Abstract
Background
Tumor cell dissemination after needle biopsy has been reported in a variety of malignancies, including non-small-cell lung cancer (NSCLC). However, there is little clinical evidence in regard to whether preoperative biopsy increases the risk of recurrence in completely resected NSCLC.
Patients and methods
A total of 322 patients diagnosed as pathological stage I NSCLC using intraoperative biopsy (IOB) (control group), preoperative percutaneous needle biopsy (PNB) or bronchoscopic biopsy were included in this study. Baseline characteristics were collected and compared. The disease-free survival (DFS) of patients was analyzed using Kaplan–Meier method. Subgroup analysis and Cox regression were performed to evaluate the effect of preoperative biopsy on recurrence risk with adjustment for potential confounders.
Results
Among these patients, 202 (63%) underwent IOB, 66 (20%) underwent PNB, and 54 (17%) underwent bronchoscopic biopsy. DFS of patients who had preoperative PNB or bronchoscopic biopsy was similar to those who had IOB (P=0.514 and 0.869). Neither preoperative PNB nor transbronchial biopsy significantly affected recurrence incidence across all the relevant subgroups. Furthermore, multivariate analysis showed that preoperative biopsy was not associated with increased recurrence risk in NSCLC patients with adjustment for confounders, while squamous cell carcinoma and adjuvant chemotherapy were associated with prolonged DFS.
Conclusion
Neither preoperative PNB nor bronchoscopic biopsy increased the recurrence risk in patients with resected stage I NSCLC, indicating that these procedures could be safely used for diagnosis of early-stage NSCLC.
Introduction
Lung cancer is the leading cause of cancer death among both men and women.Citation1 Non-small-cell lung cancer (NSCLC) accounts for ~85% of lung cancer.Citation2 Currently, preoperative biopsy, including percutaneous needle biopsy (PNB) and bronchoscopic biopsy, has been widely used for pathological diagnosis of pulmonary nodules suspected as lung cancer. These diagnostic methods are highly accurate, minimally invasive, and can potentially benefit patients by providing a definite diagnosis and more flexible treatment options before surgery.Citation3
While preoperative PNB and bronchoscopic biopsy are believed to improve the accuracy and efficiency in NSCLC diagnosis, there has been a concern that these procedures may cause tumor cell dissemination, thus increasing recurrence incidence after surgery. Tumor cells, on one hand, may contaminate the biopsy needles and seed along the biopsy route.Citation4–Citation8 On the other hand, since malignancies are enriched in blood supply, there is a possibility that tumor cells displaced during biopsy, such as PNB and bronchoscopic biopsy, may enter the bloodstream and lead to metastasis in certain organs.Citation9–Citation11 Previously, several retrospective studies have explored the potential influence of preoperative PNB on pleural recurrence risk in early-stage NSCLC. But the clinical outcome assessed in these studies was only local recurrence limited to pleura, and their conclusions were contradictory.Citation12–Citation16 Besides, patients who had bronchoscopic biopsy were either excluded or used as control subjects for analysis together with those who had intraoperative biopsy (IOB). Therefore, whether preoperative biopsy could increase the risk of total recurrence by triggering hematogenous dissemination in NSCLC patients still remains unknown.
Herein, we retrospectively investigated the potential influence of preoperative PNB and bronchoscopic biopsy on recurrence risk in stage I NSCLC patients who underwent curative surgeries at our hospital.
Patients and methods
Patients
A review of the medical records was made of all 868 NSCLC patients who underwent complete resection between January 2010 and September 2014 at Xiangya Hospital, Central South University (Changsha, China). The inclusion criteria for patient were listed as follows: solitary pulmonary lesions on radiological images presenting as suspected lung cancer; completely resected NSCLC; diagnosis of NSCLC confirmed by pathological examination of surgical tissue specimens; and pathological stage I NSCLC (the seventh edition of tumor-node-metastasis staging system). Patients with comorbidity of other malignancies or who were lost to follow-up were excluded. A total of 466 patients confirmed as stage II NSCLC, 71 patients lost to follow-up, 3 patients with comorbidity of other malignancies, and 6 patients with incomplete medical records were excluded. Finally, 322 patients were included in this study. The median follow-up period for the entire cohort was 78 months. This study was approved by the Institutional Review Board and Ethics Committee of Central South University. Written informed consent was waived as this was a retrospective study.
Diagnostic methods
PNB, bronchoscopic biopsy, and IOB were performed for pathologic diagnosis of NSCLC in a single-center (Xiangya Hospital) setting using standard methods.
For PNB, computed tomography (CT) scans were performed at 110 kV, 25 mA using a 4×1.25 mm collimation (Philips, Amsterdam, The Netherlands). After an initial CT examination for biopsy planning, needle access was chosen individually according to the tumor localization. The semiautomatic biopsy system 18G (TSK Laboratory, Tochigi-Ken, Japan) was used to take tumor samples.
Bronchoscopic biopsy was performed under local anesthesia in standard fashion as previously described.Citation17 BF-260 video bronchoscope (Olympus, Tokyo, Japan) was used and tumor samples were taken with regular disposable biopsy forceps. In patients whose lesions could not be directly seen under bronchoscope, biopsy was performed under the guidance of endobronchial ultrasonography.
IOB was performed by the Department of Thoracic Surgery, Xiangya Hospital.Citation18 Briefly, the pulmonary lesion was completely resected, with a 2 cm surgical margin or a margin equivalent to the maximal diameter of the lesion. Tissue samples were subsequently obtained from the resected pulmonary lesions, immediately put into 10% neutral buffered formalin, and sent to the Department of Pathology for rapid frozen section analysis. If the pulmonary lesion was confirmed as malignancy, lobectomy or pneumonectomy and systemic lymphadenectomy were further performed to achieve a curative resection. Tumor tissue samples were obtained from the resected tumors and again sent to the Department of Pathology for pathological examination, the result of which was used as the golden standard of final diagnosis for patients.
Bronchoscopic evaluation of all the patients was made by pulmonologists at the hospital.
Assessment of recurrence
We reviewed the medical records of all patients to analyze disease-free survival (DFS). DFS was defined as the period of time that patients were left with no detectable disease after complete surgical resection of primary tumors in lung. Recurrence events were determined if any of the following happened after curative surgery: malignant pleural or pericardial effusion; pleural malignant nodules in thoracic imaging; intrapulmonary recurrences or metastases; and extrathoracic metastases. Local recurrences were defined as any recurrences within the ipsilateral thorax.
Statistical analysis
The normal distribution of tumor sizes in NSCLC patients was confirmed by one-sample Kolmogorov–Smirnov test, and subsequently the unpaired t-test was used to compare the tumor sizes of patients who underwent PNB, bronchoscopic biopsy, or IOB. Otherwise, the differences in baseline characteristics were compared using the chi-square test. The Kaplan–Meier method was used to estimate survival rates. Using the log-rank test, we compared DFS of patients in different groups. The association of PNB or bronchoscopic biopsy with recurrence incidence was evaluated by conducting subgroup analysis and Cox proportional hazard regression model. All statistical analyses were carried out using the SPSS Statistics version 24.0 (IBM Corp, Armonk, NY, USA). A P-value <0.05 was considered to be statistically significant.
Results
Baseline characteristics of patients
As shown in , a total of 322 NSCLC patients were included in this study. Among them, 66 underwent preoperative PNB, 54 underwent preoperative bronchoscopic biopsy, and 202 underwent IOB as part of the diagnostic workup. Compared with IOB, PNB was more frequently performed in patients with larger-sized tumor (2.52±0.07 vs 2.33±0.06 cm, P=0.015) and squamous cell carcinoma (SCC) (30% vs 17%, P=0.031). All other baseline clinicopathologic characteristics were not significantly different between PNB and IOB groups. In contrast, bronchoscopic biopsy was far more frequently used in male (96% vs 57%, P<0.001) patients of SCC (86% vs 17%, P<0.001), and those with smoking history (83% vs 44%, P<0.001) and later T stage (59% vs 23%, P<0.001) in comparison with IOB. Additionally, thoracotomy (87% vs 51%, P<0.001) was more frequently applied in patients of bronchoscopic biopsy group.
Table 1 Baseline characteristics of patients with resected early-stage NSCLC
Unadjusted survival analysis
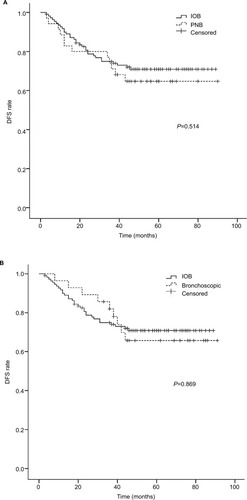
Recurrence pattern of NSCLC patients included in this study is shown in . Brain, bone, and ipsilateral thorax were the most frequent recurrence locations. DFS curves were similar among patients who underwent PNB, bronchoscopic biopsy, or IOB, as determined by Kaplan–Meier analysis (). No significant difference in overall DFS was observed.
Table 2 Recurrence patterns of patients with resected early-stage NSCLC
Figure 1 Preoperative percutaneous needle biopsy and bronchoscopic biopsy did not increase recurrence incidence in NSCLC patients.
Notes: (A) DFS curves for patients who had percutaneous needle biopsy or intraoperative biopsy. (B) DFS curves for patients who had bronchoscopic biopsy or intraoperative biopsy.
Abbreviations: DFS, disease-free survival; IOB, intraoperative biopsy; NSCLC, non-small-cell lung cancer; PNB, percutaneous needle biopsy.
Subgroup analysis
Since some baseline characteristics of patients who underwent PNB or bronchoscopic biopsy were significantly different from that of the patients in IOB group, we further performed subgroup analysis to control the influences of confounders. Neither PNB nor bronchoscopic biopsy affected the risk of recurrence across relevant subgroups ( and ). Several subgroups (female, adenocarcinoma and video-assisted thoracoscopic surgery subgroups, etc.) were not included in the comparison between bronchoscopic biopsy and IOB because sample number was too small.
Table 3 Subgroup analysis of recurrence risk in NSCLC patients who underwent percutaneous needle biopsy
Table 4 Subgroup analysis of recurrence risk in NSCLC patients who underwent bronchoscopic biopsy
Cox regression analysis
Univariate analysis was performed to determine the potential prognostic factors of DFS in patients with resected stage I NSCLC. It revealed that tumor size, histologic subtypes, surgery types, and adjuvant chemotherapy were associated with DFS (). Subsequently, multivariate analysis demonstrated that SCC (hazard ratio [HR], 0.65; 95% CI: 0.41–0.96; P=0.036) and adjuvant chemotherapy (HR, 0.56; 95% CI: 0.44–0.73; P<0.001) were independent prognostic factors of DFS (). Preoperative PNB (HR, 1.08; 95% CI: 0.55–2.15; P=0.821) and bronchoscopic biopsy (HR, 0.78; 95% CI: 0.48–1.26; P=0.302) were not associated with DFS after adjusting for tumor size, histologic subtypes, surgery types, and adjuvant chemotherapy ().
Table 5 Univariate analysis of prognostic factors for DFS in NSCLC patients after radical resection
Table 6 Multivariate analysis of prognostic factors for DFS in NSCLC patients after radical resection
Discussion
PNB and bronchoscopic biopsy are commonly used diagnostic methods for pulmonary lesions when there is a suspicion of lung cancer. However, there has been a concern that invasive biopsy procedures may cause tumor cell dissemination, thus increasing the recurrence risk after radical resection. In the present study, we found that preoperative PNB and bronchoscopic biopsy did not increase recurrence incidence in early-stage NSCLC patients after curative surgery.
Malignancies are usually well vascularized. When a needle or forceps for biopsy is inserted into the tumor, displacement of a small amount of tumor cells is theoretically unavoidable. These cells may enter the bloodstream and colonize in certain sites if they are not eliminated by human immune system.Citation9 In this case, preoperative biopsy might cause recurrence in NSCLC patients, but this possibility has been barely investigated yet. In this study, we retrospectively analyzed the recurrence outcomes of NSCLC patients who underwent preoperative PNB, bronchoscopic biopsy, or IOB at our hospital. Our data revealed that preoperative PNB or bronchoscopic biopsy did not significantly affect DFS in NSCLC patients after radical resection. Since bronchoscopic biopsy is much more applicable to the diagnosis of central type lung cancer, it was not surprising that most cases in the bronchoscopic biopsy group were male smoker patients with SCC, and the therapeutic methods used in these patients were also different from other groups. Therefore, subgroup analysis and Cox regression were performed to control the imbalance of unmatched baseline characteristics. Compared with IOB, no significant difference in recurrence risk was observed in patients who had preoperative PNB or bronchoscopic biopsy, suggesting that these procedures are safe diagnostic methods without increasing recurrence incidence. In addition, based on our data, histologic subtype and adjuvant chemotherapy were independent prognostic factors of DFS in NSCLC patients, which was consistent with previous studies.Citation19,Citation20 It is worth noting that, in the present study, 38.8% (125/322) of NSCLC patients underwent adjuvant chemotherapy, and all of them were evaluated as pathological stage IB NSCLC. Actually, whether adjuvant chemotherapy should be applied in stage IB NSCLC has remained controversial so far. According to National Comprehensive Cancer Network guidelines, adjuvant chemotherapy could be appropriate for stage IB NSCLC patients with high-risk factors, such as poorly differentiated tumors, vascular invasion, tumors >4 cm, and visceral pleural involvement. Recently, increasing amount of clinical studies has revealed the benefits of adjuvant chemotherapy for stage IB NSCLC patients.Citation21,Citation22 These studies and our data consistently supported the beneficial effect of adjuvant chemotherapy in high-risk stage IB NSCLC patients.
Whether biopsy can cause cancer cells to spread is controversial. It has been reported that needle biopsy may shed cancer cells into peripheral blood, as indicated by significantly increased mRNA levels of tumor markers after biopsy.Citation10,Citation11 However, entry of a small number of tumor cells into blood does not necessarily lead to recurrence or metastases. Instead, in most cases, these cells die soon due to anoikis or are eliminated by immune systems, and successful metastatic colonization is a more inefficient business even though several cells survive in the bloodstream.Citation23 Indeed, there is increasing amount of clinical evidence strongly supporting that preoperative biopsy does not spread cancer or affect survival outcomes in patients with malignancies.Citation24–Citation26
In terms of lung cancer, several cases of tumor cell implantationCitation4–Citation8 and increased incidence of pleural recurrence after needle biopsy have been reported in NSCLC patients.Citation12,Citation13,Citation15 However, Sano et al reported that preoperative percutaneous CT-guided needle biopsy did not lead to pleural dissemination, as determined by intraoperative pleural lavage cytology in patients with lung cancer.Citation27 Recent studies also demonstrated that PNB was not associated with increased pleural recurrence or impaired survival outcomes in NSCLC patients.Citation14,Citation16,Citation28 Importantly, a recent meta-analysis, including these related observational studies revealed that PNB did not increase the risk of pleural recurrence compared with non-percutaneous transthoracic needle biopsy strategies in early-stage lung cancer patients.Citation29 The difference between the present study and the previous ones is that, since the application of coaxial biopsy needles has greatly reduced the incidence of needle track implantation, we are now focusing on the possibility of hematogenous dissemination after biopsy. Therefore, total recurrence, instead of pleural recurrence, was used as an endpoint event of interest in the present study. Additionally, we also investigated the potential influence of bronchoscopic biopsy on recurrence, because bronchoscopic biopsy is also invasive and could theoretically cause tumor cell displacement like PNB. Collectively, previous studies and our data support that preoperative PNB and bronchoscopic biopsy are safe diagnostic methods without increasing recurrence in NSCLC. But this knowledge should not interfere with surgical techniques that may benefit the patient by limiting procedural morbidity.
Several limitations should be noted in the present study. First, patient selection bias is unavoidable because this is a single-center retrospective study. The relatively small number of patients, which is partially caused by the strict inclusion criteria and long-term follow-up, also reduces the validity when interpreting the results. In addition, the subjects in this study were stage I NSCLC patients presenting as solitary pulmonary lesions in radiological images. Thus, we could not conclude whether these findings are applicable to other NSCLC populations. More studies with larger patient numbers are required to solve this issue.
Conclusion
The present study showed that preoperative biopsy, including PNB or bronchoscopic biopsy, was not associated with increased risk of recurrence and did not affect DFS in resected stage I NSCLC patients. Therefore, the use of these diagnostic procedures should be considered in the management of patients with pulmonary nodules suspected as early-stage NSCLC.
Acknowledgments
This work was supported in part by grants from the National Natural Science Foundation of China (81600025) and the National Key Research and Development Program of China (SQ2016YFSF110276).
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2016CA Cancer J Clin201666173026742998
- LiJJLiRWangWIDH2 is a novel diagnostic and prognostic serum biomarker for non-small-cell lung cancerMol Oncol201812560261029465809
- XiaoDLuCZhuWComparison of small biopsy specimens and surgical specimens for the detection of EGFR mutations and EML4-ALK in non-small-cell lung cancerOncotarget2016737590495905727322143
- KaraMAlverGSakSDKavukcuSImplantation metastasis caused by fine needle aspiration biopsy following curative resection of stage IB non-small cell lung cancerEur J Cardiothorac Surg200120486887011574245
- ScottiVDi CataldoVFalchiniMIsolated chest wall implantation of non-small cell lung cancer after fine-needle aspiration: a case report and review of the literatureTumori2012985126e129e22495713
- VoravudNShinDMDekmezianRHDimeryILeeJSHongWKImplantation metastasis of carcinoma after percutaneous fine-needle aspiration biopsyChest199210213133151623781
- RaftopoulosYFureyWWKaceyDJPodbielskiFJTumor implantation after computed tomography-guided biopsy of lung cancerJ Thorac Cardiovasc Surg200011961288128910838551
- YoshikawaTYoshidaJNishimuraMYokoseTNishiwakiYNagaiKLung cancer implantation in the chest wall following percutaneous fine needle aspiration biopsyJpn J Clin Oncol2000301045045211185892
- ShyamalaKGirishHCMurgodSRisk of tumor cell seeding through biopsy and aspiration cytologyJ Int Soc Prev Community Dent20144151124818087
- LouhaMNicoletJZylberbergHLiver resection and needle liver biopsy cause hematogenous dissemination of liver cellsHepatology199929387988210051492
- HuXCChowLWFine needle aspiration may shed breast cells into peripheral blood as determined by RT-PCROncology200059321722211053989
- MatsugumaHNakaharaRKondoTKamiyamaYMoriKYokoiKRisk of pleural recurrence after needle biopsy in patients with resected early stage lung cancerAnn Thorac Surg20058062026203116305838
- InoueMHondaOTomiyamaNRisk of pleural recurrence after computed tomographic-guided percutaneous needle biopsy in stage I lung cancer patientsAnn Thorac Surg20119141066107121440124
- FlechsigPKunzJHeusselCPInvasive lung cancer staging: influence of CT-guided core needle biopsy on onset of pleural carcinomatosisClin Imaging2015391566125457543
- KashiwabaraKSembaHFujiiSTsumuraSPreoperative percutaneous transthoracic needle biopsy increased the risk of pleural recurrence in pathological stage I lung cancer patients with sub-pleural pure solid nodulesCancer Invest201634837337727532604
- AsakuraKIzumiYYamauchiYIncidence of pleural recurrence after computed tomography-guided needle biopsy in stage I lung cancerPLoS One201278e4204322876299
- AnJYangHPHuCPMultinodule abnormalities of the tracheo-bronchus: bronchoscopy findings and clinical diagnosisClin Respir J201711444044726260022
- LuoFYLiuZHHuQHAssociation of BTBD7 with metastasis and poor prognosis in non-small-cell lung cancer patientsJ Cancer20156547748125874012
- ChanskyKSculierJPCrowleyJJGirouxDVanMeerbeeck JGold-strawPInternational Staging Committee and Participating InstitutionsThe International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancerJ Thorac Oncol20094779280119458556
- WuFLiuXMaJAGaoYHuCAdjuvant therapy for resected EGFR-mutant non-small-cell lung cancerLancet Oncol2018193e12429508749
- BurdettSPignonJPTierneyJNon-Small Cell Lung Cancer Collaborative GroupAdjuvant chemotherapy for resected early-stage non-small cell lung cancerCochrane Database Syst Rev20153CD011430
- MorgenszternDDuLWaqarSNAdjuvant chemotherapy for patients with T2N0M0 NSCLCJ Thorac Oncol201611101729173527287414
- SteegPSTumor metastasis: mechanistic insights and clinical challengesNat Med200612889590416892035
- NgamruengphongSXuCWoodwardTARisk of gastric or peritoneal recurrence, and long-term outcomes, following pancreatic cancer resection with preoperative endosonographically guided fine needle aspirationEndoscopy201345861962623881804
- NgamruengphongSSwansonKMShahNDWallaceMBPreoperative endoscopic ultrasound-guided fine needle aspiration does not impair survival of patients with resected pancreatic cancerGut20156471105111025575893
- LoughranCFKeelingCRSeeding of tumour cells following breast biopsy: a literature reviewBr J Radiol201184100686987421933978
- SanoYDateHToyookaSPercutaneous computed tomography-guided lung biopsy and pleural dissemination: an assessment by intraoperative pleural lavage cytologyCancer2009115235526553319685526
- WisniveskyJPHenschkeCIYankelevitzDFDiagnostic percutaneous transthoracic needle biopsy does not affect survival in stage I lung cancerAm J Respir Crit Care Med2006174668468816799079
- WangTLuoLZhouQRisk of pleural recurrence in early stage lung cancer patients after percutaneous transthoracic needle biopsy: a meta-analysisSci Rep201774276228202941
