBRAF V600E and TERT promoter mutations in papillary thyroid carcinoma in Chinese patientsPLoS One2016114e015331927064992
- SongYSLimJAChoiHPrognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patientsCancer201612291370137926969876
- MatsuseMYabutaTSaenkoVTERT promoter mutations and Ki-67 labeling index as a prognostic marker of papillary thyroid carcinomas: combination of two independent factorsSci Rep201774175228150740
- HongARLimJAKimTHThe frequency and clinical implications of the BRAF(V600E) mutation in papillary thyroid cancer patients in Korea over the past two decadesEndocrinol Metab (Seoul)201429450551325325273
- LiuSZhangBZhaoYAssociation of BRAFV600E mutation with clinicopathological features of papillary thyroid carcinoma: a study on a Chinese populationInt J Clin Exp Pathol20147106922692825400776
- ItoYYoshidaHMaruoRBRAF mutation in papillary thyroid carcinoma in a Japanese population: its lack of correlation with high-risk clinicopathological features and disease-free survival of patientsEndocr J2009561899718840924
- GuerraAFugazzolaLMarottaVA high percentage of BRAFV600E alleles in papillary thyroid carcinoma predicts a poorer outcomeJ Clin Endocrinol Metab20129772333234022508706
- ZengRCJinLPChenEDPotential relationship between Hashimoto’s thyroiditis and BRAF(V600E) mutation status in papillary thyroid cancerHead Eck201638Suppl 1E1019E1025
- KimSJMyongJPJeeHGCombined effect of Hashimoto’s thyroiditis and BRAF(V600E) mutation status on aggressiveness in papillary thyroid cancerHead Neck20163819510125213729
- KimSKSongKHLimSDClinical and pathological features and the BRAF(V600E) mutation in patients with papillary thyroid carcinoma with and without concurrent Hashimoto thyroiditisThyroid200919213714119014278
- MarottaVGuerraAZatelliMCBRAF mutation positive papillary thyroid carcinoma is less advanced when Hashimoto’s thyroiditis lymphocytic infiltration is presentClin Endocrinol (Oxf)201379573373823469895
- KimSKWooJWLeeJHChronic lymphocytic thyroiditis and BRAF V600E in papillary thyroid carcinomaEndocr Relat Cancer2016231273426598713
- SongYSLimJAParkYJMutation profile of well-differentiated thyroid cancer in AsiansEndocrinol Metab (Seoul)201530325226226435130
- VuongHGAltibiAMADuongUNPHassellLPrognostic implication of BRAF and TERT promoter mutation combination in papillary thyroid carcinoma-A meta-analysisClin Endocrinol (Oxf)201787541141728666074
- MoonSSongYSKimYAEffects of coexistent BRAFV600E and TERT promoter mutations on poor clinical outcomes in papillary thyroid cancer: a meta-analysisThyroid201727565166028181854
- HornSFiglARachakondaPSTERT promoter mutations in familial and sporadic melanomaScience2013339612295996123348503
- VinagreJAlmeidaAPopuloHFrequency of TERT promoter mutations in human cancersNature commun20134218523887589
- RohJLKimJMParkCICentral lymph node metastasis of unilateral papillary thyroid carcinoma: patterns and factors predictive of nodal metastasis, morbidity, and recurrenceAnn Surg Oncol20111882245225021327454
- LimYSLeeJCLeeYSLateral cervical lymph node metastases from papillary thyroid carcinoma: predictive factors of nodal metastasisSurgery2011150111612121507446
- YoonJHLeeHSKimEKShort-term follow-up US leads to higher false-positive results without detection of structural recurrences in PTMCMedicine2016951e243526735548
- de BiaseDGandolfiGRagazziMTERT promoter mutations in papillary thyroid microcarcinomasThyroid20152591013101926148423
