Abstract
Purpose
To discuss new therapeutic strategies for chemotherapy-induced nausea and vomiting (CINV) involving 5-hydroxytryptamine type 3 (5HT3)-receptor antagonists (RAs).
Summary
CINV remains poorly controlled in patients receiving moderately emetogenic chemotherapy (MEC) or highly emetogenic chemotherapy (HEC); nausea and delayed-phase CINV (24–120 hours after chemotherapy) are the most difficult to control. National Comprehensive Cancer Network (NCCN) and American Society of Clinical Oncology (ASCO) antiemesis-guideline recommendations for HEC include a four-drug regimen (5HT3 RA, neurokinin 1 [NK1] RA, dexamethasone, and olanzapine). For some MEC regimens, a three-drug regimen (5HT3 RA, NK1 RA, and dexamethasone) is recommended. While 5HT3 RAs have dramatically improved CINV in the acute phase (0–24 hours after chemotherapy), their efficacy declines in the delayed phase. Newer formulations have been developed to extend 5HT3-RA efficacy into the delayed phase. Granisetron extended-release subcutaneous (GERSC), the most recently approved 5HT3 RA, provides slow, controlled release of therapeutic granisetron concentrations for ≥5 days. GERSC is included in the NCCN and ASCO guidelines for MEC and HEC, with NCCN-preferred status for MEC in the absence of an NK1 RA. Efficacy and safety of 5HT3 RAs in the context of guideline-recommended antiemetic therapy are reviewed.
Conclusion
Recent updates in antiemetic guidelines and the development of newer antiemet-ics should help mitigate CINV, this dreaded side effect of chemotherapy. GERSC, the most recently approved 5HT3-RA formulation, is indicated for use with other antiemetics to prevent acute and delayed nausea and vomiting associated with initial and repeat courses of MEC and anthracycline–cyclophosphamide combination-chemotherapy regimens.
Plain-language summary
This review article discusses different treatments that are used to prevent nausea and vomiting (NV) experienced by people with cancer who are being treated with chemotherapy: chemotherapy-induced NV (CINV). The article is about chemotherapy treatments that are the most likely to cause NV in people with cancer, called moderately emetogenic chemotherapy (MEC) and highly emetogenic chemotherapy (HEC). Guidelines from major medical organizations on how to prevent CINV are compared, and the problems in preventing CINV are discussed. Different types of drugs used to treat CINV and different combinations of these drugs are described. Even though several types of drugs are available to prevent CINV, many patients with cancer still suffer from NV. The advantages and disadvantages of drugs used to treat CINV are explained, and information about recently approved new drugs and new forms of existing drugs is provided. The article mainly describes a class of drugs called 5HT3-receptor antagonists, which are often used to prevent nausea and vomiting. New drugs and new combinations of drugs may help control CINV in people with cancer who are treated with chemotherapy.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) adversely affects patient health,Citation1,Citation2 quality of life,Citation3,Citation4 and chemotherapy compliance,Citation5 and is one of the most dreaded effects of chemotherapy.Citation6 This review focuses on current approaches and future directions involving 5-hydroxytryptamine type 3 (5HT3)-receptor antagonists (RAs) for the management of CINV. Use of 5HT3 RAs in the prevention of radiotherapy-induced and chemoradiation-induced NV is discussed briefly.
Chemotherapeutics differ in their emetogenicity. In the absence of antiemetic prophylaxis, agents associated with >90% risk of emesis are classified as highly emetogenic chemotherapy (HEC) and those associated with 30%–90% risk of emesis classified as moderately emetogenic chemotherapy (MEC).Citation7–Citation9 Several oral targeted anticancer therapies, such as crizotinib, lenvatinib, olaparib, and panobinostat, are also recognized as being highly or moderately emetogenic.Citation7 The emetogenicity of combination regimens is determined by identifying the most emetogenic agent in the combination, then assessing the relative contribution of the other agents.Citation7,Citation8 This review discusses treatment options for the prevention of CINV associated with MEC and HEC, as these are the most challenging settings; it does not discuss management of CINV associated with agents that have low-to-minimal emetogenicity.
In clinical trials, measurement of antiemetic efficacy covers a range of end points, involving emesis, nausea, and assessments of rescue medication use for breakthrough CINV (ie, CINV occurring despite the use of prophylactic anti-emetics). A commonly used end point is complete response (CR; no emesis and no rescue medication use) during the acute phase (first 24 hours), delayed phase (>24–120 hours), and overall phase (0–120 hours) following chemotherapy administration. End points that also specifically involve nausea include complete control (CC; CR and only mild nausea), total response (CR and no nausea), and no nausea or no significant nausea (typically measured as <25 mm on a 100 mm visual analogue scale).
Despite considerable advances in antiemetics over the past 20 years, beginning with the introduction of the 5HT3 RAs, prevention of CINV remains suboptimal. For example, one study in a community hospital setting found that chemotherapy-induced nausea and vomiting was reported in approximately 50% and up to a quarter of patients, respectively, despite antiemetic prophylaxis.Citation10 Delayed CINV seems to be less well managed than acute CINV. In a US prospective study, 34% of patients reported experiencing acute CINV, whereas 58% of patients reported experiencing delayed CINV.Citation4 Both nausea and emesis appear to be more prevalent during the delayed phase than in the acute phase of CINV.Citation1 Lack of adequate CINV control has multiple adverse consequences. The negative effects of CINV on patient health and quality of life are well known, including reducing patient daily functioning, increasing patient anxiety and depression, and decreasing the patient’s chemotherapy treatment adherence, leading to chemotherapy reductions or delays.Citation1,Citation3,Citation5,Citation10–Citation12 In addition, the costs of poorly controlled CINV are considerable. Analysis of data from 2018 working-age patients in a large US employment database with information from 45 large employers and more than 100 health-insurance payers (1997–2002) found that 28% of patients had uncontrolled CINV, and the associated monthly medical costs for these patients were approximately US$1,300 higher and indirect costs US$433 higher than for patients whose CINV was well controlled.Citation13 Using another database of more than 19,000 patients, 14% of patients had a CINV-associated hospital visit (inpatient, outpatient, or emergency room), and the mean cost per visit was US$4,043.Citation14 Therefore, prevention of CINV, especially delayed CINV, is critical in patients receiving chemotherapy, for both economic reasons and patient welfare.
Most 5HT3-RA trials have focused on emesis control, using CR as a primary end point and nausea-control measures as secondary end points. Although emesis control has improved using new classes of antiemetics, the management of nausea, particularly in the delayed phase following HEC, is difficult. However, recent clinical trial focus has turned toward nausea control and the use of different classes of agents to prevent nausea. In a recent Phase III placebo-controlled trial of the antipsychotic olanzapine plus a three-drug antiemetic regimen (a neurokinin 1 [NK1] RA plus a 5HT3 RA plus dexamethasone) in patients receiving HEC, nausea prevention was the primary end point. The proportion of patients with no nausea was significantly higher with the addition of olanzapine across acute, delayed, and overall phases of CINV.Citation15
Granisetron (Kytril; Roche Laboratories Inc., Nutley, NJ, USA [oral] and Genentech, San Francisco, CA, USA [intravenous]), ondansetron (Zofran; GlaxoSmithKline, Research Triangle, NC, USA), and palonosetron (Aloxi; Eisai, Woodcliff Lake, NJ, USA), are the most commonly used 5HT3 RAs and the focus of this review. Dolasetron IV (Anzemet; Sanofi-Aventis, Bridgewater, NJ, USA) is no longer approved in the USA to prevent CINV, because of potential cardiac effects. Other first-generation 5HT3 RAs include azasetron, ramosetron, and tropisetron; however, they are not commercially available in the USA.Citation11,Citation16 This review discusses new therapeutic strategies involving 5HT3 RAs to improve management of delayed CINV following HEC and challenges associated with nausea prevention.
Antiemetic-guideline recommendations
The goal of CINV prophylaxis is to prevent NV following MEC or HEC across both acute and delayed phases. Because CINV is multifactorial, antiemetic prophylaxis often involves agents targeting neurotransmitters with complementary mechanisms of action, such as 5HT3 RAs, NK1 RAs, and the atypical antipsychotic olanzapine. Several antiemesis guidelines for clinical practice are available, based on clinical evidence and expert consensus, from the National Comprehensive Cancer Network (NCCN),Citation7 Multinational Association for Supportive Care in Cancer (MASCC) and European Society of Medical Oncology (ESMO),Citation17,Citation18 and American Society of Clinical Oncology (ASCO; Citation7,Citation17–Citation19).Citation19
Table 1 Antiemesis-guideline recommendations for CINV prevention following HEC or MEC
However, despite the availability of comprehensive CINV-prevention guidelines and a wide range of antiemetics, guideline adherence remains low. The INSPIRE study, involving 1,295 patients from US community practices, found that guideline-consistent CINV prophylaxis was administered in only 57% of patients.Citation20 Corticosteroids were commonly omitted in the delayed phase in patients receiving HEC, as were NK1 RAs on day 1 in patients receiving MEC regimens, although NK1 RAs are not uniformly recommended with MEC in antiemetic guidelines.Citation7,Citation17–Citation20 Data from the Pan European Emesis Registry of 991 patients found that guideline-consistent CINV prophylaxis during single-day chemotherapy was administered in only 55% of patients in the acute phase and 46% of patients in the delayed phase, and that guideline-consistent CINV prophylaxis significantly reduced the incidence of CINV compared with guideline-inconsistent prophylaxis.Citation21 More recently, in a retrospective analysis of a US claims database, of 1,059 patients receiving MEC or HEC, those who were given a guideline-recommended three-drug antiemetic regimen of an NK1 RA plus a 5HT3 RA plus dexamethasone had fewer CINV-related emergency-department visits (9%) than patients receiving a two-drug regimen of a 5HT3 RA plus dexamethasone (15%) and had fewer CINV-related hospitalizations (4% vs 6%, respectively).Citation22 Poor patient adherence to antiemetic regimens, especially to prescribed oral antiemetics, may also be a barrier to effective CINV control, as reported in a multinational survey of 2,388 health-care providers.Citation5 Clearly, patients experiencing NV may be reluctant to take oral medications, even if they are prescribed to control CINV, and many patients undergoing chemotherapy have oral mucositis, which reduces their ability to swallow.Citation23
Highly emetogenic chemotherapy
For the prevention of CINV associated with single-day HEC, the NCCN and MASCC/ESMO guidelines are in agreement in recommending a three-drug regimen of a 5HT3 RA plus an NK1 RA plus a steroid (typically dexamethasone) on day 1 of chemotherapy, followed by dexamethasone on the subsequent 2 or 3 days, with an oral NK1 RA on days 2 and 3 only if an oral NK1 RA is given on day 1 (). The 2017 ASCO and 2018 NCCN guidelines recommend a four-drug regimen that incorporates olanzapine on day 1, in addition to the three-drug regimen just mentioned above, followed by dexamethasone on days 2 and 3, and an oral NK1 RA on days 2 and 3 only if an oral NK1 RA is given on day 1; this is also an option in the NCCN guidelines.Citation7,Citation19 The NCCN also recommends olanzapine plus palonosetron and dexamethasone as an alternative option on day 1,Citation7 and MASCC/ESMO guidelines recommend olanzapine plus a 5HT3 RA plus dexamethasone for nausea control, but give a low-grade recommendation for this regimen.Citation7,Citation17
Since 2011, the high emetogenicity of the combination of an anthracycline and cyclophosphamide (AC) has been recognized by the NCCN, ASCO, and MASCC/ESMO. AC-based regimens were reclassified from MEC to HEC in the ASCO antiemesis guidelines, and AC-based chemotherapy for patients with breast cancer has a distinct recommendation for CINV prevention within the HEC category in the MASCC/ESMO guidelines.Citation7,Citation17,Citation19 Consequently, the ASCO-recommended antiemetic regimen for AC-based regimens is now the same as that recommended by NCCN guidelines for any HEC regimen: primarily a four-drug combination of a 5HT3 RA, dexamethasone, an NK1 RA, and olanzapine on day 1, with olanzapine on days 2–4 and dexamethasone on days 2–4 depending on the NK1 RA administered on day 1.Citation7,Citation19 The MASCC/ESMO guidelines recommend the three-drug regimen for AC-based chemotherapy on day 1 for acute CINV, with aprepitant (if aprepitant was given on day 1) or dexamethasone on days 2 and 3 to prevent delayed CINV. However, if any NK1 RA was given other than aprepitant on day 1, MASCC/ESMO guidelines state that no other treatment is necessary on subsequent days.Citation17
Carboplatin
Although carboplatin is classified as moderately emetogenic in MASCC/ESMO and ASCO guidelines,Citation17–Citation19 NCCN guidelines note that carboplatin may be highly emetogenic in some patients, depending on dose (ie, if the carboplatin area under the concentration–time curve [AUC] is ≥4 mg/mL/min), and ASCO recommends an adjusted antiemetic regimen for patients receiving carboplatin with an area under the curve ≥4 mg/mL/min.Citation7,Citation19 For patients receiving carboplatin, MASCC/ESMO guidelines recommend a three-drug antiemetic regimen of a 5HT3 RA plus an NK1 RA plus dexamethasone, similar to the regimen recommended for patients receiving HEC, with aprepitant on days 2 and 3 if given on day 1.Citation17 In a retrospective analysis of 1,059 patients in a US claims database, 73% of those treated with AC (classified as HEC) and 56% treated with cisplatin (HEC) received an antiemetic regimen containing an NK1 RA compared with 23% treated with carboplatin. However, health-care-resource utilization (eg, emergency-room visits, hospitalization) was lower in patients whose antiemetic regimen included an NK1 RA, suggesting that a three-drug antiemetic regimen may be more effective in preventing CINV in patients receiving carboplatin.Citation22
Moderately emetogenic chemotherapy
For the prevention of CINV associated with single-day MEC, all three guidelines are in agreement in recommending a two-drug regimen of a 5HT3 RA plus dexamethasone on day 1 and dexamethasone on days 2 and 3 (with some exceptions), although ASCO and MASCC/ESMO guidelines have separate recommendations for patients receiving carboplatin ().Citation7,Citation17–Citation19 The NCCN guidelines also recommend this two-drug regimen plus an NK1 RA on day 1 or a three-drug combination of olanzapine, palonosetron, and dexamethasone on day 1.Citation7 If the 5HT3 RA palonosetron, extended-release (ER) granisetron transdermal delivery system (GTDS; Sancuso, ProStrakan, Bridgewater, NJ, USA), or granisetron ER subcutaneous (GERSC; Sustol, Heron Therapeutics, Redwood City, CA, USA) is given on day 1 with dexamethasone, NCCN guidelines state that no further antiemetic therapy is necessary on days 2 and 3.Citation7 Details of recommended prophylaxis on days 2 and 3 by all guidelines are shown in . For MEC, NCCN guidelines list palonosetron and GERSC as preferred 5HT3 RAs in the absence of an NK1 RA.Citation7
Multiday chemotherapy
For multiday chemotherapy, treatment guidelines are less definitive, in part because there is a lack of evidence to support recommendations in this setting. ASCO guidelines suggest using an antiemetic agent appropriate for the emetogenic risk of the chemotherapeutic agent administered on each day and for 2 days after completion of the chemotherapy regimen, and suggest that the ER agents GTDS or GERSC may be preferable to a daily 5HT3 RA. For patients receiving multiday cisplatin regimens, the three-drug combination of an NK1 RA, 5HT3 RA, and dexamethasone is recommended.Citation19 The NCCN guidelines propose taking several factors into account when determining the appropriate antiemetic regimen, including route of administration, duration of action, and dosage regimen of the antiemetic(s); administration in an inpatient or outpatient setting; patient adherence to a regimen; and individual patient risk factors. Several general principles are proposed for each type of antiemetic agent. A 5HT3 RA should be used before each dose of MEC or HEC, and the need for subsequent doses should be based on the antiemetic agent and route of administration. Dexamethasone is recommended for each day of chemotherapy, continuing for 2–3 days afterward for HEC or MEC regimens known to have a higher risk of causing delayed emesis, but dexamethasone administration only on day 1 is an option for patients receiving MEC or noncisplatin HEC who have few CINV risk factors. An NK1 RA may be used with MEC or HEC regimens that have a significant risk of causing delayed CINV, but there are limited data on the use of NK1 RAs over multiple days.Citation7 The most recent updates to the MASCC/ESMO guidelines do not offer specific recommendations for antiemetics to prevent CINV associated with multiday HEC or MEC chemotherapy.Citation17,Citation18
Breakthrough CINV
For breakthrough CINV (ie, CINV occurring despite the use of guideline-recommended prophylactic antiemetics), ASCO and NCCN guidelines suggest adding an antiemetic agent with a different mechanism of action to that of the antiemetic(s) used during the previous chemotherapy cycle. Olanzapine may be added if it was not part of the initial antiemetic regimen, and other dopamine antagonists (eg, metoclopramide, phenothiazines) may also be considered.Citation7,Citation19 Multiple concurrent antiemetics may be required and administered continuously, rather than as needed. If a combination of a 5HT3 RA and dexamethasone with or without an NK1 RA has previously been used, other options of different classes include olanzapine, benzodiazepine, or metoclopramide.Citation7,Citation19 The use of 5HT3 RAs in the breakthrough setting is based on their efficacy as prophylactic antiemetics, as there are no published data from prospective Phase III clinical trials on the use of 5HT3 RAs to treat breakthrough CINV. The most recent updates to the MASCC/ESMO guidelines have no specific recommendations for the prevention of breakthrough CINV associated with HEC or MEC.Citation17,Citation18
Benefits and limitations of 5HT3-RA formulations
The introduction of 5HT3 RAs dramatically improved the prevention of CINV, particularly in the acute phase.Citation24 Granisetron is a potent and highly selective 5HT3 RA with little or no affinity for other serotonin receptors or for dopaminergic, adrenergic, benzodiazepine, histaminic, or opioid receptors.Citation25 In contrast, in addition to binding with 5HT3 receptors, ondansetron binds with low affinity to 5HT1B, 5HT1C, α1-adrenergic, and µ-opioid receptors.Citation26 Ondansetron is available for intravenous (IV) or oral administration only, whereas granisetron is available in a wide range of formulations (Citation25,Citation27–Citation37). Dolasetron is only available in the oral formulation in the USA for CINV prevention () due to the potential cardiac adverse events (AEs) observed with the IV formulation. Formulations of 5HT3 RAs may affect their utility. Injectable forms have an adherence advantage, whereas compliance with oral antiemetics may be suboptimal because of difficulty ingesting pills, especially for patients with head and neck cancer or those afraid to swallow pills for fear of inducing further emesis.Citation38,Citation39
Table 2 Characteristics of 5HT3-RA formulations
Extending the efficacy of 5HT3 RAs into the delayed phase: palonosetron
Palonosetron, a potent and selective 5HT3 RA, is a second-generation agent with a half-life (t½) that is longer (~40 hours)Citation40 than first-generation agents (). Additionally, in vitro research has suggested that palonosetron causes 5HT3-receptor internalization, leading to prolonged inhibition of 5HT3 RAs.Citation41 These properties (t½, binding affinity, and receptor internalization) of palonosetron are believed to form the basis of its pharmacologic activity in the acute and delayed CINV phases.Citation26,Citation41–Citation43 However, direct evidence linking biochemical properties of palonosetron with its clinical efficacy is lacking. Notably, palonosetron IV is indicated for CINV prevention in both acute and delayed phases following MEC, but only in the acute phase following HEC.Citation34 In the USA, palonosetron is available only for IV administration.Citation34 At the time of its approval in the USA, palonosetron had not been investigated in combination with an NK1 RA.
In 2014, the oral fixed-dose combination of the NK1 RA netupitant 300 mg and palonosetron 0.5 mg (NEPA; Akynzeo, Helsinn Therapeutics Inc, Iselin, NJ, USA) became available for CINV prevention in both acute and delayed phases in patients treated with MEC or HEC.Citation36 Approval was based on three clinical trials in patients receiving HEC, MEC, and repeated cycles of chemotherapy.Citation37,Citation44,Citation45 In single-cycle trials in patients receiving MEC or HEC, NEPA plus dexamethasone was significantly superior to the comparator regimen across the delayed and overall CINV phases in terms of CR.Citation44,Citation45 However, in these trials, NEPA plus dexamethasone was compared with a two-drug regimen of oral palonosetron plus dexamethasone, not the three-drug regimen recommended by all guidelines for patients receiving HEC and by some guidelines for patients receiving MEC.Citation7,Citation17–Citation19 Over multiple cycles, NEPA plus dexamethasone had similar CR rates to a three-drug regimen of aprepitant, palonosetron, and dexamethasone, but a small numerical advantage in the delayed and overall phases.Citation37 In clinical trials, the NK1 RA provided additional control of delayed CINV in combination with a 5HT3 RA, suggesting that the NK1 RA is providing the ben efit in the delayed phase.Citation46,Citation47 In fact, the NEPA prescribing information states that palonosetron prevents CINV in the acute phase and netupitant prevents CINV in the acute and delayed phases.Citation36 Since NEPA provides an NK1 RA and a 5HT3 RA as a single oral dose, it has the potential to improve the prevention of delayed CINV and improve adherence to guidelines for CINV prevention following HEC, which recommend combined use of NK1 RA and 5HT3 RA plus a corticosteroid.Citation48 However, oral administration of NEPA may pose a challenge to some patients, as discussed earlier.
Palonosetron and its place in antiemetic treatment guidelines
Antiemesis guidelines recommend palonosetron as a preferred 5HT3 RA in some settings. In patients with breast cancer receiving AC-based chemotherapy (ie, a HEC regimen), a three-drug regimen of a 5HT3 RA, dexamethasone, and an NK1 RA is recommended by the MASCC/ESMO guidelines, but palonosetron is stated as the preferred 5HT3 RA if an NK1 RA is not available.Citation17 These recommendations were based on the results of Phase III trials of palonosetron in patients receiving HEC (Citation49–Citation60).Citation49,Citation50 In patients receiving MEC or multiday chemotherapy, either palonosetron or GERSC is recommended in NCCN guidelines as a preferred 5HT3 RA in combination with dexamethasone for CINV prevention (ie, when no NK1 RA is given).Citation7 In a recent trial in 341 patients with breast cancer receiving AC-based chemotherapy (reported as an abstract), palonosetron failed to show superiority over granisetron IV (CR 62.3% vs 60.4%, P=0.8) in the delayed phase, when each agent was combined with fosaprepitant and dexamethasone.Citation61 Recently, a double-blind, randomized study comparing a three-drug palonosetron regimen with a three-drug granisetron regimen in patients receiving AC-based HEC showed no significant difference between study arms in terms of delayed-phase CR, despite again using the 0.75 mg palonosetron dose.Citation62
Table 3 Results of Phase III clinical trials evaluating antiemetic efficacy in CINV prevention following HEC
The NCCN guidelines considered two Phase III trials in the MEC setting to support palonosetron as a preferred 5HT3 RA in combination with dexamethasone to prevent CINV associated with MEC.Citation63,Citation64 However, one study in patients receiving MEC reported that palonosetron IV was noninferior to dolasetron IV, which is no longer approved by the US Food and Drug Administration (FDA) for CINV prevention because of associated cardiac adverse effects (Citation45,Citation59,Citation60,Citation63–Citation73).Citation63,Citation74 The other study demonstrated superior CR in acute and delayed CINV phases of single-dose palonosetron 0.25 mg IV compared with single-dose ondansetron 32 mg IV in patients receiving MEC ().Citation64 However, this study did not use the recommended combination of ondansetron with dexamethasone. In a subsequent Phase III trial in patients receiving HEC, palonosetron 0.25 mg IV was noninferior to a first-generation 5HT3 RA (ondansetron 32 mg IV) in acute, delayed, and overall phases. Superiority of palonosetron to ondansetron was shown only in a post hoc analysis, where CR with palonosetron was significantly higher in the delayed and overall phases compared with ondansetron when both agents were administered with dexamethasone.Citation49
Table 4 Results of Phase III clinical trials evaluating antiemetic efficacy in CINV prevention following MEC
As such, the evidence to support the preference for palonosetron in CINV prevention in these settings has been questioned, and it has been suggested that there are insufficient data to support this designation.Citation75,Citation76 Phase III trials evaluated single-dose palonosetron compared with single-dose dolasetron or ondansetron, both of which have shorter t½ than palonosetron (), so would be expected to be less effective at controlling CINV beyond day 1 of chemotherapy. Meta-analyses of CINV trials have been used to support the choice of palonosetron as the preferred 5HT3 RA in CINV prevention.Citation77,Citation78 However, such analyses may result in pooling data from trials with significant differences in study populations and dexamethasone usage, so their conclusions require validation in prospective, randomized trials.
Extending the efficacy of 5HT3 RAs into the delayed phase: granisetron
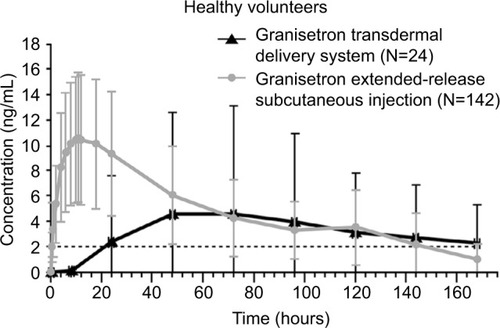
The short t½ of the first-generation 5HT3 RAs (), such as granisetron, has limited their use in CINV prevention, especially for the delayed phase. Currently, two ER formulations of granisetron are available: GTDS and GERSC (previously APF530). The GTDS patch, which is 52 cm2 in size, provides exposure similar to that of 2 mg oral granisetron. However, in contrast to oral granisetron, where maximum plasma concentration (Cmax) is reached 2 hours after administration, drug exposure with GTDS is slower, with Cmax reached 48 hours after patch application and granisetron exposure continuing over 5 days (Citation29–Citation31,Citation79).Citation79 Therefore, GTDS is a convenient option for CINV prevention, because it reduces pill burden for patients, particularly those who have difficulty in swallowing oral medication.Citation23 However, GTDS has potential drawbacks, including patch detachment,Citation80–Citation82 which may result in a lack of antiemetic efficacy, and the need for patch application 24–48 hours prior to chemotherapy, which may result in unnecessary exposure to antiemetic if the patient does not proceed with their next round of chemotherapy.Citation83
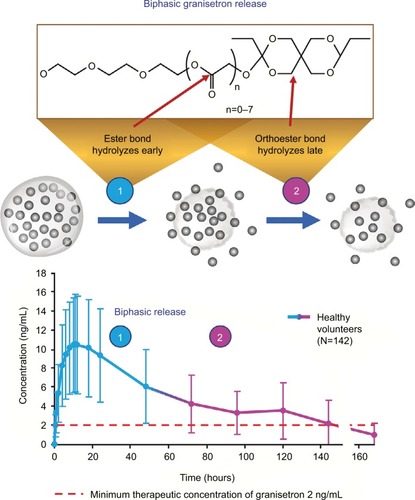
Figure 1 Extended-release formulations of granisetron: plasma granisetron concentrations following administration.
Notes: Data from these studies.Citation29–Citation31,Citation79 Dashed line indicates minimum therapeutic concentration of granisetron 2 ng/mL (data from patent application 20120258164 for granisetron transdermal delivery system).
GERSC was developed as a long-acting formulation to provide prolonged 5HT3-RA action through sustained granisetron release, and was recently FDA-approved for use in adults in combination with other antiemetics for prevention of acute and delayed NV associated with initial and repeat courses of MEC or AC-combination chemotherapy regimens.Citation29 Extended granisetron release is achieved through Biochronomer technology () using a viscous, bioerodible tri(ethylene glycol) poly(orthoester) (TEG-POE) polymer.Citation84 The POE polymer contains an orthoester linkage that is hydrolyzed to excreted fragments on exposure to an aqueous environment. The acid-labile nature of the TEG-POE polymer allows granisetron release by diffusion from the depot followed by controlled polymer hydrolysis, resulting in further release of granisetron.Citation85 In healthy subjects, granisetron is released from the polymer over an extended period (Cmax occurring approximately 10 hours after administration) (). The biphasic drug release from the polymer provides granisetron exposure into the delayed phase of CINV, with mean plasma concentration of 3.5 ng/mL (range 0–14 ng/mL) 5 days after a single 10 mg dose ().Citation29 Following a single SC injection of GERSC to patients with cancer, time to Cmax was reported as occurring approximately 24 hours postdose, and the t½ of granis-etron was 26–34 hours.Citation30 The difference in reported time to Cmax in healthy subjects and patients with cancer may be an artifact of the small number of participants in these analyses and the timing of blood samples used to estimate these phar-macokinetic parameters. Overall, GERSC provides slow, controlled release of therapeutic granisetron concentrations for ≥5 days following a single SC injection.Citation30,Citation84 The efficacy of GERSC in CINV prevention during acute and delayed phases following MEC or HEC has been shown in two large Phase III trials.Citation54,Citation59,Citation86 In contrast to GTDS, which is applied 24–48 hours prior to chemotherapy,Citation31 GERSC may be given shortly (≥30 minutes) before chemotherapy (). However, the GERSC single-dose syringe must be prepared at least 60 minutes prior to SC administration by warming the viscous liquid to room temperature, and the contents must be injected slowly by a health-care professional. A topical anesthetic may be applied to the injection site (IS) prior to administration.Citation29
Clinical trial experience has established that 5HT3 RAs effectively control emesis in the acute phase,Citation87–Citation90 but their efficacy in the delayed phase is limited.Citation91,Citation92 In Phase III trials, the addition of the NK1 RA aprepitant to a regimen of a 5HT3 RA plus dexamethasone improved CINV control over both acute and delayed phases following MEC or HEC ( and ).Citation55,Citation56,Citation68,Citation93 In the Phase III MAGIC trial of GERSC versus ondansetron, each combined with an NK1 RA and dexamethasone, the GERSC regimen provided superior delayed-phase CR in patients receiving HEC compared with the ondansetron regimen,Citation54 suggesting the involvement of 5HT3 RAs in mitigating delayed as well as acute CINV.
Efficacy of combination therapies for CINV
The three-drug antiemetic regimen (5HT3 RA + NK1 RA + dexamethasone)
Currently, guidelines for CINV prevention following HEC recommend a three-drug regimen of a 5HT3 RA, an NK1 RA, and dexamethasone as an option, with NCCN and ASCO guidelines also proposing a four-drug regimen with the addition of olanzapine (). No 5HT3 RA as a part of a three-drug regimen is preferred by any guideline for HEC.Citation7,Citation17,Citation19 Efficacy results from key Phase III trials in patients receiving HEC are summarized in . The MAGIC trial was the first Phase III registrational efficacy study to compare single doses of two 5HT3 RAs – GERSC and ondansetron – in a guideline-recommended three-drug versus three-drug regimen in 942 randomized patients receiving HECCitation54 (). As expected from the ER design, compared with the ondansetron arm, the GERSC arm demonstrated a superior delayed-phase CR rate (64.7% vs 56.6%, P=0.014, absolute treatment difference 8.0%), challenging the view that 5HT3 RAs have equal efficacy in delayed-phase CINV prevention following HEC.Citation11,Citation94 Consistent with the primary end point of CR, the GERSC arm versus the ondansetron arm also showed benefit in reducing rescue medication use and nausea frequency in the delayed and overall CINV phases.Citation54 Additionally, patient-reported satisfaction with antiemetic therapy was significantly higher in the GERSC arm versus the ondansetron arm in the delayed phase.Citation54
Because all patients in the MAGIC trial were from US community practices, these results are likely to be representative of outcomes in that practice setting. Importantly, the GERSC regimen demonstrated superiority to the ondansetron regimen in a population where the majority of patients were women (GERSC arm 79.6%, ondansetron arm 82.5%), a high-risk group for CINV.Citation54 Moreover, patients were stratified by planned use of cisplatin (≥50 mg/m2, yes/no), and the delayed-phase CR rate was higher in the GERSC arm (65.3%) versus the ondansetron arm (54.7%) in the 252 patients in the cisplatin stratum.Citation54 In a post hoc subgroup analysis of the 589 patients (65%) receiving AC-based HEC, at least 98% of whom were women, delayed-phase CR rates showed a trend favoring the GERSC arm (64%) over the ondansetron arm (56%).Citation95 A similar trend was found in the subgroup of 252 patients scheduled to receive cisplatin, where delayed-phase CR rates were numerically higher in the GERSC arm (65%) than in the ondansetron arm (55%).Citation96 These subgroup findings were consistent with the overall population, although the analyses were not powered to show a significant difference between groups.
The two-drug antiemetic regimen (5HT3 RA + dexamethasone)
Guidelines for CINV prevention following MEC generally recommend a two-drug regimen of a 5HT3 RA plus dexa-methasone on day 1 (with or without an NK1 RA) and dexa-methasone on days 2 and 3, with NCCN guidelines proposing the addition of an NK1 RA for patients with additional CINV risk factors or prior treatment failure.Citation7,Citation18,Citation19 Efficacy results from key Phase III trials in patients receiving MEC are sum-marized in . In the Phase III trial of GERSC compared with palonosetron, both in combination with dexamethasone, in patients receiving MEC (reanalyzed according to 2011 ASCO criteriaCitation97), both doses of GERSC (5 mg and 10 mg SC) were noninferior to palonosetron (0.25 mg IV) in preventing acute-phase CINV as determined by CR in cycle 1 of chemotherapy, and the 10 mg dose of GERSC was noninferior to palonosetron in preventing CINV in the delayed phase.Citation86
Of interest, because of AEs associated with dexametha-sone, such as insomnia, gastrointestinal symptoms, agitation, increased appetite, and weight gain,Citation98 several studies have investigated the efficacy of a dexamethasone-sparing palono-setron regimen involving 1-day versus 3-day dexamethasone in the MEC setting and reported the noninferiority of these regimens ().Citation67,Citation69,Citation72
Nausea control
Significant advances have been made in emesis control following MEC or HEC with the use of newer agents, but nausea control following HEC remains suboptimal, especially in the delayed phase of CINV, with delayed nausea being reported by 68% of patients in one study.Citation10 A multinational survey of 2,388 health-care providers found delayed nausea to be most challenging to prevent,Citation5 and several patient-related factors, including younger age, type of cancer, and patient’s perceived susceptibility to nausea, can contribute to increased incidence of chemotherapy-induced nausea.Citation99 Subjectivity in the measurement of nausea and its severity and a lack of understanding of underlying mechanisms pose barriers to optimal nausea control. In clinical trials, such end points as CC (CR with no more than mild nausea) and total response (CR with no nausea) provide some measure of nausea control, but rely on the patient’s self-reporting of nausea frequency and severity.
In the MAGIC trial of GERSC, patients with CC in the delayed phase comprised 60.7% in the GERSC regimen and 53.1% in the ondansetron regimen, a treatment difference of 7.6% (95% CI 1.1%–14.0%, unadjusted P=0.022). Percentages of CC were also higher in the GERSC regimen than the ondansetron regimen in the overall phase, but the difference was not significant (54.7% vs 49.6%, respectively; unadjusted P=0.123). The proportions of patients with no nausea in the GERSC and ondansetron regimens were similar in the delayed (49.7% vs 44.2%, respectively; P=0.099) and overall phases (45.3% vs 40.5%, respectively; P=0.138).Citation54
A recent Phase III trial of olanzapine versus placebo, each with a three-drug regimen of a 5HT3 RA, an NK1 RA, and dexamethasone, focused specifically on nausea control as the primary end point, and showed effective nausea control with the olanzapine regimen compared with the placebo regimen in patients receiving cisplatin or AC-based HEC. For patients in the olanzapine group compared with the placebo group, the proportions of patients with no nausea (acute phase 74% vs 45%, respectively, P=0.002; delayed phase 42% vs 25%, P=0.002; overall phase 37% vs 22%, P=0.002) and with CR (acute phase 86% vs 65%, P<0.001; delayed phase 67% vs 52%, P=0.007; overall phase 64% vs 41%, P<0.001) were significantly higher across all CINV phases.Citation15
Efficacy of 5HT3 RAs in multicycle chemotherapy
In trials evaluating antiemetics in multicycle-chemotherapy settings, the efficacy of 5HT3 RAs in CINV prevention was generally maintained over multiple cycles (Citation37,Citation80,Citation100–Citation102). Palonosetron at a dose of 0.75 mg, which is three times the approved dosage in the USA, was generally well tolerated in a Phase III trial in patients receiving cisplatin or AC-based HEC in Japan ().Citation100 CR rates were maintained across four cycles in the acute (range 72%–77%), delayed (range 56%–63%), and overall (range 52%–56%) phases.Citation100 A single-arm study in 156 patients receiving four to six cycles of cisplatin-based HEC evaluated CINV-prevention efficacy of palonosetron 0.25 mg IV in a three-drug regimen with oral aprepitant 125 mg and dexamethasone 20 mg IV on day 1. Overall-phase CR rates were maintained over six cycles, ranging from 74.4% to 82.0%. At least 90% of patients had no emesis, and at least 60% had no nausea across cycles.Citation103 The proportion of high-risk female patients in this study was 36%.
Table 5 Results of prospective Phase III clinical trials evaluating antiemetic efficacy in CINV prevention following multicycle or multiday chemotherapy
In a Phase III study of NEPA over multiple cycles, oral NEPA plus oral dexamethasone was compared with a control regimen of oral aprepitant plus oral palonosetron plus oral dexamethasoneCitation37 (). Overall-phase CR rates were maintained over multiple cycles in patients receiving the NEPA regimen (HEC 79%–91%, MEC 80%–93%) or the control regimen (HEC 58–86%, MEC 82%–89%), and were slightly lower with the latter regimen. In the delayed phase, there was a small but consistent numerical advantage in CR of 2%–6% with the NEPA regimen compared with the control regimen. The proportion of patients with no significant nausea in the overall phase remained high in both groups. Around 50% of study patients were women, and overall-phase CR rates with the NEPA regimen were generally similar in men and women across cycles (data not shown).Citation37
In a Phase III trial in patients receiving multiple cycles of MEC or HEC, sustainability of GERSC efficacy in CINV prevention was examined. Patients received GERSC 5 or 10 mg SC or palonosetron 0.25 mg IV in cycle 1. After cycle 1, no palonosetron was administered, and upon consent patients previously receiving palonosetron were rerandomized to receive GERSC 5 or 10 mg SC. CR rates were sustained over four cycles in patients receiving GERSC 10 mg once per cycle, with CR rates of 68.4% in patients in the acute phase of CINV, and 57.9% in the delayed phase following HEC; corresponding CR rates in patients receiving MEC were 56.5% and 41.3%, respectively. Emesis control was comparable to nausea control in patients receiving GERSC. Among patients in the MEC and HEC groups, respectively, 84%–88% and 63%–67% were women.Citation101 The higher proportion of women in the MEC group versus the HEC group and inclusion of AC-based regimens as MEC (as per guidelines at the time of the study designCitation8) instead of HEC (as per current guidelinesCitation7,Citation17,Citation19) may have contributed to the lower CR rates in the MEC group compared with those in the HEC group. At the time of this study, the recommended antiemetic regimen for patients receiving multiple cycles of HEC did not include an NK1 RA. The efficacy of GERSC as a part of a three-drug regimen remains to be investigated in the setting of multicycle MEC or HEC.
Efficacy of 5HT3 RAs in multiday chemotherapy
There have been fewer trials evaluating the antiemetic efficacy of 5HT3 RAs in patients receiving multiday chemotherapy than in those receiving single-day chemotherapy, and the NCCN guidelines recognize the lack of evidence to support recommendations in multiday-chemotherapy settings.Citation7 The efficacy of transdermal (GTDS) versus oral granisetron in preventing CINV was investigated in a Phase III study of 641 patients receiving their first cycle of a multiday MEC or HEC regimen ().Citation80 GTDS was noninferior to daily oral granisetron, and efficacy was maintained across the multiday-chemotherapy regimen (CC, GTDS 60%, oral granisetron 65%, 95% CI –13 to 3).Citation80 A small Phase III CINV-prevention study in 69 patients receiving 5-day cisplatin showed a significantly higher CR rate with a regimen of the NK1 RA aprepitant plus a 5HT3 RA (excluding palonosetron) plus dexamethasone versus a 5HT3 RA plus dexamethasone (42% vs 13%, P<0.001) ().Citation102
In a retrospective trial of palonosetron in comparison with ondansetron in patients receiving multiday cisplatin or carboplatin, palonosetron therapy was associated with a 63% lower risk of uncontrolled CINV (odds ratio 0.37, 95% CI 0.25–0.54; P<0.0001).Citation104 A pilot study involving administration of palo-nosetron 0.25 mg IV for 1, 2, or 3 days in patients receiving multiday high-dose melphalan and hematopoietic stem-cell transplantation suggested that multiple doses may be more effective than a single dose.Citation105 However, these results need confirmation in larger trials in combination with NK1 RAs.
Factors affecting the efficacy of 5HT3 RAs in prevention of acute and delayed CINV
In addition to chemotherapy emetogenicity, patient-related risk factors influence CINV and may affect outcomes in clinical studies. These factors include female sex,Citation106 younger age,Citation99 history of low alcohol consumption,Citation107 history of motion sickness,Citation107 and history of previous chemotherapy-induced emesis.Citation11 As such, young female patients receiving HEC are at particularly high risk of experiencing CINV,Citation108 and differences in proportions of female patients in clinical studies may affect results. The development of predictive tools with a simple scoring system to identify patients at higher risk for CINV, based on a combination of patient- and chemotherapy-related risk factors, may assist in determining the most suitable antiemetic regimen for patients.Citation109
Genetic variation in 5HT3 receptors or molecules involved in their transport and metabolism may also lead to differences in antiemetic efficacy.Citation110,Citation111 5HT3-RA metabolism is dependent on enzymes in the CYP450 family, with certain enzymes playing a dominant role in certain drugs.Citation112–Citation114 For example, palonosetron and dolasetron are metabolized primarily by CYP2D6, whereas granisetron is metabolized primarily by CYP1A1;Citation110,Citation115,Citation116 there is no dominant CYP450 enzyme for metabolizing ondansetron.Citation110,Citation112 CYP2D6 is also involved in the metabolism of a variety of drug classes, and genetic polymorphisms lead to alleles with different activities (defective, decreased, or increased), resulting in poor, intermediate, extensive, or ultrarapid metabolizers (UMs).Citation111,Citation115 The frequency of UMs varies by race, and control of chemotherapy-induced vomiting using ondansetron or tropisetron is weakest in UM patients compared with poor or intermediate metabolizers.Citation117 A pharmacogenetically driven treatment pathway that accounts for CYP2D6 genotype has been proposed, suggesting that patients classed as CYP2D6 UMs receiving MEC or HEC should be given granisetron as the first-line 5HT3 RA to circumvent the CYP2D6 pathway and reduce pharmacokinetic and pharmacodynamic variability.Citation115 It has been suggested that genetic polymorphisms of CYP3A4 among patients from different races influence granisetron exposure and thereby its efficacy.Citation114 Because the ER formulation of GERSC allows continuous exposure to granisetron, it may reduce interpatient variability in antiemetic efficacy resulting from differences in granisetron metabolism. Genetic variations in 5HT3R may also exist, which may explain in part the decreased efficacy of 5HT3 RAs in some patients.Citation115 Additional prospective studies investigating 5HT3-receptor polymorphisms and drug transporters may further elucidate the role of genetics in antiemetic response.
Safety of 5HT3 RAs
Most common AEs
The safety profile of 5HT3 RAs is well documented, and these agents are typically well tolerated. The most commonly reported AEs associated with IV or PO 5HT3 RAs are headache (0–24%, depending on the agent and formulation) and constipation (0–14%) (Citation25,Citation27–Citation29,Citation31–Citation33,Citation36).Citation25,Citation28,Citation29,Citation31–Citation34,Citation36 Diarrhea, asthenia, and dyspepsia are other known AEs. The incidence of these AEs is generally similar across the 5HT3 RAsCitation59,Citation118,Citation119 (), and they are generally mild and manageable. In IV and oral formulations of 5HT3 RAs, headache is reported more frequently than constipation, but in the MAGIC trial of GERSC, this finding was reversed. In the Phase III MAGIC trial, constipation occurred in 22% of patients in the GERSC arm and 15% of patients in the ondansetron IV arm, while headache was less common (12% vs 18%, respectively).Citation54 Similar trends were observed with GTDS, which also provides ER granisetron: a higher proportion of GTDS-treated versus oral granisetron-treated patients (7% vs 3%) had constipation, whereas a lower proportion had headache (0.3% vs 2.5%).Citation80
Table 6 Percentage of patients (≥2%) with AEs using 5HT3 RA, based on drug-prescribing information
Hypersensitivity and injection-site reactions
Hypersensitivity reactions and IS reactions (ISRs) have been reported with all 5HT3 RAs. Extremely rare instances (<1/10,000) of hypersensitivity, including anaphylaxis and ISRs, have been reported from postmarketing experience with palonosetron IV.Citation34 Rare and sometimes severe cases of hypersensitivity have been reported with IV granisetron (eg, anaphylaxis, hypotension, shortness of breath, and urticaria)Citation25 and ondansetron IV (eg, anaphylactic reactions, angioedema, bronchospasm, cardiopulmonary arrest, hypotension, laryngospasm, shock, shortness of breath, and stridor).Citation32 ISRs, including pain, redness, and burning, have also been reported with ondansetron IV.Citation32
The Phase III trial of GERSC versus palonosetron IV in preventing CINV in patients receiving MEC or HEC showed that the safety profiles of these two agents were similar.Citation59 The most common treatment-related AEs were ISRs (GERSC 10 mg SC 38.9%, palonosetron 11.2%), constipation (GERSC 10 mg SC 4.5%, palonosetron 3.0%), and headache (GERSC 10 mg SC 2.8%, palonosetron 1.9%).Citation59
In the Phase III MAGIC trial, GERSC was also generally well tolerated compared with ondansetron IV, and no unexpected safety findings were observed.Citation54 All ISRs were conservatively considered treatment-related, regardless of their time of occurrence following study-drug administration, and were graded by investigators on days 1 and 6 and at the final safety follow-up visit according to prespecified criteria representing only size differences, not functional impairment.Citation54 Additionally, patients were instructed to evaluate the IS on days 2 to 5 and record the ISR status in a provided diary. ISRs occurred in patients in both arms at a similar frequency (61.8% in the GERSC arm, 59.5% in the ondansetron arm), presumably because of the double-dummy design of the study, where both arms received SC and IV drug or vehicle placebo; most ISRs were mild–moderate and resolved over time.Citation54 No ISRs led to study discontinuation or death.Citation54
Compared with the earlier Phase III trial, a greater proportion of patients receiving GERSC in the MAGIC trial reported ISRs.Citation54,Citation59 Several factors may have contributed to this finding, including the assumption that all ISRs were treatment-related, grading only according to size and not functional impairment (which may have led investigators to pay greater attention to ISRs), and the instruction for patients to evaluate the site and record any signs of ISRs in patient diaries. Further, the MAGIC trial was conducted only in the USA, whereas the previous trial also included patients from Poland and India;Citation54,Citation59 potential differences in ISR reporting across countries may have led to the observed ISR-reporting differences in the studies. The most common ISR in both trials was bruising, which was higher in the MAGIC trial (GERSC arm 41.9%, ondansetron arm 33.6%) than in the previous Phase III trial (GERSC 10 mg, related 15.6%, unrelated 4.3%; palonosetron 0.25 mg IV, related 6.5%, unrelated 2.6%).Citation54,Citation59 However, if ISRs in the MAGIC trial were graded by National Cancer Institute Common Terminology Criteria for AEs instead of size, all instances of IS bruising of grades 2 and 3 would have been classified as grade 1. A Phase I crossover study demonstrated that GERSC may be administered to the abdomen or nondominant upper arm, but ISR incidence was higher in patients receiving abdominal (84.5%) versus upper-arm injections (69.2%).Citation120 Therefore, upper-arm administration may be preferable.
Serotonin syndrome
5HT3 RAs have been associated with serious AEs, such as serotonin syndrome (involving symptoms of mental status changes), autonomic instability, neuromuscular symptoms, and seizures with or without gastrointestinal symptoms.Citation25,Citation32–Citation34,Citation36 Serotonin syndrome results from increased serotonin signaling in the central nervous system.Citation121 Occurrences of serotonin syndrome have generally been associated with concomitant use of serotonergic drugs and 5HT RAs or with an overdose of 5HT3 RAs.Citation33 Some fatalities resulting from serotonin syndrome have been reported with 5HT3 RAs.Citation121 Patient monitoring for symptoms of serotonin syndrome and discontinuation of 5HT3 RAs on their occurrence is recommended.
Cardiac effects
As a class, 5HT3 RAs block cardiac hERG potassium channels with varying effectiveness,Citation83 so may be associated with abnormal cardiac electrical activity, such as a prolonged QT interval on electrocardiography (ECG). Dolasetron and ondansetron have lower clinical cardiac safety margins compared with granisetron and palonosetron.Citation83 In 2010, the FDA requested the withdrawal of the CINV indication for dolasetron IV, because of the possibility of life-threatening cardiac arrhythmias, such as QT-interval prolongation.Citation74 In 2012, the FDA restricted IV doses of ondansetron to three doses of 0.15 mg/kg administered every 4 hours to a maximum of 16 mg, given the risk of QT-interval prolongation.Citation122 Postmarketing reports of torsades de pointes have also been documented in patients using ondansetron.Citation32
Other 5HT3 RAs have demonstrated no clinically significant changes in QT interval. Palonosetron 0.25, 0.75, or 2.25 mg IV showed no significant effect on cardiac QT intervals in healthy volunteers.Citation123 Supratherapeutic doses of NEPA (netupitant 600 mg and palonosetron 1.5 mg) produced no significant change in QTc, PR, or QRS intervals in healthy volunteers.Citation124 In a thorough QT/QTc study in healthy volunteers, granisetron administered via GTDS achieved prolonged therapeutic plasma concentrations compared with granisetron IV, but this did not lead to significant or progressive QT prolongation.Citation125 In a similar study, GERSC 20 mg (twice the approved dose) elicited no clinically significant effect on QT interval compared with placebo.Citation126 Although ECG changes induced by 5HT3 RAs can be asymptomatic and reversible, fatal cardiac arrhythmias can occur in certain cases.Citation127 NCCN guidelines recommend that patients at high risk of developing life-threatening cardiac arrhythmias, such as those with torsades de pointes, receive routine ECG monitoring during treatment with 5HT3 RAs.Citation7
Special populations
For ondansetron IV and PO and palonosetron IV, no dosage adjustment is recommended for patients with renal impairment or mild–moderate hepatic impairment.Citation32–Citation34 In patients with severe hepatic impairment (Child–Pugh score ≥10), the maximum total daily dose of ondansetron should not exceed 8 mg.Citation32,Citation33 For NEPA, no dosage adjustment is necessary in patients with mild–moderate renal or hepatic impairment, but NEPA should be avoided in patients with severe hepatic or renal impairment or end-stage renal disease.Citation36 Total granisetron clearance is not affected in patients with severe renal failure receiving a single IV dose of granisetron, and no dosage adjustment is necessary in patients with hepatic impairment.Citation25 For granisetron PO, no dosage adjustment is recommended for patients with either renal or hepatic impairment.Citation28 No studies have investigated the pharmacokinetics of GTDS in patients with renal or hepatic impairment.Citation31 For GERSC, there are no pharmacokinetic data on the elimination of the TEG-POE polymer vehicle in patients with renal impairment, and the clinical significance of prolonged granisetron elimination in patients with cancer is unknown. Consequently, current recommendations are that GERSC should not be administered more than once every 14 days in patients with moderate renal impairment and should be avoided in patients with severe renal impairment.Citation29
Clearance of ondansetron IV in pediatric patients aged 1–4 months is slower, and the t½ is ~2.5-fold longer than in patients aged >4–24 months, so patients <4 months old receiving ondansetron IV should be closely monitored.Citation32 The safety and effectiveness of ondansetron PO have been established in pediatric patients ≥4 years of age receiving MEC, but not HEC.Citation33 The safety and effectiveness of palonosetron IV have been established in pediatric patients aged 1 month to <17 years. Pediatric patients require a higher IV dose of palono-setron than adults to prevent CINV, but the safety profile is consistent with that in adults.Citation34 For NEPA, safety and effectiveness in patients <18 years of age have not been established. For granisetron, the recommended IV dose in pediatric patients 2–16 years of age is 10 µg/kg. Benzyl alcohol, a component of granisetron 1 mg/mL IV, has been associated with serious adverse reactions and death, particularly in neonates.Citation25 The safety and effectiveness of granisetron PO, GTDS, and GERSC in pediatric patients have not been established.Citation28,Citation29,Citation31
For ondansetron IV and PO, no overall differences in safety or effectiveness have been observed between elderly and younger patients, and no dosage adjustment is needed in patients >65 years of age. However, greater sensitivity of some older patients to ondansetron cannot be ruled out.Citation32,Citation33 Population pharmacokinetic analyses of palonosetron IV have not revealed any differences between patients ≥65 years of age and younger adult patients.Citation34 For NEPA PO, the safety profiles in elderly and younger patients are similar.Citation36 For different formulations of granisetron, the safety and effectiveness of granisetron IV and PO are similar in patients of various ages.Citation25,Citation28 For GTDS and GERSC, clinical experience has not identified any differences in safety or effectiveness between elderly and younger patients.Citation29,Citation31 In general, caution should be used when administering any of these agents to elderly patients, because of their increased likelihood of having decreased hepatic, renal, or cardiac function, concomitant diseases, and receiving other drug therapies.
Safety of 5HT3 RAs in multicycle chemotherapy
5HT3 RAs have generally been well tolerated in multicycle CINV-prevention trials. NEPA was generally safe when administered over multiple cycles to patients receiving MEC: the incidence of AEs did not increase across cycles.Citation37 Serious treatment-emergent AEs occurred in 16.2% of patients in the NEPA group (two were treatment-related) and 18.3% of patients in the aprepitant plus palonosetron group. GERSC was generally safe in patients receiving up to four cycles of MEC or HEC.Citation101 No treatment-related serious AEs were observed. Most ISRs were mild, and those of moderate severity occurred in less than 3% of patients. ISRs included bruising (GERSC 500 mg, 29.7%), nodules (GERSC 500 mg, 17.5%), erythema (GERSC 500 mg, 11.8%), pain (GERSC 500 mg, 7.2%), and bleeding (GERSC 500 mg, 6.6%).Citation101
Safety of 5HT3 RAs in multiday chemotherapy
In a Phase III trial of GTDS in patients receiving multiday chemotherapy, the most common AE was constipation, occurring in 7% of patients,Citation80 and no QTc prolongation occurred with GTDS. Multiple doses of palonosetron 0.25 mg IV administered with dexamethasone were well tolerated in patients with multiple myeloma receiving multiday melphalan and hematopoietic stem-cell transplantation, and no serious drug-related AEs were reported.Citation105 In patients receiving up to six cycles of cisplatin, a combination of palonosetron 0.25 mg IV, aprepitant 125 mg PO, and dexamethasone 20 mg IV on day 1 of chemotherapy was well tolerated.Citation103
5HT3 RAs for radiotherapy-induced NV and chemoradiation-induced NV
In addition to chemotherapy, other causes of NV include radiotherapy and concomitant chemotherapy and radiotherapy. Among patients receiving radiotherapy, 50%–80% may experience radiotherapy-induced NV (RINV), depending on the site of irradiation, dose, fractionation schedule, and patient-related risk factors.Citation128 5HT3 RAs have been widely used for RINV prophylaxis,Citation128 and NCCN guidelines recommend pretreatment with oral granisetron or oral ondansetron, with or without oral dexamethasone, for each day of upper-abdomen, localized, or total-body irradiation.Citation7
Despite widespread use of chemoradiation to treat gynecologic and other cancers, prophylactic antiemetic regimens for RINV and chemoradiation-induced NV (CRINV) are relatively underexplored.Citation129 The standard of care for women with cervical cancer involves weekly cisplatin-based HEC and concomitant fractionated radiotherapy, resulting in increased CINV risk in these patients, due to their young age, female sex, and the high emetogenicity of cisplatin.Citation129 In a Phase II study in 48 women receiving concomitant radiotherapy and cisplatin therapy for gynecologic cancers, prophylaxis with palonosetron 0.25 mg IV once on day 1 and prednisolone 100 mg once on day 1 followed by 50 mg twice on day 2, 25 mg twice on day 3, and 25 mg once on day 4 was insufficient for emesis control.Citation130 The primary end point, cumulative probability of patients completing five cycles of chemoradiation without emesis, was 57%.Citation130 A recent Phase III study in 246 women with cervical cancer receiving fractionated radiotherapy and weekly cisplatin for 5 weeks compared fosaprepitant 150 mg IV versus placebo, each combined with palonosetron 0.25 mg IV and dexamethasone 16 mg PO, for the control of CRINV.Citation131 All patients received dexamethasone 8 mg PO twice on day 2, 4 mg PO twice on day 3, and 4 mg once on day 4. This was the first study to investigate the efficacy of a three-drug regimen in CRINV prevention. A greater proportion of patients receiving the fosaprepitant three-drug regimen did not experience emesis at 5 weeks (65.7% vs 48.7%), with a lower cumulative risk of emesis in these patients (P=0.008). These results represent a significant advance in CRINV management.
Conclusion
With the recent addition of new formulations of 5HT3 RAs, health-care providers have a variety of agents to choose from. However, these agents have important differences in efficacy and safety, and the appropriate antiemetic regimen must be selected carefully, considering both chemotherapy regimen and schedule and patient-related risk factors.
For patients receiving single-day HEC, a three-drug anti-emetic regimen is recommended, and the 5HT3 RA included in this regimen may influence the combination’s efficacy in preventing CINV. Ideally, the 5HT3 RA in combination with an NK1 RA and a corticosteroid should prevent CINV across both acute and delayed phases following HEC. Based on clinical experience, first-generation 5HT3 RAs such as ondansetron, older formulations of granisetron, and dolasetron IV (no longer approved in the USA to prevent CINV, because of potential cardiac effects) are considered similar in efficacy for preventing acute CINV following MEC or HEC, but considered inadequate for preventing delayed CINV following HEC.Citation11 Recent results with GERSC, the ER polymer formulation of granisetron using novel Biochronomer technology, change this view.Citation54 Two large Phase III trials in 2,370 patients have demonstrated that a single dose of GERSC is effective in preventing CINV following MEC and HEC regimens.Citation54,Citation59,Citation86 GERSC was noninferior to the standard of care, palonosetron, in preventing CINV in the acute and delayed phases following MEC and in the acute phase following HEC, and numerically superior to palonosetron in preventing delayed CINV following HEC.Citation86 GERSC was also superior to ondansetron (when both agents were administered in the guideline-recommended three-drug regimen) in preventing delayed CINV following HEC.Citation54 In light of the need for better therapies to manage delayed CINV following HEC, GERSC provides a new and effective option. Furthermore, GERSC efficacy has been maintained over successive cycles of MEC or HEC.Citation101 Therefore, GERSC is a convenient and effective SC option for acute and delayed CINV prevention following MEC or HEC administered over single or multiple cycles. In the USA, GERSC is indicated for use in combination with other antiemetics for the prevention of acute and delayed NV associated with initial and repeat courses of MEC and AC combination-chemotherapy regimens.
Another recent formulation, NEPA (fixed-dose netupitant and palonosetron), in combination with dexamethasone, has shown superiority to palonosetron plus dexamethasone in controlling CINV following MEC in acute, delayed, and overall phases and was effective over multiple cycles of MEC or HEC.Citation37,Citation45 As such, NEPA is an additional oral antiemetic option for patients receiving MEC or HEC.
Historically, CINV-prevention trials have typically used CR as the primary end point and focused on emesis control in both the acute and delayed phases of CINV, but delayed nausea, especially following HEC, continues to be inadequately controlled. The subjectivity in measuring nausea and the lack of understanding of its underlying pathophysiology have hindered the study of nausea control and the development of effective agents to prevent nausea. The use of a stringent no-nausea end point in clinical trials and a better understanding of the underlying pathophysiology are likely to lead to improved nausea control. Given the efficacy of olanzapine in nausea control, its addition to the existing three-drug regimen may improve nausea management and may be the next step in CINV-prevention regimens. The continued occurrence of CINV, despite the availability of effective antiemetic regimens and comprehensive clinical practice guidelines, suggests the need for greater adherence to guidelines and better understanding of patient characteristics and genetic differences. Future research on 5HT3-RA pharmacogenetics may improve the understanding of variability in patient response to antiemetics.Citation110,Citation132
Acknowledgments
Research support was provided by Heron Therapeutics Inc (Redwood City, CA, USA). Medical writing assistance was provided by Doyel Mitra, PhD, Yvonne E Yarker, PhD, and Joanna Sandilos Rega, PhD, of SciStrategy Communications (Conshohocken, PA, USA).
Disclosure
JG has served as a member of a speaker bureau or advisory committee for Heron Therapeutics. LS has acted in a consultant/advisory role for Eisai, Helsinn, Merck, and Tesaro, received research funding from Helsinn, and served as a member of a speaker bureau or advisory committee for Helsinn and Merck. NG has served as a member of a speaker bureau or advisory committee for Eisai, Heron, and Tesaro. The authors report no other conflicts of interest in this work.
References
- Bloechl-DaumBDeusonRRMavrosPHansenMHerrstedtJDelayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatmentJ Clin Oncol200624274472447816983116
- NavariRMManagement of chemotherapy-induced nausea and vomiting: focus on newer agents and new uses for older agentsDrugs201373324926223404093
- CohenLde MoorCAEisenbergPMingEEHuHChemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settingsSupport Care Cancer200715549750317103197
- HaideraliAMendittoLGoodMTeitelbaumAWegnerJImpact on daily functioning and indirect/direct costs associated with chemotherapy-induced nausea and vomiting (CINV) in a U.S. populationSupport Care Cancer201119684385120532923
- van LaarESDesaiJMJatoiAProfessional educational needs for chemotherapy-induced nausea and vomiting (CINV): multinational survey results from 2388 health care providersSupport Care Cancer201523115115725015057
- CoatesAAbrahamSKayeSBOn the receiving end: patient perception of the side-effects of cancer chemotherapyEur J Cancer Clin Oncol19831922032086681766
- National Comprehensive Cancer NetworkNCCN clinical practice guidelines in oncology: antiemesis – v2.20182018 Available from: https://www.nccn.org/professionals/physician_gls/default.aspxAccessed May 25, 2018
- HeskethPJKrisMGGrunbergSMProposal for classifying the acute emetogenicity of cancer chemotherapyJ Clin Oncol19971511031098996130
- GrunbergSMWarrDGrallaRJEvaluation of new antiemetic agents and definition of antineoplastic agent emetogenicity: state of the artSupport Care Cancer201119Suppl 1S43S4720972805
- HilariusDLKloegPHvan der WallEvan den HeuvelJJGundyCMAaronsonNKChemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based studySupport Care Cancer201220110711721258948
- NavariRM5-HT receptors as important mediators of nausea and vomiting due to chemotherapyBiochim Biophys Acta2015184810 Pt B2738274625838122
- JanelsinsMCTejaniMAKamenCPeoplesARMustianKMMorrowGRCurrent pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patientsExpert Opin Pharmacother201314675776623496347
- ShihTYXuYEltingLSCosts of uncontrolled chemotherapy-induced nausea and vomiting among working-age cancer patients receiving highly or moderately emetogenic chemotherapyCancer2007110367868517567835
- BurkeTAWisniewskiTErnstFRResource utilization and costs associated with chemotherapy-induced nausea and vomiting (CINV) following highly or moderately emetogenic chemotherapy administered in the US outpatient hospital settingSupport Care Cancer201119113114020101417
- NavariRMQinRRuddyKJOlanzapine for the prevention of chemotherapy-induced nausea and vomitingN Engl J Med2016375213414227410922
- NavariRMManagement of Chemotherapy-Induced Nausea and Vomiting: New Agents and New Uses of Current AgentsBasel, HeidelbergSpringer2016
- HerrstedtJRoilaFWarrD2016 Updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following high emetic risk chemotherapySupport Care Cancer201725127728827443154
- RoilaFWarrDHeskethPJ2016 updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following moderately emetogenic chemotherapySupport Care Cancer201725128929427510316
- HeskethPJKrisMGBaschEAntiemetics: American Society of Clinical Oncology clinical practice guideline updateJ Clin Oncol201735283240326128759346
- GilmoreJWPeacockNWGuAAntiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE studyJ Oncol Pract2014101687424065402
- AaproMMolassiotisADicatoMThe effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER)Ann Oncol20122381986199222396444
- SchwartzbergLHarrowBLalLSRadtchenkoJLymanGHResource utilization for chemotherapy-induced nausea and vomiting events in patients with solid tumors treated with antiemetic regimensAm Health Drug Benefits20158527328226380034
- KrautLFauserAAAnti-emetics for cancer chemotherapy-induced emesis: potential of alternative delivery systemsDrugs200161111553156211577793
- HeskethPJGandaraDRSerotonin antagonists: a new class of anti-emetic agentsJ Natl Cancer Inst19918396136201850806
- Kytril (granisetron) injection, for intravenous use [prescribing information]San FranciscoGenentech2011
- van WijngaardenITulpMTSoudijnWThe concept of selectivity in 5-HT receptor researchEur J Pharmacol199018863013122164935
- Anzemet tablets (dolasetron mesylate) [prescribing information]Bridgewater (NJ)Sanofi-Aventis2013
- Kytril (granisetron hydrochloride) tablets, oral solution [prescribing information]Nutley (NJ)Roche Laboratories2010
- Sustol (granisetron) extended-release injection, for subcutaneous use [prescribing information]Redwood (CA)Heron Therapeutics2016
- GabrailNYanagiharaRSpaczy skiMPharmacokinetics, safety, and efficacy of APF530 (extended-release granisetron) in patients receiving moderately or highly emetogenic chemotherapy: results of two phase II trialsCancer Manag Res20157839225834466
- Sancuso (granisetron transdermal system) [prescribing information]Bridgewater (NJ)ProStrakan2015
- Zofran (ondansetron hydrochloride) injection for intravenous use [prescribing information]Research Triangle Park (NC)GlaxoSmithKline2014
- Zofran (ondansetron hydrochloride) tablets [prescribing information]Research Triangle Park (NC)GlaxoSmithKline2016
- Aloxi (palonosetron HCl) injection for intravenous use [prescribing information]Woodcliff Lake (NJ)Eisai2015
- SmithHSCoxLRSmithEJ5-HT3 receptor antagonists for the treatment of nausea/vomitingAnn Palliat Med20121211512025841471
- Akynzeo (netupitant and palonosetron) capsules for oral use [prescribing information]Woodcliff Lake (NJ)Eisai2016
- GrallaRJBosnjakSMHontsaAA phase III study evaluating the safety and efficacy of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting over repeated cycles of chemotherapyAnn Oncol20142571333133924631949
- TsukaharaKNakamuraKMotohashiRAntiemetic therapy of fosaprepitant, palonosetron, and dexamethasone combined with cisplatin-based chemotherapy for head and neck carcinomasActa Otolaryngol2014134111198120425315920
- VidallCFernandez-OrtegaPCortinovisDJahnPAmlaniBScotteFImpact and management of chemotherapy/radiotherapy-induced nausea and vomiting and the perceptual gap between oncologists/oncology nurses and patients: a cross-sectional multinational surveySupport Care Cancer201523113297330525953380
- EglenRMLeeCHSmithWLPharmacological characterization of RS 25259-197, a novel and selective 5-HT3 receptor antagonist, in vivoBr J Pharmacol199511448608667773547
- RojasCThomasAGAltJPalonosetron triggers 5-HT3 receptor internalization and causes prolonged inhibition of receptor functionEur J Pharmacol20106262–319319919836386
- WongEHClarkRLeungEThe interaction of RS 25259-197, a potent and selective antagonist, with 5-HT3 receptors, in vitroBr J Pharmacol199511448518597773546
- StoltzRParisiSShahAMacciocchiAPharmacokinetics, metabolism and excretion of intravenous [14C]-palonosetron in healthy human volunteersBiopharm Drug Dispos200425832933715378559
- HeskethPJRossiGRizziGEfficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal studyAnn Oncol20142571340134624608196
- AaproMRugoHRossiGA randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapyAnn Oncol20142571328133324603643
- RapoportBLChasenMRGridelliCSafety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trialsLancet Oncol20151691079108926272769
- SchwartzbergLSModianoMRRapoportBLSafety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of moderately emetogenic chemotherapy or anthracycline and cyclophosphamide regimens in patients with cancer: a randomised, active-controlled, double-blind, phase 3 trialLancet Oncol20151691071107826272768
- HeskethPJAaproMJordanKSchwartzbergLBosnjakSRugoHA review of NEPA, a novel fixed antiemetic combination with the potential for enhancing guideline adherence and improving control of chemotherapy-induced nausea and vomitingBiomed Res Int2015201565187926421300
- AaproMSGrunbergSMManikhasGMA phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapyAnn Oncol20061791441144916766588
- SaitoMAogiKSekineIPalonosetron plus dexametha-sone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trialLancet Oncol200910211512419135415
- NavariRMNagyCKGraySEThe use of olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapySupport Care Cancer20132161655166323314603
- GrunbergSChuaDMaruASingle-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with cisplatin therapy: randomized, double-blind study protocol – EASEJ Clin Oncol201129111495150121383291
- NavariRMGraySEKerrACOlanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trialJ Support Oncol20119518819522024310
- SchnadigIDAgajanianRDakhilSRAPF530 (granisetron injection extended-release) in a three-drug regimen for delayed CINV in highly emetogenic chemotherapyFuture Oncol201612121469148126997579
- HeskethPJGrunbergSMGrallaRJThe oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin – the Aprepitant Protocol 052 study groupJ Clin Oncol200321224112411914559886
- Poli-BigelliSRodrigues-PereiraJCaridesADAddition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting: results from a randomized, double-blind, placebo-controlled trial in Latin AmericaCancer200397123090309812784346
- SaitoHYoshizawaHYoshimoriKEfficacy and safety of single-dose fosaprepitant in the prevention of chemotherapy-induced nausea and vomiting in patients receiving high-dose cisplatin: a multicentre, randomised, double-blind, placebo-controlled phase 3 trialAnn Oncol20132441067107323117073
- HuZChengYZhangHAprepitant triple therapy for the prevention of chemotherapy-induced nausea and vomiting following high-dose cisplatin in Chinese patients: a randomized, double-blind, placebo-controlled phase III trialSupport Care Cancer201422497998724276953
- RaftopoulosHCooperWO’BoyleEGabrailNBocciaRGrallaRJComparison of an extended-release formulation of granisetron (APF530) versus palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapy: results of a prospective, randomized, double-blind, noninferiority phase 3 trialSupport Care Cancer201523372373225179689
- GebbiaVCannataGTestaAOndansetron versus granisetron in the prevention of chemotherapy-induced nausea and vomiting: results of a prospective randomized trialCancer1994747194519528082100
- MatsumotoKTakahashiMSatoKPalonosetron or granisetron for prevention of CINV in patients with breast cancer receiving dexamethasone and fosaprepitant following anthracycline plus cyclophosphamide (AC) regimenJ Clin Oncol20153315 Suppl9598
- SaitoMUomoriTMiuraKComparison between 1st and 2nd generation serotonin receptor antagonists in triplet antiemetic therapy in breast cancer patients: according to recent multi-institutional double-blind randomized studySupport Care Cancer201725Suppl 2S054
- EisenbergPFigueroa-VadilloJZamoraRImproved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetronCancer200398112473248214635083
- GrallaRLichinitserMvan der VegtSPalonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetronAnn Oncol200314101570157714504060
- WarrDGHeskethPJGrallaRJEfficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapyJ Clin Oncol200523122822283015837996
- GrunbergSMDuganMMussHEffectiveness of a single-day three-drug regimen of dexamethasone, palonosetron, and aprepitant for the prevention of acute and delayed nausea and vomiting caused by moderately emetogenic chemotherapySupport Care Cancer200917558959419037667
- AaproMFabiANoleFDouble-blind, randomised, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapyAnn Oncol20102151083108820080830
- RapoportBLJordanKBoiceJAAprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: a ran-domized, double-blind studySupport Care Cancer201018442343119568773
- CelioLFrustaciSDenaroAPalonosetron in combination with 1-day versus 3-day dexamethasone for prevention of nausea and vomiting following moderately emetogenic chemotherapy: a randomized, multicenter, phase III trialSupport Care Cancer20111981217122520574663
- BocciaRGrunbergSFranco-GonzalesERubensteinEVoisinDEfficacy of oral palonosetron compared to intravenous palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapy: a phase 3 trialSupport Care Cancer20132151453146023354552
- SchmittTGoldschmidtHNebenKAprepitant, granisetron, and dexamethasone for prevention of chemotherapy-induced nausea and vomiting after high-dose melphalan in autologous transplantation for multiple myeloma: results of a randomized, placebo-controlled phase III trialJ Clin Oncol201432303413342025225424
- KomatsuYOkitaKYukiSOpen-label, randomized, comparative, phase III study on effects of reducing steroid use in combination with palonosetronCancer Sci2015106789189525872578
- NishimuraJSatohTFukunagaMCombination antiemetic therapy with aprepitant/fosaprepitant in patients with colorectal cancer receiving oxaliplatin-based chemotherapy (SENRI trial): a multicentre, randomised, controlled phase 3 trialEur J Cancer201551101274128225922233
- US Food and Drug AdministrationFDA drug safety communication: abnormal heart rhythms associated with use of Anzemet (dolasetron mesylate)2010 Available from: https://www.fda.gov/Drugs/Drug-Safety/ucm237081.htmAccessed October 10, 2017
- KolesarJMEickhoffJVermeulenLCSerotonin type 3-receptor antagonists for chemotherapy-induced nausea and vomiting: therapeutically equivalent or meaningfully different?Am J Health Syst Pharm201471650751024589542
- YehYCBlouinGCReddyPEvidence to support use of palo-nosetron over generic serotonin type 3-receptor antagonists for chemotherapy-induced nausea and vomitingAm J Health Syst Pharm201471650050624589541
- BotrelTEClarkOAClarkLPaladiniLFaleirosEPegorettiBEfficacy of palonosetron (PAL) compared to other serotonin inhibitors (5-HT3R) in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic (MoHE) treatment: systematic review and meta-analysisSupport Care Cancer201119682383220495832
- PopovicMWarrDGDeangelisCEfficacy and safety of palonosetron for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV): a systematic review and meta-analysis of randomized controlled trialsSupport Care Cancer20142261685169724590374
- HowellJSmeetsJDrenthHJGillDPharmacokinetics of a granisetron transdermal system for the treatment of chemotherapy-induced nausea and vomitingJ Oncol Pharm Pract200915422323119304880
- BocciaRVGordanLNClarkGHowellJDGrunbergSMEfficacy and tolerability of transdermal granisetron for the control of chemotherapy-induced nausea and vomiting associated with moderately and highly emetogenic multi-day chemotherapy: a randomized, double-blind, phase III studySupport Care Cancer201119101609161720835873
- SeolYMKimHJChoiYJTransdermal granisetron versus palonosetron for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: a multicenter, randomized, open-label, cross-over, active-controlled, and phase IV studySupport Care Cancer201624294595226265119
- WokovichAMProdduturiSDoubWHHussainASBuhseLFTransdermal drug delivery system (TDDS) adhesion as a critical safety, efficacy and quality attributeEur J Pharm Biopharm20066411816797171
- MasonJWMoonTEUse and cardiovascular safety of transdermal and other granisetron preparations in cancer managementCancer Manag Res2013517918523930078
- OttoboniTGelderMSO’BoyleEBiochronomer technology and the development of APF530, a sustained release formulation of granisetronJ Exp Pharmacol20146152127186139
- HellerJBarrJBiochronomer technologyExpert Opin Drug Deliv20052116918316296743
- RaftopoulosHBocciaRCooperWO’BoyleEGrallaRJSlow-release granisetron (APF530) versus palonosetron for chemotherapy-induced nausea/vomiting: analysis by American Society of Clinical Oncology emetogenicity criteriaFuture Oncol201511182541255126289588
- CupissolDRSerrouBCaubelMThe efficacy of granisetron as a prophylactic anti-emetic and intervention agent in high-dose cisplatin-induced emesisEur J Cancer199026Suppl 1S23S272169782
- de MulderPHSeynaeveCVermorkenJBOndansetron compared with high-dose metoclopramide in prophylaxis of acute and delayed cisplatin-induced nausea and vomiting: a multicenter, randomized, double-blind, crossover studyAnn Intern Med1990113118348402146911
- GrunbergSMStevensonLLRussellCAMcDermedJEDose ranging phase I study of the serotonin antagonist GR38032F for prevention of cisplatin-induced nausea and vomitingJ Clin Oncol198978113711412526864
- HeskethPNavariRGroteTDouble-blind, randomized comparison of the antiemetic efficacy of intravenous dolasetron mesylate and intravenous ondansetron in the prevention of acute cisplatin-induced emesis in patients with cancerJ Clin Oncol1996148224222498708713
- GelingOEichlerHGShould 5-hydroxytryptamine-3 receptor antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Systematic re-evaluation of clinical evidence and drug cost implicationsJ Clin Oncol20052361289129415718327
- RoilaFWarrDClark-SnowRADelayed emesis: moderately emetogenic chemotherapySupport Care Cancer200513210410815549426
- GrallaRJde WitRHerrstedtJAntiemetic efficacy of the neurokinin-1 antagonist, aprepitant, plus a 5HT3 antagonist and a corticosteroid in patients receiving anthracyclines or cyclophosphamide in addition to high-dose cisplatin: analysis of combined data from two phase III randomized clinical trialsCancer2005104486486815973669
- HeskethPJComparative review of 5-HT3 receptor antagonists in the treatment of acute chemotherapy-induced nausea and vomitingCancer Invest200018216317310705879
- SchnadigIDAgajanianRDakhilSRPhase 3 comparison of APF530 versus ondansetron, each in a guideline-recommended three-drug regimen, for prevention of chemotherapy-induced nausea and vomiting due to anthracycline plus cyclophosphamide-based highly emetogenic chemotherapy regimens: a post hoc subgroup analysis of the Phase III randomized MAGIC trialCancer Manag Res2017917918728579832
- SchwartzbergLMosierMGellerRBKlepperMJSchnadigIVogelzangNJAPF530 for nausea and vomiting prevention following cisplatin: phase 3 MAGIC trial analysisJ Community Support Oncol20171528288
- BaschEPrestrudAAHeskethPJAntiemetics: American Society of Clinical Oncology clinical practice guideline updateJ Clin Oncol201129314189419821947834
- VardyJChiewKSGalicaJPondGRTannockIFSide effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapyBr J Cancer20069471011101516552437
- RoscoeJAMorrowGRColagiuriBInsight in the prediction of chemotherapy-induced nauseaSupport Care Cancer201018786987619701781
- AogiKSakaiHYoshizawaHA phase III open-label study to assess safety and efficacy of palonosetron for preventing chemotherapy-induced nausea and vomiting (CINV) in repeated cycles of emetogenic chemotherapySupport Care Cancer20122071507151421808994
- BocciaRCooperWO’BoyleESustained antiemetic responses with APF530 (sustained-release granisetron) during multiple cycles of emetogenic chemotherapyJ Community Support Oncol2015132384625866983
- AlbanyCBramesMJFauselCJohnsonCSPicusJEinhornLHRandomized, double-blind, placebo-controlled, phase III cross-over study evaluating the oral neurokinin-1 antagonist aprepitant in combination with a 5HT3 receptor antagonist and dexamethasone in patients with germ cell tumors receiving 5-day cisplatin combination chemotherapy regimens: a Hoosier Oncology Group studyJ Clin Oncol201230323998400322915652
- LongoFMansuetoGLapadulaVCombination of aprepitant, palonosetron and dexamethasone as antiemetic prophylaxis in lung cancer patients receiving multiple cycles of cisplatin-based chemotherapyInt J Clin Pract201266875375722805267
- FeinbergBGilmoreJHaislipSImpact of initiating antiemetic prophylaxis with palonosetron versus ondansetron on risk of uncontrolled chemotherapy-induced nausea and vomiting in patients with lung cancer receiving multi-day chemotherapySupport Care Cancer201220361562321761096
- GiraltSAManganKFMaziarzRTThree palonosetron regimens to prevent CINV in myeloma patients receiving multiple-day high-dose melphalan and hematopoietic stem cell transplantationAnn Oncol201122493994620935058
- du BoisAMeerpohlHGVachWKommossFGFenzlEPfleidererACourse, patterns, and risk-factors for chemotherapy-induced emesis in cisplatin-pretreated patients: a study with ondansetronEur J Cancer1992282–34504571534250
- WarrDGStreetJCCaridesADEvaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of phase 3 trial of aprepitant in patients receiving adriamycin-cyclophosphamide-based chemotherapySupport Care Cancer201119680781320461438
- TakeshimaNMatodaMAbeMEfficacy and safety of triple therapy with aprepitant, palonosetron, and dexamethasone for preventing nausea and vomiting induced by cisplatin-based chemotherapy for gynecological cancer: KCOG-G1003 phase II trialSupport Care Cancer201422112891289824825735
- DranitsarisGMolassiotisAClemonsMThe development of a prediction tool to identify cancer patients at high risk for chemotherapy-induced nausea and vomitingAnn Oncol20172861260126728398530
- TrammelMRoedererMPatelJMcLeodHDoes pharmacogenomics account for variability in control of acute chemotherapy-induced nausea and vomiting with 5-hydroxytryptamine type 3 receptor antagonists?Curr Oncol Rep201315327628523512709
- de WitRAaproMBlowerPRIs there a pharmacological basis for differences in 5-HT3-receptor antagonist efficacy in refractory patients?Cancer Chemother Pharmacol200556323123815838653
- DixonCMColthupPVSerabjit-SinghCJMultiple forms of cytochrome P450 are involved in the metabolism of ondansetron in humansDrug Metab Dispos19952311122512308591723
- SanwaldPDavidMDowJCharacterization of the cytochrome P450 enzymes involved in the in vitro metabolism of dolasetron. Comparison with other indole-containing 5-HT3 antagonistsDrug Metab Dispos19962456026098723743
- HassanBAYusoffZBGenetic polymorphisms in the three Malaysian races effect granisetron clinical antiemetic actions in breast cancer patients receiving chemotherapyAsian Pac J Cancer Prev201112118519121517255
- AndersenRLJohnsonDJPatelJNPersonalizing supportive care in oncology patients using pharmacogenetic-driven treatment pathwaysPharmacogenomics201617441743426871520
- NakamuraHAriyoshiNOkadaKNakasaHNakazawaKKitadaMCYP1A1 is a major enzyme responsible for the metabolism of granisetron in human liver microsomesCurr Drug Metab20056546948016248838
- KaiserRSezerOPapiesAPatient-tailored antiemetic treatment with 5-hydroxytryptamine type 3 receptor antagonists according to cytochrome P-450 2D6 genotypesJ Clin Oncol200220122805281112065557
- AaproMGranisetron: an update on its clinical use in the management of nausea and vomitingOncologist20049667368615561811
- de LeonAPalonosetron (Aloxi): a second-generation 5-HT3 receptor antagonist for chemotherapy-induced nausea and vomitingProc (Bayl Univ Med Cent)200619441341617106506
- MorrisonDAndersonASlamaMPhase 1 bioavailability study comparing 2 different subcutaneous routes of administration for APF530Support Care Cancer201523Suppl111625315366
- BoyerEWShannonMThe serotonin syndromeN Engl J Med2005352111112112015784664
- US Food and Drug AdministrationFDA drug safety communication: new information regarding QT prolongation with ondansetron (Zofran)2012 Available from: http://www.fda.gov/Drugs/Drug-Safety/ucm310190.htmAccessed March 2, 2017
- MorganrothJFlahartyKKParisiSMoresinoCEffect of single doses of IV palonosetron, up to 2.25 mg, on the QTc interval duration: a double-blind, randomized, parallel group study in healthy volunteersSupport Care Cancer201524262162726111957
- SpinelliTMoresinoCBaumannSTimmerWSchultzAEffects of combined netupitant and palonosetron (NEPA), a cancer supportive care antiemetic, on the ECG of healthy subjects: an ICH E14 thorough QT trialSpringerplus2014338925105088
- MasonJWSelnessDSMoonTEO’MahonyBDonachiePHowellJPharmacokinetics and repolarization effects of intravenous and transdermal granisetronClin Cancer Res201218102913292122452942
- MasonJWMoonTEO’BoyleEDietzAA randomized, placebo-controlled, four-period crossover, definitive QT study of the effects of APF530 exposure, high-dose intravenous granisetron, and moxifloxacin on QTc prolongationCancer Manag Res2014618119024741326
- BryggerLHerrstedtJ5-Hydroxytryptamine 3 receptor antagonists and cardiac side effectsExpert Opin Drug Saf201413101407142225196083
- FeyerPJahnFJordanKProphylactic management of radiation-induced nausea and vomitingBiomed Res Int2015201589301326425557
- SchwartzbergLProgress in chemoradiotherapy-induced nausea and vomitingLancet Oncol201617441241326952946
- RuhlmannCHBelliCDahlTHerrstedtJPalonosetron and prednisolone for the prevention of nausea and emesis during fractionated radiotherapy and 5 cycles of concomitant weekly cisplatin: a phase II studySupport Care Cancer201321123425343123949739
- RuhlmannCHChristensenTBDohnLHEfficacy and safety of fosaprepitant for the prevention of nausea and emesis during 5 weeks of chemoradiotherapy for cervical cancer (the GAND-emesis study): a multinational, randomised, placebo-controlled, double-blind, phase 3 trialLancet Oncol201617450951826952945
- SuginoSJanickiPKPharmacogenetics of chemotherapy-induced nausea and vomitingPharmacogenomics201516214916025616101
- TesaroTesaro announces fourth-quarter and full-year 2017 operating results2018 Available from: http://ir.tesarobio.com/news-releases/news-release-details/tesaro-announces-fourth-quarter-and-full-year-2017-operatingAccessed March 5, 2018