Abstract
Introduction
The timing of postmastectomy radiotherapy (PMRT) may influence locoregional recurrence and survival outcomes. In this study, we assessed the long-term survival effect of the interval between surgery and PMRT in locally advanced breast cancer treated with mastectomy and adjuvant chemotherapy.
Methods
In this retrospective study, we included women with locally advanced breast cancer who underwent adjuvant chemotherapy and PMRT after mastectomy between 1999 and 2007. Based on the interval between surgery and PMRT, the patients were classified into three groups: Group 1 (≤4 vs >4 months), Group 2 (≤5 vs >5 months), and Group 3 (≤6 vs >6 months). Univariate and multivariate regression analyses were performed to determine the prognostic factors of survival outcomes.
Results
A total of 340 women were included in this study, and the median follow-up duration was 79.8 months. The median surgery–PMRT interval was 5 months. The surgery–PMRT interval including Group 1, Group 2, and Group 3 was not significantly associated with locoregional recurrence-free survival, distant metastasis-free survival, disease-free survival, and overall survival. In addition, in the subgroup analysis of the effect of surgery–PMRT interval on survival outcomes according to various clinicopathologic factors, the surgery–PMRT interval was also not associated with survival outcomes in different age groups, tumor stage, and breast cancer subtypes.
Conclusion
Our findings suggest that the delay in the start of PMRT in locally advanced breast cancer does not increase the likelihood of locoregional recurrence, distant metastasis, and death.
Introduction
Patients with locally advanced breast cancer, which is defined as stage III disease, had a higher risk for locoregional recurrence (LRR). Approximately 30% of patients with high-risk breast cancer develop LRR after mastectomy, but the administration of postmastectomy radiotherapy (PMRT) may reduce LRR and improve survival outcomes.Citation1–Citation3 In these high-risk patients, the interval between PMRT administration and mastectomy may affect LRR and survival outcomes. However, the optimal time between surgery and PMRT remains unclear.
There are conflicting results regarding the optimal interval between surgery and radiotherapy. However, it has been reported that an interval >6–12 weeks in patients not receiving chemotherapy, and an interval >6–7 months in patients receiving adjuvant chemotherapy after surgery, may lead to a higher risk of recurrence.Citation4–Citation9 Several retrospective studies have yielded variable findings in women receiving breast-conserving surgery.Citation5,Citation10–Citation14 Adjuvant chemotherapy and radiotherapy is the standard treatment for high-risk breast cancer after mastectomy.Citation15 In this study, we retrospectively assessed the long-term survival effect of the surgery–PMRT interval in women with locally advanced breast cancer treated with mastectomy and adjuvant chemotherapy.
Materials and methods
Patients
We retrospectively analyzed the medical data of patients with breast cancer who underwent mastectomy between 1999 and 2009 at the Sun Yat-Sen University Cancer Center, Guangzhou, China. Patients were eligible for inclusion in this study if:
in accordance with the current tumor (T) node (N) metastasis (M) staging system, they had stage III breast cancer;
they underwent mastectomy, and at least 4 cycles adjuvant chemotherapy followed by PMRT;
PMRT was administrated to the chest wall and supraclavicular lymph nodes to a prescription dose of 50 Gy in 25 fractions; and
they had complete clinicopathologic and follow-up data.
Clinicopathologic factors
The following clinicopathologic factors were included: age; menopausal status; tumor stage; hormone receptor (HoR) status; and human epidermal growth factor receptor-2 (HER2) status. The expressions of estrogen receptor (ER), progesterone receptor (PR), and HER2 were assessed in accordance with our previous study.Citation16 ER and PR positivity were defined as immunohistochemistry findings of >1% positive cells. HER2 positivity was defined as an immunohistochemistry score of 3+ or 2+ with confirmation by fluorescence in situ hybridization. The breast cancer subtypes (BCS) were classified as four subtypes according to HoR and HER2 status: HoR+/HER2−, HoR+/HER2+, HoR−/HER2+, and HoR−/HER2− subtypes. Based on the interval between mastectomy and radiotherapy, the patients were classified into three groups: Group 1 (≤4 vs >4 months), Group 2 (≤5 vs >5 months), and Group 3 (≤6 vs >6 months). The primary endpoints of this study were locoregional recurrence-free survival (LRFS), distant metastasis-free survival (DMFS), disease-free survival (DFS), and overall survival (OS). LRR was defined as pathologically confirmed recurrence including ipsilateral chest wall, axillary lymph nodes, supraclavicular and subclavian lymph nodes, or internal mammary lymph nodes. Distant metastasis was defined as tumor recurrence at a site distal to the primary cancer. DFS referred to absence of LRR or distant metastasis. OS was defined as the time from initial diagnosis to the date of death or last follow-up.
Statistical analysis
The χ2-test or Fisher’s exact test was performed to compare differences between the three interval groups. The survival curves were estimated using the Kaplan–Meier method and compared using the log-rank test. Univariate and multivariate Cox regression analyses were performed to investigate the risk factors for survival outcomes. In the univariate analysis, the survival differences were investigated according to age, menopausal status, tumor stage, BCS, and timing of PMRT administration. Multivariate Cox regression analyses were then performed to identify the independent predictors from those statistically significant in the univariate analysis. Statistical analyses were performed using SPSS (version 21.0; IBM Corporation, Armonk, NY, USA). A P value <0.05 was considered statistically significant.
Results
A total of 340 patients were included in this study. The characteristics of the patients are presented in . Their median age was 45 years (range, 24–74 years), and 65.3% were premenopausal. There were 168 (49.4%), 14 (4.1%), and 158 (46.5%) patients with IIIA, IIIB, and IIIC stage disease, respectively. There were 52.1%, 21.2%, 14.1%, and 12.6% of patients with HoR+/HER2−, HoR+/HER2+, HoR−/HER2+, and HoR−/HER2− subtypes, respectively.
Table 1 Baseline characteristics according to interval category (n = 340)
All patients underwent mastectomy, axillary lymph node dissection, adjuvant chemotherapy, and PMRT. Most of the patients (98.2%) received anthracycline-and-taxane-based chemotherapy, and the median of chemotherapy courses was 6 (range, 4–12). Patients with HoR-positive disease received endocrine therapy, and only two patients with HER2-positive disease were treated with trastuzumab-containing regimens. All patients received PMRT to the chest wall and supraclavicular lymph nodes. The internal mammary lymph nodes were not routinely irradiated.
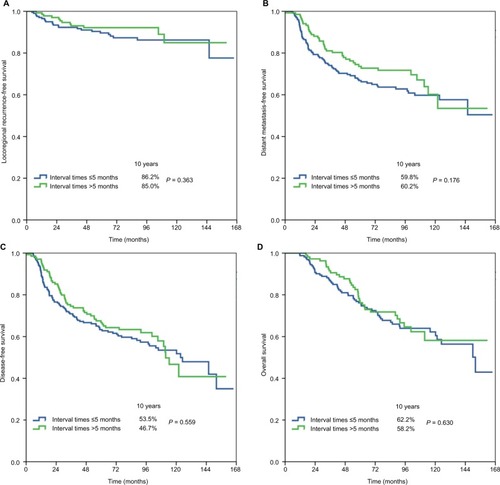
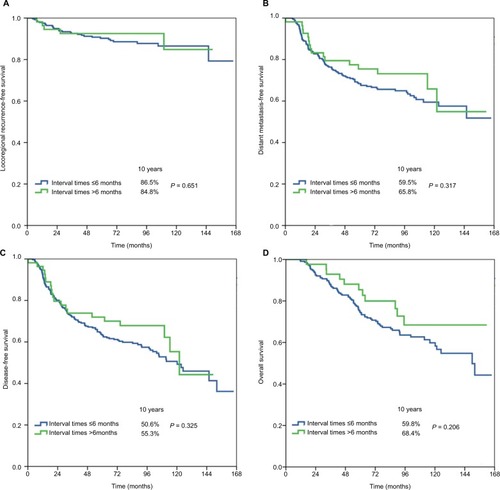
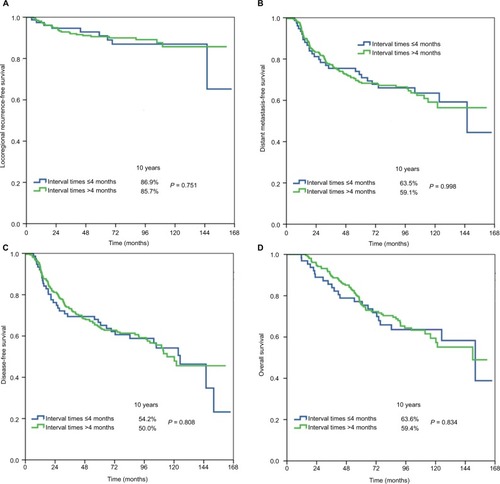
The median surgery–PMRT interval was 5 months (range, 3–15 months). Patient characteristics by surgery–PMRT interval are listed in . In Group 1 (n = 78 in ≤4 months, n = 262 in >4 months), there were no significant differences in patient characteristics between the two cohorts. In Group 2 (n = 204 in ≤5 months, n = 136 in >5 months), patients with a surgery–PMRT interval >5 months were more likely to be aged ≥50 years and receive >6 cycles of chemotherapy. In addition, in Group 3 (n = 285 in ≤6 months, n = 55 in >6 months), patients with a surgery–PMRT interval >6 months were more likely to receive >6 cycles of chemotherapy.
The median follow-up time was 79.8 months (range, 5–166 months). A total of 36 patients developed LRR, and the 10-year LRFS was 86.3%. The details on event distribution of LRR and distant metastasis are listed in . The 10-year DMFS, DFS, and OS were 60.6%, 51.5%, and 61.1%, respectively.
Table 2 The details on event distribution between groups
shows the univariate analyses of survival outcomes. The surgery–PMRT interval including Group 1, Group 2, and Group 3 was not significantly associated with LRFS, DMFS, DFS, or OS. In addition, in the subgroup analysis of the effect of surgery–PMRT interval on survival outcomes according to various clinicopathologic factors, the surgery–PMRT interval was also not associated with survival outcomes in different age groups, tumor stage, and BCS. The survival curves in Group 1, Group 2, and Group 3 are shown in –, respectively. The BCS was the independent significant predictor for LRFS, menopausal status, tumor stage, and BCS were the independent significant predictors for DMFS and DFS, while age, tumor stage, and BCS were the independent significant predictors for OS.
Table 3 Univariate Cox regression analysis of prognostic factors
Figure 1 Locoregional recurrence-free survival (A), distant metastasis-free survival (B), disease-free survival (C), and overall survival (D) in Group 1 (≤4 vs >4 months).
Discussion
In this study, we assessed the long-term survival effect of the interval between surgery and PMRT in women with locally advanced breast cancer. Our results showed that the delay in the start of PMRT in locally advanced breast cancer is not related to an increased risk of LRR, distant recurrence, or death.
Reportedly, ~50% of patients experience delayed postoperative radiotherapy.Citation17 For ethical reasons, it is impossible to conduct a randomized controlled trial to assess the effect of the surgery–radiotherapy interval on survival outcomes in patients with breast cancer. Hence, the optimal surgery–radiotherapy interval remains unclear. Theoretically, the risk of LRR or distant recurrence is related to the residual tumor burden in the surgical bed. Therefore, a long interval before PMRT after breast surgery may increase the likelihood of residual tumor growth and the development of radioresistance, consequently leading to a poorer outcome.Citation18,Citation19
Controversy continues to surround the surgery–radiotherapy interval in patients with breast cancer who receive breast-conserving surgery. In the International Breast Cancer Study Group trials, the timing of radiotherapy after breast-conserving surgery was not significantly associated with the LRFS, DFS, and OS of patients who received initial chemotherapy or endocrine therapy.Citation10,Citation11 Two population-based cohort studies also found that starting radiotherapy shortly after breast-conserving surgery did not improve the long-term survival outcomes of patients with or without chemotherapy.Citation13,Citation14 However, a meta-analysis indicated that delayed radiotherapy after breast-conserving surgery was associated with a significantly higher risk of LRR.Citation12 In addition, a population-based study of the Surveillance Epidemiology and End Results Program that included 18,050 women with stage 0–II disease after breast-conserving surgery and radiotherapy (but not chemotherapy) showed that an interval >6 weeks was associated with an increased likelihood of LRR.Citation5 These results suggest that radiotherapy should be initiated as soon as possible after breast-conserving surgery in patients not receiving chemotherapy.
Approximately 30% of locally advanced breast cancer may develop LRR after mastectomy, but the administration of PMRT may reduce LRR and decrease the incidence of systemic relapse.Citation1–Citation3 The optimal interval between mastectomy and PMRT in patients with locally advanced disease remains unclear. However, several retrospective studies have shown that the interval between mastectomy and PMRT in patients with locally advanced breast cancer is not associated with survival outcomes. Kim et alCitation20 after examining 275 patients with stage I–IIIB disease treated with chemotherapy and PMRT found that delaying the start of PMRT (≤2 vs >2 months and ≤6 vs >6 months) did not affect LRR or survival outcomes. Metz et alCitation21 assessed 221 patients with locally advanced breast cancer who received mastectomy and showed that delayed PMRT did not adversely affect LRR at 8 years. The rates of LRR at 8 years were 13%, 4%, and 12% in patients with surgery–PMRT intervals of ≤2, 2.1–6, and >6 months, respectively (P = 0.51).Citation21 Desai et alCitation22 examined 248 patients and demonstrated no significant effect of the surgery–PMRT interval on LRR in patients who underwent neoadjuvant chemotherapy and mastectomy.
The participants of this study were classified based on the interval between surgery and the initiation of PMRT. Our findings indicated that the survival benefit of PMRT is maintained up to 6 months. Our findings were similar to those of the aforementioned studies.Citation20–Citation22 That is, the survival outcomes and patterns of treatment failure did not differ according to the interval between mastectomy and PMRT. The German Society for Radiooncology recommends that PMRT should be started 4–6 weeks after surgery or completion of the primary or adjuvant chemotherapy.Citation23 Although the optimal interval between mastectomy and PMRT cannot be determined from the available evidence, patient anxiety is related to the waiting time for PMRT; thus, we propose that patients start PMRT as soon as possible after mastectomy.
Delayed administration of PMRT can result from various social and patient factors, including a lack of adequate equipment and personnel, treatment at cancer centers, a desire for breast reconstruction, and the problem of wound healing, which lead to long waiting lists.Citation24 Although timely access to PMRT remains a priority for all centers, the clinical efficacy of PMRT is maintained even when the start of PMRT is delayed. PMRT should still be administered to patients who have experienced a delay but have not developed LRR.Citation22
The primary limitation of our study is its retrospective nature from a single institution; however, the effect of the surgery–radiotherapy interval on survival is a subject ethically inappropriate for randomized studies. Therefore, retrospective studies are a reasonable choice to confirm the impact of the surgery–radiotherapy interval on breast cancer recurrence. In addition, this study was limited by its small sample size and limited follow-up time. Moreover, most of the patients with HER2-positive disease did not receive trastuzumab-based treatment, which was the standard of care in the modern series; therefore, the results of this study are less applicable to the general population of breast cancer patients.
Conclusion
In conclusion, our results suggest that the delay in the start of PMRT in locally advanced breast cancer does not increase the likelihood of LRR, distant metastasis, and death.
Acknowledgments
This work was partly supported by the Natural Science Foundation of Fujian Province (No. 2016J01635), and the Science and Technology Planning Projects of Xiamen Science & Technology Bureau (No. 3502Z20174070), and Guangdong Medical Research Foundation (No. A2017023). The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit (RDD) public platform (www.researchdata.org.cn), with the approval RDD number as RDDA2018000693.
Disclosure
The authors report no conflicts of interest in this work.
References
- EBCTCG (Early Breast Cancer Trialists’ Collaborative Group)McGalePTaylorCEffect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trialsLancet201438399352127213524656685
- OvergaardMHansenPSOvergaardJPostoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b TrialN Engl J Med1997337149499559395428
- RagazJJacksonSMLeNAdjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancerN Engl J Med1997337149569629309100
- NixonAJRechtANeubergDThe relation between the surgery-radiotherapy interval and treatment outcome in patients treated with breast-conserving surgery and radiation therapy without systemic therapyInt J Radiat Oncol Biol Phys199430117218083111
- PungliaRSSaitoAMNevilleBAEarleCCWeeksJCImpact of interval from breast conserving surgery to radiotherapy on local recurrence in older women with breast cancer: retrospective cohort analysisBMJ2010340c84520197326
- TsoutsouPGKoukourakisMIAzriaDBelkacémiYOptimal timing for adjuvant radiation therapy in breast cancer: a comprehensive review and perspectivesCrit Rev Oncol Hematol200971210211618922700
- VujovicOCherianAYuEDarARStittLPereraFThe effect of timing of radiotherapy after breast-conserving surgery in patients with positive or close resection margins, young age, and node-negative disease, with long term follow-upInt J Radiat Oncol Biol Phys200666368769016949764
- WhelanTJJulianJWrightJJadadARLevineMLDoes locoregional radiation therapy improve survival in breast cancer? A meta-analysisJ Clin Oncol20001861220122910715291
- HuangJBarberaLBrouwersMBrowmanGMackillopWJDoes delay in starting treatment affect the outcomes of radiotherapy? A systematic reviewJ Clin Oncol200321355556312560449
- KarlssonPColeBFColleoniMTiming of radiotherapy and outcome in patients receiving adjuvant endocrine therapyInt J Radiat Oncol Biol Phys201180239840220729007
- KarlssonPColeBFPriceKNTiming of radiation therapy and chemotherapy after breast-conserving surgery for node-positive breast cancer: long-term results from international breast cancer study group trials VI and VIIInt J Radiat Oncol Biol Phys201696227327927598802
- GuptaSKingWDKorzeniowskiMWallaceDLMackillopWJThe effect of waiting times for postoperative radiotherapy on outcomes for women receiving partial mastectomy for breast cancer: a systematic review and meta-analysisClin Oncol (R Coll Radiol)2016281273974927498044
- van MaarenMCBretveldRWJobsenJJThe influence of timing of radiation therapy following breast-conserving surgery on 10-year disease-free survivalBr J Cancer2017117217918828588320
- CorradiniSNiemoellerOMNiyaziMTiming of radiotherapy following breast-conserving surgery: outcome of 1393 patients at a single institutionStrahlenther Onkol2014190435235724638237
- National Comprehensive Cancer Network, Breast cancer. Version 22017 Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdfAccessed September 2, 2017
- WuSGSunJYZhouJNumber of negative lymph nodes can predict survival of breast cancer patients with four or more positive lymph nodes after postmastectomy radiotherapyRadiat Oncol2014928425511525
- BenkVMLevintonCFortinPR2055 Effect of delay in initiating radiotherapy for patients with early stage breast cancer: results of a natural experimentInt J Radiat Oncol Biol Phys1999453305306
- MackillopWJBatesJHO’SullivanBWithersHRThe effect of delay in treatment on local control by radiotherapyInt J Radiat Oncol Biol Phys199634124325012118558
- FletcherGHImplications of the density of clonogenic infestation in radiotherapyInt J Radiat Oncol Biol Phys1986129167516803531119
- KimHJKimJSChieEKNohDYBangYJHaSWThe sequencing of chemotherapy and radiotherapy in breast cancer patients after mastectomyTumori2010961283320437854
- MetzJMSchultzDJFoxKMathewsAGlickJSolinLJAnalysis of outcomes for high-risk breast cancer based on interval from surgery to postmastectomy radiation therapyCancer J20006532433011079172
- DesaiSHurleyJTakitaCImpact of surgery-radiation interval on locoregional outcome in patients receiving neo-adjuvant therapy and mastectomyBreast J201319442743023750652
- Sautter-BihlMLSouchonRBudachWDEGRO practical guidelines for radiotherapy of breast cancer II. Postmastectomy radiotherapy, irradiation of regional lymphatics, and treatment of locally advanced diseaseStrahlenther Onkol2008184734735319016032
- BelkacémiYFourquetACutuliBExpert Review Board of Nice/Saint-Paul de VenceRadiotherapy for invasive breast cancer: guidelines for clinical practice from the French expert review board of Nice/Saint-Paul de VenceCrit Rev Oncol Hematol20117929110220615725