Abstract
Background and objective
Upregulated T-cell immunoglobulin and mucin domain containing molecule-3 (Tim-3) in hepatitis B virus (HBV)-specific CD8+ T-cells contributes to CD8+ T-cell exhaustion during chronic HBV infection. The membrane-bound Tim-3 can be cleaved from the cell surface by sheddase, yielding soluble Tim-3 (sTim-3). This study investigated serum sTim-3 levels in patients with chronic HBV infection of various liver diseases.
Methods
Serum sTim-3 levels were quantitatively determined in 288 patients with chronic HBV infection of various liver diseases. The sTim-3 levels were analyzed in relation to liver diseases including HBV-related hepatocellular carcinoma (HCC) and overall survival of HCC patients.
Results
Serum sTim-3 levels in the patients with chronic HBV infection were significantly elevated compared with healthy controls (P<0.001) and the levels from asymptomatic HBV carrier status, chronic hepatitis, liver cirrhosis to HCC were progressively increased. Serum sTim-3 levels were closely associated with the severity of liver function abnormalities. Importantly, serum sTim-3 levels were independently associated with HCC risk (OR, 4.310; 95% CI, 2.141–8.676, P<0.001) in comparison to non-HCC diseases in chronic HBV infection and significantly associated with the overall survival of HCC patients, with a level >3000 pg/mL being related to shorter overall survival than a level ≤3000 pg/mL (P=0.019).
Conclusion
Serum sTim-3 is involved in disease progression and HCC development in chronic HBV infection and its quantitative determination may be potentially used as a marker for monitoring the disease progression and predicting the HCC prognosis in chronic HBV infection.
Introduction
Hepatitis B virus (HBV) infection is a severe public health problem worldwide. Chronic HBV infection is related to a variety of diseases including asymptomatic HBV carrier status (ASC), chronic hepatitis (CH), liver cirrhosis (LC), and hepatocellular carcinoma (HCC), constituting a major cause of liver-related morbidity and mortality.Citation1–Citation3 The various outcomes in HBV infection result from the complex interplay between HBV replication and host immune response.Citation4 It is widely recognized that CD8+ T-cells play an important role in the host immune response to HBV infection. In acute HBV infection, CD8+ T-cells are primarily involved in virus clearance by producing cytokines interferon (IFN)-γ and tumor necrosis factor-α.Citation5,Citation6 In chronic HBV infection, however, CD8+ T-cells exhibit exhausted phenotype suffering from loss of functionCitation7–Citation10 and the condition of HBV-specific CD8+ T-cell exhaustion is related to different clinical outcomes.Citation11
T-cell immunoglobulin and mucin domain containing molecule-3 (Tim-3), a type I transmembrane protein negatively regulating immune responses, is involved in the dysfunction and exhaustion of CD8+ T-cells in chronic HBV infection. The expression of Tim-3 is significantly increased in HBV-specific CD8+ T-cells and this is associated with the exhaustion of CD8+ T-cells in patients with chronic HBV infection.Citation12–Citation16 Blocking Tim-3 signaling pathway is able to reverse the impaired function of CD8+ T-cells.Citation12,Citation13,Citation17 Increased Tim-3 expression on both peripheral T-cells and monocytes and lymphocytes in liver tissues is closely related to the disease progression of chronic HBV infection and HBV-related HCC.Citation12,Citation14,Citation15,Citation18 Therefore, Tim-3 plays important roles in the pathogenesis of chronic HBV infection.
In addition to the membrane-bound form, Tim-3 has a soluble form (sTim-3), which may be produced by alternative splicing,Citation19,Citation20 cleavage from the cell surface by matrix metal-loproteinases, a disintegrin, and metalloprotease (ADAM)10 and ADAM17,Citation21 and even the passive release of a soluble fragment from apoptotic cells.Citation22 sTim-3 is also functionally relevant to the regulation of T-cell-mediated immune response. For example, recombinant mouse sTim-3 is able to inhibit T-cell responses to antigen-specific stimulation.Citation20 sTim-3 is involved in Toll-like receptor-mediated immune responses of CD14+ monocytes.Citation21 sTim-3 shed from CD8+ T-cells in HIV infection functions as a coinhibitory receptor.Citation23
The mechanism of CD8+ T-cell exhaustion by Tim-3 and sTim-3 expression remains largely unknown. Recent studies showed that Tim-3 may contribute to exhaustion by restricting the development of long-lived memory T-cellsCitation24 and Tim-3 engagement during antigen stimulation can directly influence T-cell differentiation through mammalian target of rapamycin complex-1.Citation25 In addition, another recent study showed that human liver cancer tissues contained a high ratio of Tim-3 expressing hepatocytes and HBV was involved in Tim-3 upregulation in malignant hepatocytes. The tumor cell-intrinsic Tim-3 can promote liver cancer through nuclear factor-κB/interleukin-6/signal transducer and activator of transcription 3 axis.Citation26
Although the role of the membrane-bound form of Tim-3 in immune dysfunction and disease progression during HBV infection has been extensively documented, the potential influences of the soluble form, namely sTim-3, on HBV infection remain unknown. Therefore, this study examined the levels of serum sTim-3 in patients with chronic HBV infection of various liver diseases and analyzed the association between sTim-3 and clinical diseases of chronic HBV infection, and overall survival of patients with HBV-related HCC.
Patients and methods
Study population
Two hundred eighty-eight patients and 92 age- and sex-matched healthy controls were included in this study. All the patients were recruited from the First Affiliated Hospital of Xi’an Jiaotong University, a tertiary hospital in northwest China. Patients who had a history of HBV infection for >6 months and had no treatment with any nucleos(t)ide analogs or immune regulatory agents including IFN at study entry were included. All the patients and healthy controls were adults. Individuals aged <18 years were excluded. Patients with positivity for hepatitis C virus or HIV, and having autoimmune, alcoholic, metabolic, or drug-induced liver diseases were excluded. The healthy controls were individuals who had normal liver biochemistries, no history of hepatitis B, and negativity for HBV markers or positivity for antibodies against hepatitis B surface antigen (anti-HBs) only due to hepatitis B vaccination.
The patients were diagnosed with ASC, CH, LC, and HCC based on the history of HBV infection, serostatus of HBsAg/anti-HBs, HBeAg/antibodies against hepatitis B e antigen (anti-HBe), and antibodies against hepatitis B core antigen (anti-HBc), serum HBV DNA levels, biochemical liver function, and findings of ultrasonography and/or computerized tomography (CT)/MRI. Patients with chronic HBV infection who had positivity of HBsAg, HBeAg, and anti-HBc, increased levels of HBV DNA, and persistently normal serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were diagnosed as having ASC. Patients with chronic HBV infection who had positivity of HBsAg, HBeAg, or anti-HBe, and anti-HBc, increased HBV DNA levels, abnormal serum ALT, and no evidence of LC and HCC on ultrasonography and CT were diagnosed as having CH. Patients with chronic HBV infection who had positivity of HBsAg, HBeAg, or anti-HBe, and anti-HBc, detectable levels of HBV DNA, evidence of LC but no evidence of HCC on liver biopsy and/or on ultrasonography and/or CT/MRI and gastroesophageal varices by endoscopy were diagnosed as having LC. And patients with chronic HBV infection who had positivity of HBsAg, HBeAg, or anti-HBe, and anti-HBc, detectable levels of HBV DNA, evidence of HCC on liver biopsy and/or ultrasonography and/or CT/MRI were diagnosed as having HCC. In all, 84 patients with HBV-related HCC were followed up for a median of 52 (31–75) months and they had a median overall survival of 28 (1–63) months. The study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University. All the study subjects gave written informed consent.
Determination of serum sTim-3 levels
Fasting blood sample was collected from all participants at the study entry, and serum samples were stored at −80°C before use.
Serum levels of sTim-3 were measured using a commercially available Quantikine Human Tim-3 enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems Inc, Minneapolis, MN, USA) according to the manufacturer’s instructions. The sensitivity, minimum detectable dose (MDD), of the assay was 0.554–8.75 pg/mL and the mean MDD was 2.17 pg/mL. The intra-assay coefficient of variation ranged from 2.2% to 2.6% and the inter-assay coefficient of variation ranged from 3.9% to 5.3%. The persons who determined the serum levels of sTim-3 were unaware of the case–control status and the patients’ clinical characteristics when they read the sTim-3 ELISA.
Laboratory tests
HBsAg, anti-HBs, HBeAg, anti-HBe, and anti-HBc were determined using ELISA kits from Beijing Wantai Biological Pharmacy (Beijing, China). Biochemical liver function, including ALT and AST levels (IU/L), was assayed on the Olympus AU5400 automatic biochemical analyzer (Olympus Corporation, Tokyo, Japan). The HBV Fluorescent Quantitative PCR Detection Kit with TaqMan probes (Da-An Gene Co, Guangzhou, China) was used to quantitatively determine the serum HBV DNA levels (IU/mL) according to the manufacturer’s instruction. Serum α-fetoprotein (AFP) levels (ng/mL) were measured by automated Eleceyes (Hoffman-La Roche Ltd, Basel, Switzerland).
Statistical analysis
Serum sTim-3 levels were logarithmically transformed to meet the normal distribution. Data were expressed as the mean ± SD or median (range). Statistical analysis was performed by SPSS software version 20.0 (IBM Corporation, Armonk, NY, USA). The two independent samples t-test was used to compare serum sTim-3 levels between patients and controls. Analysis of variance was used to compare serum sTim-3 levels among different clinical diseases of chronic HBV infection and further comparisons between groups were performed with post hoc tests followed by Bonferroni’s correction (when equal variances were assumed) or Dunnett’s T3 (when equal variances were not assumed). Correlations between values were examined by calculating Spearman correlation coefficients. Multiple stepwise logistic regression analyses were used to assess the factors independently associated with HCC in the patients. A receiver operating characteristic (ROC) curve was plotted to identify the optimal cutoff value of serum sTim-3 levels for evaluating the performances of sTim-3 in discriminating patients with HCC from patients without HCC and the area under the ROC curve (AUC) was calculated. Univariate and multiple Cox regression analyses were used to identify factors associated with the overall survival of HCC patients and to estimate the HR and 95% CI. Overall survival of HCC patients was analyzed by Kaplan–Meier analysis and compared by the log-rank test. A P-value <0.05 was considered statistically significant.
Results
Characteristics of the study subjects
The recruited patients with chronic HBV infection had a male/female ratio of 202/86 and aged 44.92±13.56 (18–78) years. The healthy controls had a male/female ratio of 65/27 and aged 44.34±13.68 (18–76) years. The gender and age between the patients and healthy controls had no significant differences (P=0.925 and P=0.724, respectively, ). The diagnosis of the clinical diseases in the 288 patients included 59 ASC, 55 CH, 90 LC, and 84 HCC ().
Association of serum sTim-3 levels with clinical diseases in chronic HBV infection
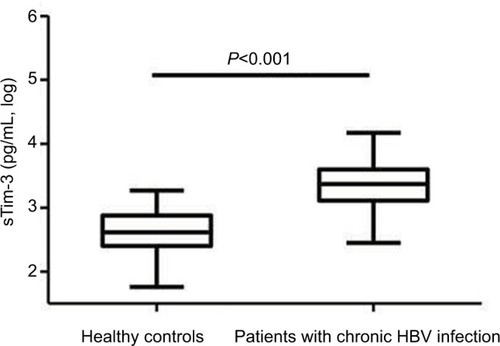
Serum sTim-3 levels in patients with chronic HBV infection (3.35±0.32 log pg/mL) were significantly elevated compared with healthy controls (2.64±0.32 log pg/mL, P<0.001, ).
Figure 1 Serum sTim-3 levels in patients with chronic HBV infection and healthy controls.
Abbreviations: HBV, hepatitis B virus; sTim-3, soluble T-cell immunoglobulin and mucin domain containing molecule-3.
The gender, age, HBeAg, HBV DNA, ALT, AST, total bilirubin, albumin, and serum sTim-3 levels between ASC, CH, LC, and HCC patients were significantly different (P=0.032, P<0.001, P=0.029, P<0.001, P<0.001, P<0.001, P<0.001, P<0.001, and P<0.001, respectively, ).
Table 1 Demographics and laboratory parameters in healthy controls and patients with different clinical diseases of chronic HBV infection
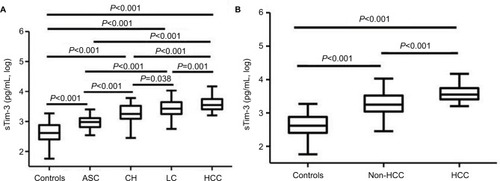
Serum sTim-3 levels from ASC, CH, LC to HCC were sequentially increased. Serum levels of sTim-3 in ASC (2.97±0.19 log pg/mL), CH (3.29±0.28 log pg/mL), LC (3.43±0.26 log pg/mL), and HCC (3.57±0.22 log pg/mL) were significantly higher than those in healthy controls (all P<0.001, ). Serum sTim-3 levels in HCC were significantly elevated compared with ASC, CH, and LC (P<0.001, P<0.001, and P=0.001, respectively); serum sTim-3 levels in LC were significantly elevated compared with ASC and CH (P<0.001 and P=0.038, respectively); and serum sTim-3 levels in CH were significantly elevated compared with ASC (P<0.001, ). Serum sTim-3 levels in patients with HCC were significantly higher than those in patients without HCC (P<0.001, ).
Figure 2 Serum sTim-3 levels in patients with (A) different clinical diseases of chronic HBV infection and (B) HBV-related HCC and patients without HCC.
Abbreviations: HBV, hepatitis B virus; ASC, chronic asymptomatic HBV carrier; CH, chronic hepatitis; LC, liver cirrhosis; HCC, hepatocellular carcinoma; sTim-3, soluble T-cell immunoglobulin and mucin domain containing molecule-3.
Correlations of serum sTim-3 levels with demographics and laboratory parameters
In ASC, there was a positive correlation between serum sTim-3 levels and age (r=0.334, P=0.010), and a negative correlation between serum sTim-3 levels and albumin (r=−0.287, P=0.028). In CH, serum sTim-3 levels were positively correlated with ALT (r=0.493, P<0.001), AST (r=0.552, P<0.001), and total bilirubin (r=0.384, P=0.004) levels and negatively correlated with albumin levels (r=−0.507, P<0.001). In LC, serum sTim-3 levels were positively correlated with HBV DNA (r=0.421, P<0.001), ALT (r=0.399, P<0.001), AST (r=0.480, P<0.001), and total bilirubin (r=0.506, P<0.001) levels, and negatively correlated with albumin levels (r=−0.474, P<0.001). In HCC, serum sTim-3 levels were also positively correlated with ALT (r=0.239, P=0.031), AST (r=0.423, P<0.001), and total bilirubin (r=0.601, P<0.001) levels, marginally positively correlated with HBV DNA levels (r=0.213, P=0.052), and negatively correlated with albumin levels (r=−0.372, P<0.001, ). When all the patients with chronic HBV infection were included in the correlation analysis, serum sTim-3 levels were positively associated with patients’ age, and ALT, AST, and bilirubin values and negatively associated with albumin levels (all P<0.001, ).
Table 2 Correlations of serum sTim-3 levels with other parameters according to clinical diseases in patients with chronic HBV infection
Performance of serum sTim-3 levels in discriminating HCC from other liver diseases
Multivariate stepwise logistic regression analysis showed that serum sTim-3 levels were independently associated with HCC risk in relation to ASC, CH, and LC (OR (95%CI)=48.476 (6.781–346.545), P<0.001; OR (95%CI)=6.315 (1.490–26.77), P=0.012; and OR (95%CI)=2.798 (1.298−6.033), P=0.009, respectively, ). When ASC, CH, and LC were combined as non-HCC, serum sTim-3 levels were also independently associated with HCC risk (OR (95%CI)=4.310 (2.141–8.676), P<0.001, ).
Table 3 Multivariate stepwise logistic regression analysis for discriminating HCC from ASC, CH, and LC in chronic HBV infection
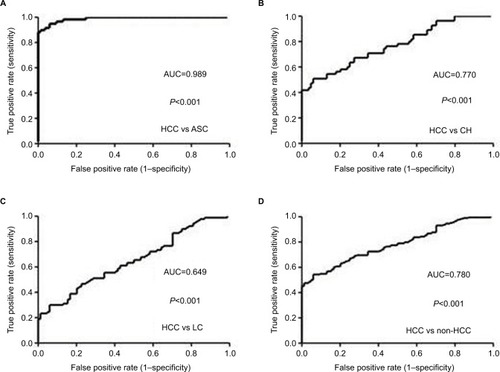
ROC curve was plotted to evaluate the performance of serum sTim-3 levels in discriminating HCC from ASC, CH, and LC. The AUC was 0.989 for HCC versus ASC (cutoff value=3.26 log pg/mL, sensitivity=94%, and specificity =94.9%, ), 0.770 for HCC versus CH (cutoff value =3.26 log pg/mL, sensitivity=94%, and specificity=50.9%, ), 0.649 for HCC versus LC (cutoff value=3.39 log pg/mL, sensitivity=77.4%, and specificity=46.7%, ), and 0.780 for HCC versus non-HCC (cutoff value=3.27 log pg/mL, sensitivity=94%, and specificity=54.4%, ).
Figure 3 ROC curves of serum sTim-3 levels for discriminating HCC from ASC (A), CH (B), LC (C), and non-HCC (D).
Abbreviations: ASC, chronic asymptomatic HBV carrier; HBV, hepatitis B virus; CH, chronic hepatitis; LC, liver cirrhosis; HCC, hepatocellular carcinoma; sTim-3, soluble T-cell immunoglobulin and mucin domain containing molecule-3; ROC, receiver operating characteristic; AUC, area under the ROC curve.
Association of serum sTim-3 levels with overall survival of HCC patients
Univariate Cox regression analysis showed that AST, total bilirubin, albumin, AFP, Child–Pugh grade, and sTim-3 levels were associated with the overall survival of patients with HBV-related HCC (). Multivariate stepwise Cox regression analysis showed that serum sTim-3 levels (HR (95%CI)=2.773 (1.474–5.219), P=0.002), together with gender and AFP (HR (95%CI)=4.329 (1.297–14.492), P=0.017; and HR (95%CI)=3.388 (1.816–6.321), P<0.001, respectively), were independently associated with the overall survival of HCC patients ().
Table 4 Univariate and multivariate analysis of factors associated with the overall survival of patients with HBV-related hepatocellular carcinoma
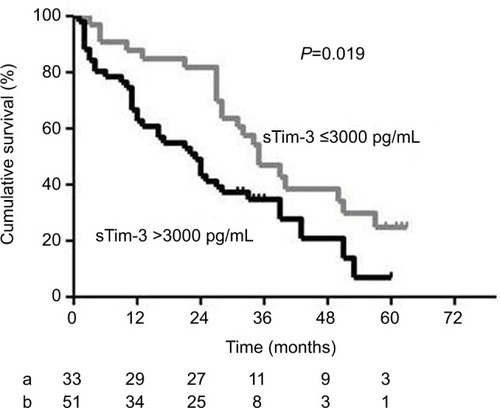
Kaplan–Meier survival curves and the log-rank test showed that the overall survival of HCC patients with serum sTim-3 levels >3000 pg/mL (3.477 log pg/mL) was significantly lower than those with serum sTim-3 levels ≤3000 pg/mL (P=0.019, ).
Figure 4 Kaplan–Meier curves of overall survival in patients with HCC according to serum sTim-3 levels of ≤3000 pg/mL (a) and >3000 pg/mL (b). At the cutoff value of 3000 pg/mL identified by the receiver operating characteristic curve, HCC patients with a sTim-3 level >3000 pg/mL had shorter cumulative survival.
Abbreviations: HCC, hepatocellular carcinoma; sTim-3, soluble T-cell immunoglobulin and mucin domain containing molecule-3.
Discussion
This is, to our knowledge, the first study to investigate serum sTim-3 in chronic HBV infection. The results showed that serum sTim-3 levels were significantly elevated in patients with chronic HBV infection, stepwisely increased from ASC, CH, LC to HCC, were closely associated with biochemical liver function, and highly discriminative of HCC from ASC, CH, and LC as well as non-HCC conditions in chronic HBV infection. Furthermore, serum sTim-3 levels were significantly associated with the overall survival of patients with HBV-related HCC.
So far, sTim-3 has been shown to be involved in several human diseases. For example, altered sTim-3 has been associated with the development of sepsisCitation27 and the regulation of immunity in unexplained recurrent spontaneous abortion.Citation28 sTim-3 levels have also been associated with diffuse cutaneous systemic sclerosis,Citation29 severe graft-versus-host disease,Citation22 and the non-relapse mortality and overall survival after allogeneic hematopoietic cell transplantation.Citation30 In infectious diseases, serum sTim-3 levels have been revealed to be involved in pulmonary tuberculosisCitation31 and disease progression in HIV infection.Citation23 The results in this study, indicating the involvement of sTim-3 in chronic HBV infection and the progression of HBV-related liver diseases, have added novel information about the role of sTim-3 in human diseases.
Among clinical diseases of chronic HBV infection including ASC, CH, LC, and HCC, this study showed that serum sTim-3 levels were progressively increased from ASC, CH, LC to HCC. These results indicate that the elevated sTim-3 levels are involved in disease progression during chronic HBV infection although the disease conditions in this study were cross-sectionally diagnosed in different individuals chronically infected with HBV.
Serum sTim-3 levels were positively correlated with ALT, AST, and total bilirubin levels in CH, LC, and HCC and negatively correlated with albumin levels in ASC, CH, LC, and HCC. These results indicate that the elevated sTim-3 may reflect the severity of necroinflammation and the impairment of albumin synthesis of the liver in patients with chronic HBV infection. Intriguingly, the sTim-3 levels seem to fully reflect the profile of liver function abnormalities in chronic HBV infection because the levels are related to not only the increased aminotransferase and total bilirubin levels, which primarily indicate the degree of necroinflammation of the liver, but also the decreased albumin level, which mainly reveals the severity of synthesis function of the liver. The currently recommended indications for treatment of chronic HBV infection are based mainly on the combination of serum HBV DNA levels, ALT levels, and severity of liver disease.Citation4 The results of this study indicate that sTim-3, an immuneresponse molecule, may reflect both the immune status and the severity of liver impairment. Therefore, sTim-3 may have the potential to be included as an indicator for patient treatment and a predictor for treatment response in chronic HBV infection.
The serum sTim-3 level in HCC was the highest and independently associated with HCC in comparison to non-HCC diseases including ASC, CH, and LC. The AUC values indicate that the serum sTim-3 level was significantly discriminative of HCC from non-HCC with high sensitivity and specificity. Furthermore, serum sTim-3 level was a significantly independent factor associated with the overall survival rate of HBV-related HCC patients. These results suggest that sTim-3 may play an important role in the pathogenesis of HBV-related HCC and the significantly increased serum sTim-3 levels may serve as a biomarker of HCC development during chronic HBV infection and a prognostic factor for patients with HBV-related HCC.
Increased membrane-bound Tim-3 expression on CD4+ and CD8+ T-cells has been associated with the severity of CH B and liver injury because of its positive correlation with ALT, AST, and total bilirubin values.Citation14 In patients with HBV-related HCC, Tim-3 expression is increased on CD4+ and CD8+ T-cells, the numbers of Tim-3+ tumor infiltrating cells are negatively associated with patient survival, and blockade of the Tim-3 signaling pathway significantly increases the functionality of tumor infiltrating Tim-3+ T-cells.Citation12 It is suggested that the level of Tim-3 expressed on T-cells and the severity of T-cell exhaustion are highly correlated in chronic HBV infection and HBV-related HCC. Similar results were obtained in the present study for sTim-3, showing its close association with disease progression, abnormal liver function, and the survival of HCC patients in chronic HBV infection. Because sTim-3 is primarily shed from cells expressing this molecule including monocytesCitation21 and CD8+ T-cells such as in HIV infection,Citation23 it is indicated that the levels of serum sTim-3 may parallel the levels of membrane-bound Tim-3 expressed on immune cells. Therefore, sTim-3 levels may reflex the quantity of membrane-bound Tim-3 expression. Serum sTim-3 may thus be used as an excellent surrogate of membrane-bound Tim-3 with the advantage over cell expressing Tim-3 in the determination of methodology.
Programmed death-1 (PD-1), another immunoinhibitory receptor, has also been associated with immune dysfunction in chronic HBV infection and HBV-related HCC.Citation10,Citation32–Citation37 Recent studies showed that the soluble form of PD-1, scilicet PD-1 (sPD-1), is involved in the disease course of chronic HBV infection and its serum levels are associated with disease conditions in chronic HBV infection including HBV-related HCC in particular.Citation38,Citation39 Similarly, the sTim-3 levels determined in this study appear to have a similar pattern of change with sPD-1 in chronic HBV infection and HBV-related HCC. As for membrane-bound forms of Tim-3 and PD-1, both of them are highly expressed in chronic HBV infection and HBV-related HCC.Citation15 However, the role of Tim-3 and PD-1 in driving T-cell exhaustion in chronic HBV infection has been indicated to be nonredundant.Citation13 Studies showed that combined targeting of both PD-1 and Tim-3, compared with targeting either PD-1 or Tim-3 alone, is more effective in controlling chronic viral infectionCitation40 and suppressing tumor proliferation.Citation41 Dual blockade of PD-1 and Tim-3 could also enhance the rate and strength of functional antiviral responses in patients with chronic HBV infection.Citation13 For soluble PD-1 and Tim-3, co-administration of these two molecules as molecular adjuvants has been revealed to be able to enhance SIV-specific cell-mediated immune responses in mice.Citation42 In this respect, the findings that both sTim-3 and sPD-1 are significantly increased in chronic HBV infection and HBV-related HCC may have implications for developing novel strategies through targeting both Tim-3 and PD-1 as an approach to improving control of chronic HBV infection and HBV-related HCC and for monitoring the effectiveness of treatment response by dual targeting Tim-3 and PD-1.
This study has several limitations including the small number of patients in the subgroups of different clinical diseases, the lack of study in replication populations, and the lack of functional studies concerning the role of sTim-3 in the CD8+ T-cell exhaustion in chronic HBV infection. We found that the sTim-3 level had very close correlation with the parameters of biochemical liver function such as ALT, AST, and albumin but it only demonstrated moderate performance for discriminating HCC from CH, LC, and non-HCC. Therefore, further studies are required to verify and extend our findings.
Conclusion
This study revealed that serum sTim-3 levels are significantly increased in patients with chronic HBV infection, and closely associated with disease progression and the severity of liver function abnormalities in patients with chronic HBV infection. Serum sTim-3 levels are independently associated with HCC risk in chronic HBV infection and significantly associated with the overall survival of patients with HBV-related HCC. These results suggest the involvement of sTim-3 in disease progression, especially hepatocarcinogenesis, in chronic HBV infection. Quantitative determination of serum sTim-3 may have the potential to be applied as a biomarker of HBV disease monitoring and HBV-related HCC prognosing. Further prospective studies are needed to validate the findings of this study and to examine the underlying mechanisms of sTim-3 elevation in HBV-related liver diseases. The potential usefulness of sTim-3 in predicting treatment response in chronic HBV infection also requires to be investigated in future studies.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (grant no 81371798).
Supplementary material
Table S1 Demographics in patients with chronic HBV infection and healthy controls, and clinical diagnoses in the patients
Disclosure
The authors report no conflicts of interest in this work.
References
- MokdadAALopezADShahrazSLiver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysisBMC Med20141214525242656
- MittalSEl-SeragHBEpidemiology of hepatocellular carcinoma: consider the populationJ Clin Gastroenterol201347SupplS2S623632345
- StanawayJDFlaxmanADNaghaviMThe global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013Lancet2016388100491081108827394647
- European Association for the Study of the LiverEASL 2017 clinical practice guidelines on the management of hepatitis B virus infectionJ Hepatol201767237039828427875
- ThimmeRWielandSSteigerCCD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infectionJ Virol2003771687612477811
- PhillipsSChokshiSRivaAEvansAWilliamsRNaoumovNVCD8(+) T cell control of hepatitis B virus replication: direct comparison between cytolytic and noncytolytic functionsJ Immunol2010184128729519949099
- GuidottiLGChisariFVImmunobiology and pathogenesis of viral hepatitisAnnu Rev Pathol20061236118039107
- WherryEJT cell exhaustionNat Immunol201112649249921739672
- WielandDHofmannMThimmeROvercoming CD8+ T-cell exhaustion in viral hepatitis: lessons from the mouse model and clinical perspectivesDig Dis201735433433828468011
- BoniCFisicaroPValdattaCCharacterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infectionJ Virol20078184215422517287266
- DinneyCMZhaoLDConradCDRegulation of HBV-specific CD8(+) T cell-mediated inflammation is diversified in different clinical presentations of HBV infectionJ Microbiol2015531071872426428923
- LiHWuKTaoKTim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinomaHepatology20125641342135122505239
- NebbiaGPeppaDSchurichAUpregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infectionPLoS One2012710e4764823112829
- WuWShiYLiJChenFChenZZhengMTim-3 expression on peripheral T cell subsets correlates with disease progression in hepatitis B infectionVirol J2011811321392402
- LiZLiNLiFImmune checkpoint proteins PD-1 and TIM-3 are both highly expressed in liver tissues and correlate with their gene polymorphisms in patients with HBV-related hepatocellular carcinomaMedicine (Baltimore)20169552e574928033288
- WangLZhaoCPengQShiJGuGExpression levels of CD28, CTLA-4, PD-1 and Tim-3 as novel indicators of T-cell immune function in patients with chronic hepatitis B virus infectionBiomed Rep20142227027424649109
- WuWShiYLiSBlockade of Tim-3 signaling restores the virus-specific CD8+ T-cell response in patients with chronic hepatitis BEur J Immunol20124251180119122539292
- RongYHWanZHSongHTim-3 expression on peripheral monocytes and CD3+CD16/CD56+natural killer-like T cells in patients with chronic hepatitis BTissue Antigens2014832768124397461
- SabatosCAChakravartiSChaEInteraction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral toleranceNat Immunol20034111102111014556006
- GengHZhangGMLiDSoluble form of T cell Ig mucin 3 is an inhibitory molecule in T cell-mediated immune responseJ Immunol200617631411142016424168
- Möller-HackbarthKDewitzCSchweigertOA disintegrin and metalloprotease (ADAM) 10 and ADAM17 are major sheddases of T cell immunoglobulin and mucin domain 3 (Tim-3)J Biol Chem201328848345293454424121505
- HansenJAHanashSMTabelliniLA novel soluble form of Tim-3 associated with severe graft-versus-host diseaseBiol Blood Marrow Transplant20131991323133023791624
- ClaytonKLDouglas-VailMBNur-ur RahmanAKSoluble T cell immunoglobulin mucin domain 3 is shed from CD8+ T cells by the sheddase ADAM10, is increased in plasma during untreated HIV infection, and correlates with HIV disease progressionJ Virol20158973723373625609823
- AveryLFildermanJSzymczak-WorkmanALKaneLPTim-3 co-stimulation promotes short-lived effector T cells, restricts memory precursors, and is dispensable for T cell exhaustionProc Natl Acad Sci U S A2018115102455246029463725
- SabinsNCChornoguzOLeanderKTIM-3 engagement promotes effector memory T cell differentiation of human antigen-specific CD8 T cells by activating mTORC1J Immunol2017199124091410229127145
- ZhangHSongYYangHTumor cell-intrinsic Tim-3 promotes liver cancer via NF-κB/IL-6/STAT3 axisOncogene Epub2018216
- RenFLiJJiangXPlasma soluble Tim-3 emerges as an inhibitor in sepsis: sepsis contrary to membrane Tim-3 on monocytesTissue Antigens201586532533226373631
- WuMZhuYZhaoJSoluble costimulatory molecule sTim3 regulates the differentiation of Th1 and Th2 in patients with unexplained recurrent spontaneous abortionInt J Clin Exp Med2015868812881926309533
- ChibaMYanabaKHayashiMYoshiharaYNakagawaHClinical significance of serum soluble T-cell immunoglobulin and mucin domain 3 levels in systemic sclerosis: association with disease severityJ Der-matol2017442194197
- Abu ZaidMWuJWuCPlasma biomarkers of risk for death in a multicenter phase 3 trial with uniform transplant characteristics post-allogeneic HCTBlood2017129216217027827824
- ZhangJZhangFLiQIncreased serum levels of soluble T cell immunoglobulin mucin molecule 3 and IL-4 and decreased IFN-γ in patients with pulmonary tuberculosisXi Bao Yu Fen Zi Mian Yi Xue Za Zhi2016327968971 Chinese27363280
- PengGLiSWuWTanXChenYChenZPD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patientsMol Immunol200845496397017868872
- ShiFShiMZengZPD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patientsInt J Cancer2011128488789620473887
- ZhangWJPengCHZhengSSProgrammed death 1 and programmed death ligand 1 expressions in patients with chronic hepatitis BHepatobiliary Pancreat Dis Int201312439439923924497
- XuPChenYJChenHThe expression of programmed death-1 in circulating CD4+ and CD8+ T cells during hepatitis B virus infection progression and its correlation with clinical baseline characteristicsGut Liver20148218619524672661
- BengschBMartinBThimmeRRestoration of HBV specific CD8+ T cell function by PD-1 blockade in inactive carrier patients is linked to T cell differentiationJ Hepatol20146161212121925016223
- HsuPNYangTCKaoJTIncreased PD-1 and decreased CD28 expression in chronic hepatitis B patients with advanced hepatocellular carcinomaLiver Int20103091379138620738778
- ChengHYKangPJChuangYHCirculating programmed death-1 as a marker for sustained high hepatitis B viral load and risk of hepatocellular carcinomaPLoS One2014911e9587025427199
- LiNZhouZLiFCirculating soluble programmed death-1 levels may differentiate immune-tolerant phase from other phases and hepatocellular carcinoma from other clinical diseases in chronic hepatitis B virus infectionOncotarget2017828460204603328545019
- JinHTAndersonACTanWGCooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infectionProc Natl Acad Sci U S A201010733147331473820679213
- SakuishiKApetohLSullivanJMBlazarBRKuchrooVKAndersonACTargeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunityJ Exp Med2010207102187219420819927
- XiaoLWangDSunCEnhancement of SIV-specific cell mediated immune responses by co-administration of soluble PD-1 and Tim-3 as molecular adjuvants in miceHum Vaccin Immunother201410372473324326266
