Abstract
Aim
This study focused on improving the American Joint Committee on Cancer TNM staging system and demonstrated an improvement in prognostic accuracy and clinical management of colon cancer using the P–TNM staging system.
Patients and methods
Eligible patients (N=56,800) were identified from the Surveillance, Epidemiology, and End Results database between January 1, 2010, and December 31, 2014. The P-stage (P0 or P1) was assigned to each patient based on age at diagnosis, tumor grade, and tumor size. The outcome of interest was cancer-specific survival (CSS). The Cox proportional hazards regression analyses were used to identify independent prognostic factors and analyze the CSS probabilities of patients with colon cancer having different P–TNM stages, respectively.
Results
A total of 29,627 patients were assigned to P0-stage and 27,173 patients were assigned to P1-stage. The P1-stage was associated with a 98.1% increased risk of cancer-specific mortality (hazard ratio =1.981, 95% confidence interval =1.891–2.076, P<0.001), which was higher in patients with nonmetastatic colon cancer. The P1-stage patients had improvement in CSS compared with those in P0-stage in respective stages (P<0.001). Moreover, CSS decreased in stage I–P1 compared with stage IIA–P0 or IIIA–P0 (P<0.001), stage IIIA–P1 compared with stage IIA–P0 (P<0.001), stage IIB–P1 compared with stage IIIB–P0 or IIC–P0 (P<0.001), stage IIIB–P1 compared with stage IIC–P0 (P<0.001), and stage IIC–P1 compared with stage IIIC–P0 (P<0.001).
Conclusion
P-stage was an independent prognostic factor for colon cancer. This study strongly supported the incorporation of P-stage into the American Joint Committee on Cancer TNM staging system for a better approach to prognostication and, thus, more individualized risk-adaptive therapies in colon cancer.
Introduction
Colon cancer is one of the most commonly diagnosed cancers among both men and women in the United States.Citation1 Presently, colon cancer is staged according to a system designed by the American Joint Committee on Cancer (AJCC) that defines the prognosis in a clear manner and is thus used for clinical treatment decisions. The AJCC staging system differentiated patients on the basis of the invasion extent of primary tumor (T-stage), lymph node status (N-stage), and distant spread (M-stage). However, the TNM staging system is not perfect for the prognostic prediction and clinical management of colon cancer. The AJCC issued a request for proposals to develop staging methods based on other available information beyond the classical TNM staging.Citation2
The present study focused on improving the AJCC TNM staging system. In 1990, Kune et alCitation3 suggested that older patients with colon cancer might have worse survival compared with younger patients. In 1984, Phillips et alCitation4 reported that the tumor grade was an independent prognostic factor in large bowel cancer. Also, Kornprat et alCitation5 analyzed 359 patients with colon cancer and reported that the tumor size was significantly associated with progression-free and cancer-specific survival (CSS) and negatively impacted survival.
Moreover, many subsequent studies revealed that age at diagnosis,Citation6–Citation9 tumor grade,Citation10,Citation11 and tumor sizeCitation12,Citation13 had a strong correlation with the prognosis of colon cancer. Yet most of them focus on the prognostic significance of one single factor and no study attempt to combine the three factors together for improved prognostic prediction. Therefore, this study proposed a novel prognostic score based on 3 patient and tumor characteristics, consequently obtaining the P-stage from the prognostic score. The present study analyzed the combined value of P-stage and the TNM staging system in predicting the prognosis and clinical management.
Patients and methods
Study design and data source
The Surveillance, Epidemiology, and End Results (SEER) database is an authoritative source of information on cancer incidence and survival in the United States. This database provides a comprehensive source of population-based information including all newly diagnosed cancer cases among people residing in areas participating in the SEER program and covering approximately 28% of the US population.
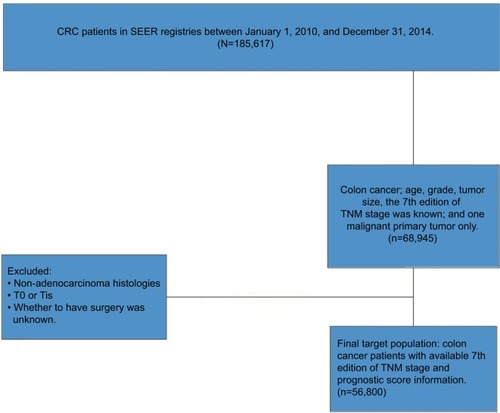
As shown in , data were obtained for 185,617 patients with a diagnosis of malignant colorectal cancer between January 1, 2010, and December 31, 2014, from the SEER program of the National Cancer Institute. Among these patients, 68,945 patients who satisfied the following inclusion criteria were identified: colon cancer, age, grade, tumor size, the seventh edition of TNM staging available, and 1 malignant primary tumor only. Patients with T0- or Tis-stage were excluded for an accurate staging. Patients with nonadenocarcinoma histology or unknown surgery status were also excluded. Finally, the target population included 56,800 colon cancer patients with diagnosis based on the seventh edition of TNM stage and 3 specific prognostic factors (age at diagnosis, tumor grade, and tumor size) available.
P-stage: risk-stratification
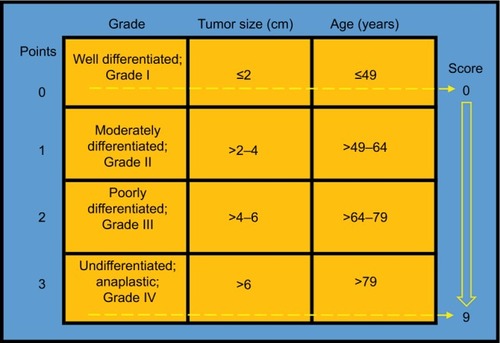
Patients were stratified based on a prognostic score incorporating 3 patient and tumor characteristics (age at diagnosis, tumor grade, and tumor size) that have been reported to influence the survival of colon cancer patients.Citation3–Citation8,Citation10–Citation13 As can be seen in , we calculated the total score with 0, 1, 2, and 3 points each given for age (≤49, >49–64, >64–79, >79 years), grade (well differentiated or grade I; moderately differentiated or grade II; poorly differentiated or grade III; undifferentiated, anaplastic or grade IV), and tumor size (≤2, >2–4, <4–6, >6 cm). The total scores ranged from 0 to 9, then a comprehensive prognostic score based on the 3 prognostic factors was obtained, with a score of 0 having the best prognosis and those with a score of 9 having the worst prognosis. These cut points were based on the prior cohort studies concerning the prognostic factors of age at diagnosis,Citation7 tumor grade,Citation11 and tumor size.Citation13 Finally, we got the P-stage of each patient according to the prognostic score – score 0–4 was assigned to P0-stage and score 5–9 was assigned to P1-stage.
Statistical analyses
Several Cox proportional hazards models were built to identify independent prognostic variables at a median survival time of 20 months (ranged 0–59 months). All the hazard ratios (HRs) are shown with 95% confidence interval (CI). The endpoint used for comparison in the present study was 59-month CSS based on selected patients with colon cancer because the longest follow-up time was 59 months, not >5 years. Variables that showed prognostic significance (log-rank, P<0.20) in the univariate analysis were included in the multivariate analysis of the selected patients. Moreover, the variables, including P-stage, TNM stage, tumor location, surgery status, histology, race, and year of diagnosis, were included in the multivariate analyses using Cox proportional hazards models. The TNM staging used in this study was the seventh edition of the AJCC cancer staging system, the newest TNM stage that could be obtained from the SEER database. This study also designed a variable called the “N–P stage,” combining the N-stage (N0-, N1-, N2a-, and N2b-stage) and the P-stage (P0 and P1, based on the prognostic score), to compare the interaction between these 2 stages in patients with nonmetastatic colon cancer. The Kaplan–Meier survival curves were used to evaluate the prognostic prediction of different factors and the log-rank tests to assess the statistical significance. A P-value <0.05 was considered statistically significant. A statistical analysis was performed using the SPSS version 22 (IBM Corporation, Armonk, NY, USA).
Ethics statement
The study was approved by the Ethical Committee and Institutional Review Board of the Fudan University Shanghai Cancer Center. The data did not include the use of human subjects or personal identifying information, and so no informed consent was required for this study.
Results
P-stage was strongly associated with the survival of colon cancer
The median follow-up time for the overall cohort was 20 months. At the end of the follow-up time, 8,841 (15.6%) patients died of colon cancer. demonstrates that higher grade, larger tumor size, and older age are associated with poorer survival, which was consistent with a prior study.Citation14 A multivariable analysis was conducted to identify the variables independently associated with CSS in the overall cohort, and it was found that the P1-stage was independently associated with 59-month CSS of 56,800 patients with colon cancer and had a 98.1% increased risk of cancer-specific mortality (HR =1.981, 95% CI =1.891–2.076, P<0.001; ). Moreover, other factors identified as independent protective factors included lower TNM stage, sigmoid colon, surgery status, adenocarcinoma histology, and later age of diagnosis. A multivariable Cox analysis was also conducted in patients with nonmetastatic colon cancer (n=50,259) selected from the overall cohort. It once again confirmed that the P1-stage was independently associated with an increased risk of CSS (HR =2.315, 95% CI =2.172–2.467, P<0.001; ) and showed a 131.5% increased risk of cancer-specific mortality in patients with nonmetastatic colon cancer (higher than that in the overall cohort), indicating that the prognostic prediction efficacy of P-stage improved in patients with AJCC stage I–III colon cancer.
Table 1 Multivariable Cox regression analyses of all independent prognostic factors
Prognostic prediction of P–TNM stage: combination of P-stage and AJCC TNM staging system
The survival curves of all P–TNM stage (AJCC TNM staging system combined with P-stage) were used to analyze the prognostic prediction of the P–TNM stage in the overall cohort (n=56,800; ). As expected, all P0-stage patients showed a statistically significant increase in the 59-month CSS compared with the P1-stage patients (P<0.001) in the respective AJCC TNM stages. Moreover, as also shows, an increased or similar 59-month CSS of stage P0–TNM patients compared with stage P1–TNM patients with higher AJCC stages was observed. A decreased CSS was also found in stage I–P1 patients compared with stage IIIA–P0 or IIA–P0 patients (P<0.001), stage IIIA–P1 patients compared with stage IIA–P0 patients (P<0.001), stage IIB–P1 patients compared with stage IIIB–P0 or IIC–P0 patients (P<0.001), stage IIIB–P1 patients compared with stage IIC–P0 patients (P<0.001), and stage IIC–P1 patients compared with stage IIIC–P0 patients (P<0.001). Thus, a considerable overlap existed between the Kaplan–Meier survival curves of adjacent AJCC TNM stages. The Kaplan–Meier survival curves of stages I–P0, I–P1, II–P0, II–P1, III–P0, and III–P1 also showed that the P0-stage patients had a statistically significant increase in the 59-month CSS compared with the P1-stage patients (P<0.001) in the respective AJCC TNM stages (). It was thus easily found that stage III–P0 had no significant difference from stage IIIA–P1 (Figure S1). shows that Kaplan–Meier survival curves of different TNM stages.
Figure 3 Kaplan–Meier survival curves of patients based on the P–TNM staging system.
Notes: (A) CSS of I–P0 stage, I–P1 stage, IIA–P0 stage, IIA–P1 stage, IIIA–P0 stage, and IIIA–P1 stage. (B) CSS of IIB–P0 stage, IIB–P1 stage, IIC–P0 stage, IIC–P1 stage, IIIB–P0 stage, and IIIB–P1 stage. (C) CSS of IIC–P0 stage, IIC–P1 stage, IIIC–P0 stage, IIIC–P1 stage, IV–P0 stage, and IV–P1 stage. (D) CSS of I–P0 stage, I–P1 stage, II–P0 stage, II–P1 stage, III–P0 stage, and III–P1 stage.
Abbreviation: CSS, cancer-specific survival.
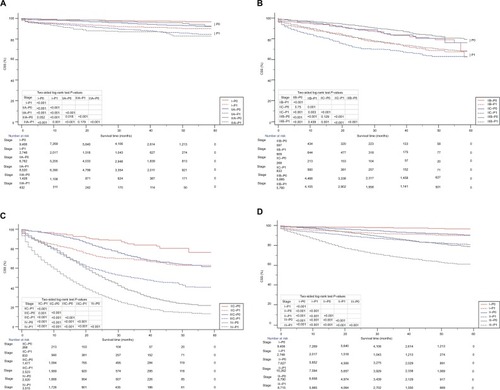
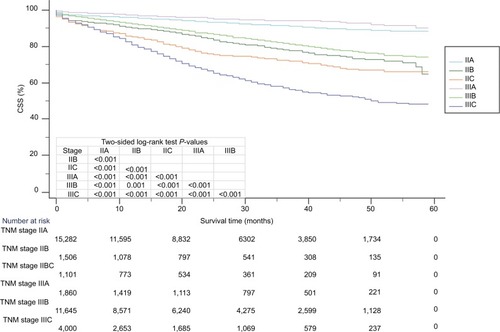
Figure 4 Kaplan–Meier survival curves of TNM staging system (including stage IIA, stage IIB, stage IIC, stage IIIA, stage IIIB, and stage IIIC).
Abbreviation: CSS, cancer-specific survival.
Multivariate Cox regression analyses were used to compare the HRs of each AJCC TNM stage and P–TNM stages. The 59-month CSS was also assigned to each P–TNM stage and TNM stage. Consistent with the Kaplan–Meier survival curves, stage P0–TNM patients showed increased 59-month CSS rates and decreased HRs compared with the respective P1–TNM stages (). Also, HRs of several stage P1–TNM patients exceeded those with stage P0–TNM, and even those with higher conventional AJCC TNM stages. The cancer-specific mortality was higher in stage I–P1 patients (HR =3.390, 95% CI =2.775–4.141) compared with stage IIA–P0 (HR =2.048, 95% CI =1.706–2.457) or IIIA–P0 patients (HR =1.445, 95% CI =1.031–2.023), stage IIIA–P1 patients (HR =5.721, 95% CI =4.192–7.808) compared with stage IIA–C0 or IIIB–P0 patients (HR =4.836, 95% CI =4.106–5.696), stage IIB–P1 patients (HR =11.180, 95% CI =9.159–13.646) compared with stage IIIB–P0 or IIC–P0 patients (HR =6.149, 95% CI =4.229–8.940), stage IIIB–P1 patients (HR =10.571, 95% CI =9.077–12.311) compared with stage IIC–P0 patients, and stage IIC–P1 patients (HR =15.022, 95% CI =12.395–18.207) compared with stage IIIC–P0 (HR =11.304, 95% CI =9.434–13.543) patients. This stage migration indicated that the P–TNM stage had a more accurate prognostic prediction than the TNM stage after the combination with P-stage. Alternatively, the P1-stage had an upstage effect that P1 patients presented higher risk of cancer-specific mortality than those P0 patients with higher TNM stages in most patients with colon cancer. The prognostic prediction efficacy was even stronger in patients with nonmetastatic colon cancer.
Table 2 Prognosis of P-stage and P–TNM stage in colon cancer
Prognosis of N-stage combined with P-stage
Multivariate Cox regression analyses were also conducted in patients with nonmetastatic colon cancer to compare the HRs of each N-stage (N0, N1, N2a, and N2b) before and after the combination of P-stage, and the 59-month CSS was also assigned to each P–N stage and N-stage (). The stage N–P0 patients showed increased 59-month CSS rates and lower HRs compared with the respective stage N–P1 patients. also shows that the number of stage N–P1 patients exceeded the number of N–P0 stage patients with the higher N stages. The cancer-specific mortality was higher in the N0–P1 stage patients (HR =2.523, 95% CI =2.241–2.817) compared with N1–P0 patients (HR =1.964, 95% CI =1.705–2.263), stage N1–P1 patients (HR =4.541, 95% CI =4.009–5.143) compared with stage N2a–P0 patients (HR =2.870, 95% CI =2.356–3.496), and stage N2a–P1 patients (HR =6.607, 95% CI =5.650–7.726) compared with stage N2b–P0 patients (HR =5.006, 95% CI =4.101–6.111). The aforementioned results indicated that P1 patients had a significantly worse prognosis than those with N1-, N2a-, and even N2b-stage.
Table 3 Prognosis of N-stage combined with P-stage in nonmetastatic colon cancer
Discussion
The AJCC TNM staging system is the most commonly used algorithm in the clinical practice of colon cancer. However, the TNM stage considers only the invasion extent of primary tumor (T-stage), lymph node status (N-stage), and distant spread (M-stage) without considering other factors that influence the prognosis of colon cancer.Citation15 This was not perfect for prognostic prediction, although several modifications in the past years had improved its predictive ability. AJCC had issued a request for staging methods based on other available information beyond the conventional TNM staging system.Citation2 Therefore, a more comprehensive staging that included other demographic and clinicopathologic variables known to impact the survival was urgently needed.
Previous studies showed that age at diagnosis,Citation3,Citation6–Citation9 tumor grade,Citation4,Citation10,Citation11 and tumor sizeCitation5,Citation12,Citation13 had a strong correlation with the prognosis of colon cancer. For example, Saha et alCitation13 found that the 5-year overall survival was 66%, 52%, 46%, and 41% in the subgroups with tumor sizes of 0–2, >2–4, >4–6, and >6 cm, respectively. In 2012, Patel et alCitation7 reported that the oldest age group (>80 years old) had a 238% increased overall mortality compared with the youngest age group (18–49 years). In 2011, Weiser et alCitation2 developed prognostic models (incorporating T-stage, N-stage, numbers of positive lymph nodes, numbers of total lymph nodes, age, gender, and tumor grade) that outperformed the current AJCC TNM staging system. Considering that HRs of male and female patients with colon cancer were not significantly different () and the numbers of positive lymph nodes and total lymph nodes had some overlap with the N-stage, these were not included in the P–TNM stage in this study.
In the present large, representative, population-based study, the AJCC TNM staging system was extended to include patient- and tumor-related variables of age of diagnosis, tumor grade, and tumor size, which are routinely available from the SEER database, and the 59-month CSS of each P–TNM stage, and thus a combination of the newly proposed P-stage and TNM stage was analyzed. The present study confirmed that all P1-stage patients had a statistically significant increase in mortality compared with the P0-stage patients with the same TNM stage. Also, the 98.1% increased HR in the overall cohort and 131.5% increased HR in the patients with nonmetastatic colon cancer also proved that the P1-stage greatly increased the 59-month cancer-specific mortality. The study also showed that several P1–TNM stages even exceeded the P0–TNM stages with higher AJCC TNM stages. The better prognosis of patients with several node-positive stages (stage IIIA–P0, IIIA–P1, or IIIB–P0) than that of several node-negative stages (stage IIC–P0, IIB–P0, IIA–P1, or IIA–P0) seemed to explain that a part of node-negative patients had a bad prognosis and that not all patients with node-positive status had a poor prognosis.Citation16,Citation17 Besides, this study showed that the P1-stage patients had a worse prognosis than the N1-, N2a-, and even N2b-stage patients, indicating that the P1-stage might be a more powerful predictor of worse prognosis compared with the node-positive status. Given the benefits of chemotherapy in node-positive patients,Citation18,Citation19 the P1-stage in this study was of great significance in indicating the use of chemotherapy. Also, in the analyses of P–TNM stage, it could be seen that the stage I (T1–T2N0M0)–P1 had a worse prognosis than stage IIIA (T1–T2N1M0)–P0. Considering almost the same in the T-stage (T1–T2), the P1-stage was once again proved to be stronger than the N1-stage for indicating a poor prognosis. However, in the clinical treatment today, stage IIIA patients are treated with adjuvant chemotherapy, while stage I patients are not.Citation20 Therefore, this study took into account the possibility of under treatment in the TNM stage I colon cancer and over-treatment in the TNM stage IIIA colon cancer. Fortunately, the P-stage could distinguish well between stages I–P0 and I–P1 in the TNM stage I, and extremely well between stages IIIA–P0 and IIIA–P1 in the TNM stage IIIA (which also seemed to account for the better prognosis of TNM stage IIIA than stage IIIA).Citation2,Citation21 Moreover, toxicity and adverse events caused by adjuvant chemotherapy could result in significant patient morbidity.Citation22 The present study suggested that the recommendation of reduced chemotherapy in stage IIIA–P0 deserves further investigation and prospective studies with the incorporation of the newly proposed P-stage. Therefore, this study strongly supported adding the P-stage into the conventional TNM staging system to generate a more refined, risk-adapted stage and thus guide the clinical treatment of colon cancer.
The adjuvant chemotherapy of TNM stage II has long been studied. At present, it has been widely accepted that patients with TNM stage II with any of high-risk factors, such as T4-stage, obstruction, perforation, poorly differentiated histology, <12 lymph nodes, presence of lymphovascular or perineural invasion, or positive margins,Citation11,Citation23–Citation26 might be considered as candidates for adjuvant chemotherapy. In 2004, the American Society of Clinical Oncology recommended the use of adjuvant chemotherapy, especially for patients with high-risk TNM stage II colon cancer, despite adequate indirect evidence of benefit.Citation18 The guidelines published by the European Society for Medical Oncology also recommend adjuvant chemotherapy for the high-risk stage II colon cancer despite insufficient scientific evidence supporting the effectiveness of adjuvant chemotherapy in this group of patients.Citation27 However, in 2011, O’Connor et alCitation24 reported that patients with stage II colon cancer with any high-risk factors (including obstruction, perforation, emergent admission, T4-stage, resection of fewer than 12 lymph nodes, and poor histology) did not get substantial survival benefit from adjuvant chemotherapy. In 2016, Verhoeff et alCitation28 showed similar results after analyzing 4,940 patients with high-risk stage II colon cancer (pT4, poor/undifferentiated grade, emergency surgery, and/or <10 evaluated lymph nodes). Given that high risks did not include the tumor size and age at diagnosis, this study considered that the P-stage might improve this situation.
Moreover, 1 distinct advantage of the simple and convenient P-stage was that the 3 prognostic factors (including age at diagnosis, tumor grade, and tumor size) were readily available and could even be specified preoperatively (tumor size known by colonoscopy, and then the colonoscopy biopsy to specify the tumor grade). After combining with the preoperative TNM stage, the P–TNM stage could be obtained, which is a more refined stage for better predicting the prognosis and guiding preoperative treatment for patients with colon cancer.
This study still had several limitations. First, the P–TNM stage did not take into account other prognostic factors, including the microsatellite instability status, treatment, carcinoembryonic antigen level, and so on. This could independently affect the survival,Citation19,Citation29,Citation30 indicating that the P–TNM stage is not perfect and needs further improvement. Anatomical staging might be less important compared with the treatment factors (patients who received adjuvant therapy and the specific regimens of the therapy). Whether the stage IIIA–P0 had a good prognosis on account of biological characteristics (discriminated by P-stage) or treatment effect (adjuvant therapy) still remains unknown. However, the P1-stage might have a worse prognosis compared with the N2a- and N2b-stage in node-positive patients, and this study still recommended less chemotherapy in the stage IIIA–P0 patients. Second, the overall cohort incorporated 56,800 patients from the SEER database; hence, the sample size still needs to be enlarged. The longest follow-up time was only 59 months, not exceeding 5 years. Besides, the analyses were merely based on retrospective data. Therefore, prospective clinical studies concerning P-stage need to be carried out for more sensitive prognosis prediction compared with N-stage and prognosis discrimination of each TNM stage.
Conclusion
The newly proposed P-stage, which is easily available even before performing operation on patients, explains the lack of clear ranking by stage in predicting outcomes using the conventional TNM stage. The present study strongly supported the incorporation of P-stage into the AJCC TNM stage (ie, the P–TNM stage) for a better approach to prognostication and, thus, more individualized risk-adaptive therapies.
Acknowledgments
This research was supported by the National Science Foundation of China (No. 81702353 and 81772599) and Shanghai Municipal Natural Science Foundation (17ZR1406400). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We have great respect for the work that goes into compiling and maintaining the SEER tumor registries, including the interpretation and reporting of these data and so on.
Supplementary materials
Figure S1 Kaplan–Meier survival curves of II–P0 stage, II–P1 stage, III–P0 stage, IIIA–P1 stage, IIIB–P1 stage, and IIIC–P1 stage.
Abbreviation: CSS, cancer-specific survival.
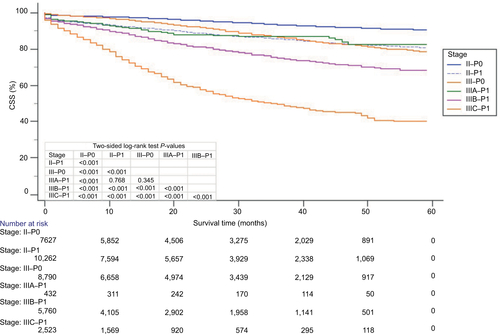
Table S1 Multivariable Cox regression analyses of all independent prognostic factors (including tumor size, tumor grade, and age of diagnosis)
Table S2 Multivariable Cox regression analyses of all independent prognostic factors in nonmetastatic colon cancer patients
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDFedewaSAColorectal cancer statistics, 2014CA Cancer J Clin201767317719328248415
- WeiserMRGönenMChouJFKattanMWSchragDPredicting survival after curative colectomy for cancer: individualizing colon cancer stagingJ Clin Oncol201129364796480222084366
- KuneGAKuneSFieldBSurvival in patients with large-bowel cancer. A population-based investigation from the Melbourne Colorectal Cancer StudyDis Colon Rectum199033119389462226081
- PhillipsRKHittingerRBlesovskyLFryJSFieldingLPLarge bowel cancer: surgical pathology and its relationship to survivalBr J Surg19847186046106743980
- KornpratPPollheimerMJLindtnerRASchlemmerARehakPLangnerCValue of tumor size as a prognostic variable in colorectal cancer: a critical reappraisalAm J Clin Oncol2011341434920101166
- AquinaCTMohileSGTejaniMAThe impact of age on complications, survival, and cause of death following colon cancer surgeryBr J Cancer2017116338939728056465
- PatelSSNelsonRSanchezJElderly patients with colon cancer have unique tumor characteristics and poor survivalCancer2013119473974723011893
- YamanoTYamauchiSKimuraKInfluence of age and comorbidity on prognosis and application of adjuvant chemotherapy in elderly Japanese patients with colorectal cancer: a retrospective multicentre studyEur J Cancer2017819010128622612
- MulcahyHEPatchettSEDalyLO’DonoghueDPPrognosis of elderly patients with large bowel cancerBr J Surg19948157367388044567
- CerottiniJPCaplinSPampallonaSGivelJCPrognostic factors in colorectal cancerOncol Rep19996240941410023012
- GillSLoprinziCLSargentDJPooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much?J Clin Oncol200422101797180615067028
- SahaSKanaanMNShaikMTumor size as a prognostic factor for patients with colon cancer undergoing sentinel lymph node mapping and conventional surgeryJ Clin Oncol2013314_suppl546
- SahaSShaikMJohnstonGTumor size predicts long-term survival in colon cancer: an analysis of the National Cancer Data BaseAm J Surg2015209357057425601557
- SagaraYFreedmanRAVaz-LuisIPatient Prognostic Score and Associations With Survival Improvement Offered by Radiotherapy After Breast-Conserving Surgery for Ductal Carcinoma In Situ: A Population-Based Longitudinal Cohort StudyJ Clin Oncol201634111190119626834064
- ComptonCFenoglio-PreiserCMPettigrewNFieldingLPAmerican Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working GroupCancer19998611243610590388
- O’ConnellJBMaggardMAKoCYColon cancer survival rates with the new American Joint Committee on Cancer sixth edition stagingJ Natl Cancer Inst200496191420142515467030
- WeiserMRLandmannRGKattanMWIndividualized prediction of colon cancer recurrence using a nomogramJ Clin Oncol200826338038518202413
- BensonAB3rdSchragDSomerfieldMRAmerican Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancerJ Clin Oncol200422163408341915199089
- GanapathiAMSpeicherPJEnglumBRAdjuvant chemotherapy for T1 node-positive colon cancers provides significant survival benefitDis Colon Rectum201457121341134825379998
- ThirunavukarasuPSukumarSSathaiahMC-stage in colon cancer: implications of carcinoembryonic antigen biomarker in staging, prognosis, and managementJ Natl Cancer Inst2011103868969721421861
- ArenaEABilchikAJWhat is the optimal means of staging colon cancer?Adv Surg201347119921124298852
- SchmollHJCartwrightTTaberneroJPhase III trial of capecitabine plus oxaliplatin as adjuvant therapy for stage III colon cancer: a planned safety analysis in 1,864 patientsJ Clin Oncol200725110210917194911
- CasadabanLRauscherGAkliluMVillenesDFreelsSMakerAVAdjuvant chemotherapy is associated with improved survival in patients with stage II colon cancerCancer2016122213277328727417445
- O’ConnorESGreenblattDYLoConteNKAdjuvant chemotherapy for stage II colon cancer with poor prognostic featuresJ Clin Oncol201129253381338821788561
- QuahHMChouJFGonenMIdentification of patients with high-risk stage II colon cancer for adjuvant therapyDis Colon Rectum200851550350718322753
- SargentDJGoldbergRMJacobsonSDA pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patientsN Engl J Med2001345151091109711596588
- LabiancaRNordlingerBBerettaGDEarly colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-upAnn Oncol201324Suppl 6vi64vi7224078664
- VerhoeffSRvan ErningFNLemmensVEde WiltJHPruijtJFAdjuvant chemotherapy is not associated with improved survival for all high-risk factors in stage II colon cancerInt J Cancer2016139118719326914273
- WolmarkNFisherBWieandHSThe prognostic significance of preoperative carcinoembryonic antigen levels in colorectal cancer. Results from NSABP (National Surgical Adjuvant Breast and Bowel Project) clinical trialsAnn Surg198419943753826370155
- RothADDelorenziMTejparSIntegrated analysis of molecular and clinical prognostic factors in stage II/III colon cancerJ Natl Cancer Inst2012104211635164623104212