Abstract
Adaptive metabolic responses toward a low oxygen environment are essential to maintain rapid proliferation and are relevant for tumorigenesis. Reprogramming of core metabolism in tumors confers a selective growth advantage such as the ability to evade apoptosis and/or enhance cell proliferation and promotes tumor growth and progression. One of the mechanisms that contributes to tumor growth is the impairment of cancer cell metabolism. In this review, we outline the small-molecule inhibitors identified over the past decade in targeting cancer cell metabolism and the usage of some of these molecules in clinical trials.
Introduction
Cancer is one of the leading causes of morbidity and mortality worldwide, with approximately 14 million new cases and 8.2 million cancer-related deaths reported in 2012.Citation1 It is a major disease burden worldwide and will be a prominent health issue globally. The inability of cells to respond to stress and to repair damage underlies many forms of cancer.
Lung, liver, stomach, and bowel cancers are the most common causes of cancer death worldwide, accounting for nearly half of all cancer death. Lung and breast cancers were reported as the most common cancers diagnosed in men and women, respectively.Citation2 Interestingly, in more developed countries (as defined by the United Nations which includes all regions of Europe, Northern America, Australia/New Zealand, and Japan), prostate and lung cancers are the leading cause of cancer death in men and women, respectively.Citation2 The regional imparity in the mix of cancers is driven largely by the availability of improved treatment and technological progress in early detection of tumor.Citation3 Cancer is also regarded differently in different settings, by which in high-income countries, it is regarded as a preventable and often curable disease. However, in many low- and middle-income countries, it is regarded as a painful death sentence. According to the World Health Organization, it is expected that the number of annual new cases to rise by 70% for the next 20 years.
Past research has identified many oncogenes and tumor suppressors that are frequently altered in human tumors. These altered genes affect key signaling pathways that govern the cell cycle and thus have the potential to be therapeutic targets. Multiple classes of chemotherapy drugs are used, mainly in combination, to target cancer cells more effectively. In recent years, it has become apparent that differences in the genetic background of cancer patients result in varying responses to chemotherapy. This has led to an increased research focus on pharmacogenomics. Cancer pharmacogenomics relates to the study of germline genetic variants that contribute to a chemotherapy-induced phenotype.Citation4
A fundamental characteristic of cancer cells is their ability to sustain indefinite cycles of proliferation by deregulating the release of growth signals. Growth-promoting signals govern entry and progression of cells through the cell cycle, thereby ensuring the homeostasis of cell number and maintenance of normal tissue architecture and function. In 2000, Hanahan and Weinberg outlined the acquired capability of “hallmarks of cancer,” which includes the ability to sustain growth signals, avoid growth suppressors, invade and metastasize to other tissues, induce angiogenesis, resist cell death, and proliferate indefinitely.Citation5 In 2011, these hallmarks were updated to include the ability of cancer cells to maintain the tumor microenvironment.Citation6 Among the ways, cancer cells achieve this by metabolic reprogramming that involves altering energy metabolism in order to fuel their growth and division.Citation6 Mitochondria have long been recognized as the powerhouses of the cell and have recently received recognition as a potential target for therapeutic intervention of cancer.
In contrast to normal undifferentiated cells, which mainly rely on oxidative phosphorylation (OXPHOS) to produce energy for cellular processes, cancer cells in the hypoxic microenvironment rely on glycolysis as their primary energy source. The phenomenon in which cancer cells preferentially use glycolysis to metabolize glucose in the presence of oxygen was identified by Otto Warburg and was later known as aerobic glycolysis. At that time, Warburg hypothesized that the switch to energy production by glycolysis in cancer cells was due to irreversible mitochondrial damage leading to permanent impairment of aerobic respiration.Citation7 However, this hypothesis was disputed as most cancer cells retain functional mitochondria. Although aerobic glycolysis is often found in malignant tumors, OXPHOS still contributes to energy production in cancers and may play a major role in energy production in some cancers.Citation8 Nonetheless, the “Warburg effect” is one of the best studied metabolic phenotype of cancer cells.
In this review, we focus on the research conducted over the past decade in identifying potential therapeutic targets of cell metabolism mainly in cancer. We summarize published in vivo, in vitro, and clinical studies of new evidences and novel small molecules that have implications in regulating cancer cell metabolism.
The OXPHOS system in mitochondria
Functionally, mitochondria are the key players in cellular adenosine triphosphate (ATP) production, fatty acid oxidation, heme biosynthesis, apoptosis induction, heat generation, and calcium homeostasis. Mitochondria are best known for their role in ATP production, which is performed via OXPHOS. The mitochondrial OXPHOS system is embedded in the mitochondrial inner membrane (MIM) and represents the final step in the conversion of nutrients to energy by catalyzing the generation of ATP. Carbon fuels are oxidized in the citric cycle to yield high-energy electrons.Citation9 This electron motive force is converted into a proton motive force, and this, in turn, is converted into a phosphoryl transfer potential. The flow of electrons from nicotinamide adenine dinucleotide (NADH) or flavin adenine dinucleotide (FADH) to oxygen through protein complexes located in the MIM leads to the pumping of protons out of the matrix. The resulting uneven distribution of protons generates a pH gradient and trans-membrane electrical potential that creates the proton motive force. ATP is synthesized when protons flow back to the mitochondrial matrix through ATP synthase (complex V).Citation10 The membrane potential is essential for other mitochondrial functions such as mitochondrial protein import and is used to trigger molecular changes that alter mitochondrial behavior in response to mitochondrial dysfunction.Citation9 Induction of cell death is influenced by changes in the amount of ATP generated by the mitochondria, and this death may occur in various ways depending on the level of ATP in the cell. In several cell types, it has been shown that a change in the amount of ATP production is sufficient in itself to initiate apoptosis.Citation10,Citation11 The intrinsic apoptotic pathway is initiated following the activation of caspase-9, which requires Apaf-1. In the absence of apoptotic stimuli, Apaf-1 exists in a monomeric form. In response to apoptotic stimuli, cytochrome c activates Apaf-1 by binding to its C-terminus. Concurrently, ATP bound to the ATPase domain of Apaf-1 is hydrolyzed, and this promotes Apaf-1 oligomerization.
Interestingly, Izyumov et al reported a two-third decrease of the initial intracellular ATP level in HeLa cells, which was achieved by using 2-deoxyglucose (DOG) and OXPHOS inhibitors, and was found to induce apoptosis.Citation12 In contrast, a severe depletion of ATP (>70%) using staurosporine (protein kinase inhibitor) in human T cells resulted in a switch in response of cells to typical apoptotic stimuli from apoptosis to necrosis.Citation12 Collectively, these data suggest that cell death is elicited under a compromised ATP level. Apoptosis inactivates PARP-1, whereas necrosis causes PARP-1 overactivation, which consumes large amount of NAD+ resulting in massive ATP depletion.Citation10,Citation13 Perhaps modulating in vivo ATP availability might be an important strategy in inducing cell death and preventing tumor growth and progression.
OXPHOS as a therapeutic target
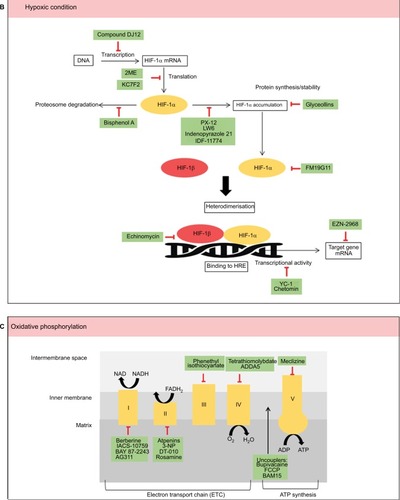
Aerobic glycolysis has long been accepted as one of the pathways utilized by proliferative cancer cells to meet their bioenergetics demand, which is essential in tumorigenesis. Elevated mitochondria-derived reactive oxygen species (ROS), a by-product of electron transport chain activity, regulate both tumor cell proliferation and quiescence and promote metastasisCitation14 and genomic instability.Citation15 This review discusses on the current small-molecule inhibitors, typically less than 900 DaCitation16 that are developed to target the mitochondrial OXPHOS in cancer cells ().
Table 1 List of OXPHOS drugs and their functions
Uncouplers transport protons into mitochondrial matrix via ATP-synthase-independent pathway. Hence, this reduces the proton motive force across the MIM and decreases the mitochondrial ROS production. Mitochondrial uncouplers such as FCCP not only inhibit mitochondrial membrane potential but may also affect the membrane potential and cell volume.Citation17 Kenwood et al reported a bona fide mitochondria uncoupler, BAM15 that has preference for protonophore activity at the mitochondria but not at the plasma membrane. The exact mechanism of BAM15 remains unclear. However, BAM15 was capable in increasing mitochondrial respiration in the presence of an ATP synthase inhibitor, providing evidence of BAM15 as a mitochondrial uncoupler.Citation18 It was suggested that the effect is due to the structural preference of BAM15 for the lipid composition of the inner mitochondrial membrane.Citation18
A high-throughput screening of small-molecule library using luciferase-driven hypoxia-inducible factor (HIF)-1 reporter HCT116 cells under hypoxic condition led to the identification of BAY 87-2243. BAY 87-2243, a class of aminoalkyl-substituted compounds, was initially found to inhibit HIF-1α and HIF-2α protein accumulation without affecting HIF target gene expression levels.Citation17 Further investigations revealed that BAY 87-2243 inhibited in vitro and in vivo mitochondrial complex I activity which prevented ROS-mediated HIF-1 inhibition.Citation19,Citation20 It was also noted that BAY 87-2243 restored prolyl hydroxylase enzyme activity, a negative regulator of HIF-1, and thus, a reduced level of HIF-1 was observed.Citation19 Combination of B-RAF inhibitor that upregulates OXPHOS and BAY 87-2243 was shown to induce augmented tumor regression in nude mice-bearing B-RAF mutant melanoma xenografts.Citation20 This evidence provides a promising future for combination of targeted therapies to prevent resistance to either agent.
A biochemical absorbance-based high-throughput study was used to identify the small-molecule inhibitor of complex IV in mitochondrial extracts. The current available complex IV inhibitors either bind to the allosteric siteCitation18 or inhibit the copper-dependent activityCitation21 within complex IV, which disrupts the electron transport chain. Tissues with high dependence on aerobic respiration, such as the central nervous system and heart, are affected upon treatment with these inhibitors. ADDA, 5(1-[2-(1-adamantyl)ethoxy]-3-(3,4-dihydro-2(1H)-isoquinolinyl)-2-propanol hydrochloride]), was found to possess a significant in vitro and mouse xenograft model (nude mouse, rear flank) antiglioblastoma multiforme activity by specifically noncompetitively inhibiting complex IV activity.Citation22 Of note, chemoresistance to temozolomide in glioblastoma multiforme is associated with an increased level of complex IV activity.Citation23
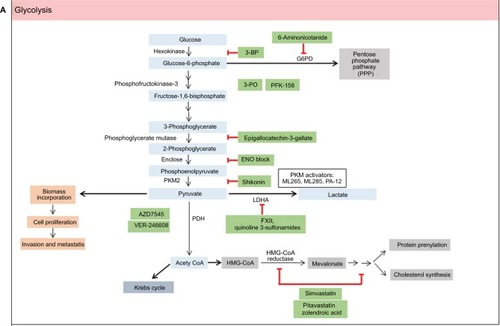
Glycolysis
Both normal and cancer cells utilize glucose and glutamine as sources of energy and also for biosynthesis of macromolecules. The high rate of glucose metabolism in cancer cells is facilitated by an increase in glucose transport by one or more isoenzymes of the glucose transporters (GLUT 1–4).Citation19 Glucose molecules are phosphorylated by hexokinases to form glucose-6-phosphate. Glucose-6-phosphate will either generate pyruvate or be fed into the pentose phosphate pathway (PPP) to generate ribose-5-phosphate and nicotinamide adenine dinucleotide phosphate (NADPH). Glutamine, on the other hand, is converted into glutamate in the cytosol by the enzyme glutaminase-1. Glutamate is then transported to the mitochondria, where it is converted into α-ketoglutarate. α-Ketoglutarate that enters the tricarboxylic acid (TCA) cycle results in the generation of malate and citrate. Glutamine serves as a source of carbon to replenish the TCA cycle, produces glutathione, and is a precursor to nucleotide and lipid synthesis via reductive carboxylation.Citation24
The inappropriate proliferation of cancer cells requires high energy, and the cells generate ATP to satisfy the biomass production. The enhanced glycolytic rate in cancer cells is required not only to meet the need for energy but also to maintain the level of glycolytic intermediates needed for biosynthesis of macromolecules. The biosynthetic activities required for proliferating cancer cells involve high rates of nucleotide synthesis, amino acid synthesis, and lipogenesis. Glycolysis provides essential molecules for both nucleotide and amino acid syntheses. The PPP consists of the oxidative generation of NADPH and nucleotide biosynthesis. The oxidation of glucose-6-phosphate generates NADPH, and the non-oxidative interconversion of phosphorylated sugars is required for nucleotide biosynthesis.
Glycolysis inhibitors
The accelerated glycolysis rate in cancer cells is associated with the overexpression of pathway enzymes, transporters, and isoenzymes with different regulatory properties.Citation25 Interestingly, overexpression of glycolytic enzymes was noted in almost 70% of human cancers.Citation26 The key enzymes involved in glycolysis are hexokinase, phosphofructokinase, and pyruvate kinase (PK). Inhibition of glycolysis severely impairs the ATP generation and renders cancer cells to be highly dependent on this metabolic pathway for survival. depicts small-molecule inhibitors of glycolysis along with their mechanisms emphasized on current molecular targets.
Table 2 List of glycolysis drugs and their functions
The generation of pyruvate is an important metabolic control point in cellular metabolism as it can be converted to either lactate and NAD+ by lactate dehydrogenase A (LDHA), which is important for continuation of glycolysis, or acetyl coenzyme A (CoA) by pyruvate dehydrogenase (PDH) for entry to glycolysis and mitochondrial respiration. NADH-competitive selective inhibitor of LDHA, quinolone-3-sulfonamide, was developed through high-throughput screening and leads optimization.Citation27 However, this compound was reported to possess an unacceptable pharmacokinetic profile that prevents it to be further investigated in in vivo models.Citation27
Acetyl CoA is involved in the synthesis of several lipid building blocks, which includes mevalonate. Protein farnesylation and geranylgeranylation are collectively known as prenylation, a lipid posttranslational modification that facilitates cell membrane anchoring and is required for oncoprotein transformation.Citation28 Statins are lipid-lowering drugs that block cholesterol biosynthesis by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the enzyme that catalyzes the rate-limiting step in the mevalonate pathway.Citation27 The role of statins in modulating the mevalonate pathway to exert the anticancer activity was reported as early as 2005.Citation29 In a recent study, mevalonate inhibitors, pitavatstatin and zolendronic acid, synergistically prevented geranylgeranylation, which resulted in inducing apoptosis in a panel of ovarian cancer cell lines.Citation30 Simvastatin exerted an antiproliferative effect in renal cancer cells through cholesterol deprivation and prenylation-associated mechanisms.Citation31
AZD3965 is a potent selective inhibitor of monocarboxylate transporter 1 (MCT1) found in cell lines displaying MCT1 and shows higher sensitivity in hypoxia.Citation32 Recent studies have demonstrated that AZD3965 inhibits lactate transport and cell growth in cancer cells which lack MCT4 protein.Citation32–Citation34 AZD3965 is currently tested in Phase I clinical trial.
The most promising drug-targeting cancer cell metabolism to date is 3-bromopyruvate, which was first discovered in the 1960s, but its anticancer properties were discovered in 2001.Citation15 3-Bromopyruvate, an alkylating agent, is a broad-spectrum inhibitor of multiple metabolic enzymes. It was shown to dampen ATP generation and inhibits multiple metabolic targets such as hexokinase 2 (HK 2), 3-phosphoglycerate kinase (PGK), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), lactate dehydrogenase (LDH), pyruvate dehydrogenase complex (PDC), succinate dehydrogenase (SDH), α-ketoglutarate dehydrogenase, isocitrate dehydrogenase (IDH), glyoxalases 1 and 2, and serine hydroxyethyltransferase.Citation35–Citation39 Currently, no clinical trials have been approved for 3-bromopyruvate even though two reports have already appeared describing the use of this inhibitor in volunteer patients with cancer.Citation40,Citation41
Small-molecule activators of glycolysis
PK is a rate-limiting glycolytic enzyme that catalyzes the final step of glycolysis, which produces pyruvate and ATP.Citation42 PK muscle isozyme, PKM2, is expressed in tissues with anabolic functions and is found both in cancer and in normal tissues. In the majority of cancer cells, the expression of PKM2 is increased, which suggests that PKM2 may be an attractive target for cancer therapy.Citation43 PKM2 is expressed in essentially all human cancers, and efforts have been made to use PKM2 as a cancer biomarker.Citation44
ML265 is a potent PKM2 activator that significantly reduces the tumor size in 7-week mouse xenograft without showing signs of acute toxicity.Citation45 ML265 was found to activate PKM2 in pervanadate-treated cells, a condition known to inhibit PKM2 activity through accumulation of phosphotyrosine peptides.Citation45 ML285, another small-molecule activator of PKM2, was found to reduce the shunt of glucose through the PPP. This sequentially allows the synthesis of NADPH, which is needed for generating reduced glutathione and required for ROS detoxification. ML285 protects the enzyme from oxidation by ROS and results in sensitization to oxidative stress and increased apoptosis.Citation46 Activation of PKM2 promotes tumor suppression in tumor growth in vivo.Citation47–Citation49 PA-12 stimulates the PK activity of recombinant PKM2 and effectively suppresses both anchorage-dependent and anchorage-independent growth of lung cancer cells in the nonessential amino acid-depleted medium.Citation50
Activation of AMP-activated protein kinase (AMPK) is required to facilitate the oxidative metabolism of non-glucose substrates, specially glutamine and lactate, to maintain cell survival. BL-AD008, a small-molecule activator of AMPK/ZIPK, showed antiproliferative activities toward cervical cancer cells. It induced apoptosis by targeting AMPK/ZIPK in cervical cancer.Citation51 AMPK direct activator MT 63-78 exerted growth inhibitory effects in prostate cancer cells in vitro as well as in xenograft models by lipogenesis inhibition.Citation52 shows the lists of small-molecule activators in glycolysis.
Table 3 List of small-molecule activators of glycolysis
Hypoxia targeting small-molecule inhibitors
Adaptive metabolic response toward a low oxygen environment is essential to maintain rapid tumor proliferation and progression.Citation53 The vascular network that surrounds the tumor creates an intermittent hypoxia, which plays a crucial role in cancer development, along with mitochondrial transformation associated with treatment failure.Citation54 When oxygen demand exceeds supply, HIF is switched on.Citation55 HIF is a heterodimer comprising α and β subunits that translocate into the nucleus.Citation56 Mammalian cells respond to hypoxia by implementing changes in gene expression controlled by the hypoxia-inducible factor 1 (HIF-1) transcription factor.Citation57,Citation58 Three different genes have been identified that encode the subunits of HIF: HIF1α, HIF2α, and HIF3α. All three HIFα subunits heterodimerized with HIF-1β subunit are subjected to posttranslational regulations dictated by the oxygen concentration in the environment.Citation59 In hypoxia, HIF-1α heterodimerized with the constitutively expressed HIF-1β [the aryl hydrocarbon receptor nuclear transporter (Arnt)] and translocated to the nucleus, where it bound to hypoxia response elements (HREs) in the promoters of various genes.Citation55 The interaction between HIF-1α and HIF-1β is critical in the process of tumor survival. A hypoxic state is important for tumor growth to start. It is thought that HIF-1 expression (HIF-1α and HIF-1β) controls the initiation of tumor growth and could be important in affecting the antitumor growth by its changing growth to be more malignant in a hypoxic state.Citation60 Therefore, therapeutic targeting of hypoxia in cancer has the potential to improve treatment efficacy.
Resistance to therapy in the presence of hypoxia was noted as early as the 1920s, with the first clinical implications observed in tumors in the lung that exhibited resistance to ionizing radiation. Under hypoxic conditions, DNA-damaging free radicals are rapidly reduced, thereby avoiding DNA damage. HIF-1 plays a central role in tumor pathology and is a target for treatment and therapy.Citation61–Citation65 To date, the small-molecule inhibitors that target HIF mainly decrease its protein levels, DNA-binding, or transactivation.Citation66,Citation67 lists various small molecules that target the hypoxic microenvironment in cancer.
Table 4 List of HIF drugs and their functions
Many different agents that specifically target HIF have been investigated for their role during hypoxia over the past few decades. PX-478 (S-2-amino-3-[4V-N,N,-bis(2-chloroethyl)amino]phenyl propionic acid N-oxide dihydrochloride) is known to suppress constitutive and hypoxia-induced levels of HIF-1α in various cancer cells, which include ovarian, colon, prostate, breast, renal, pancreatic, and lung.Citation68 It was noted to show marked tumor regression and growth delay in tumor xenografts with high level of HIF-1α.Citation69 This HIF-1α suppressor was shown to be selective in lowering HIF-1α and inhibiting its activity in multiple levels.Citation70 PX-478 has completed its Phase I clinical trial as an oral agent in lymphoma and advanced solid tumors.
Another small-molecule inhibitor that targets HIF1a is EZN-2968. EZN-2968 is an RNA antagonist composed of locked nucleic acid-based oligonucleotide that specifically binds and inhibits the expression of HIF-1α mRNA.Citation69,Citation70 A pilot study of EZN-2968 in patients with refractory solid tumors showed a reduction in HIF-1α mRNA and its target genes in tumor biopsies.Citation71 Third Phase I clinical trial has been recently initiated in patients with hepatocellular carcinoma to demonstrate the proof of mechanism of EZN-2968.
A library of aryloxy acetylaminobenzoic acid scaffold-focused screening followed by lead optimization for the identification of HIF-1α inhibitor resulted in the identification of IDF-11774, which was later shown to inhibit the expression of HIF-1α target genes. IDF-11774 inhibits angiogenesis, suppresses HIF-1α refolding, and stimulates proteosomal-mediated HIF-1α-degradation.Citation72 Additionally, IDF-11774 was shown to decrease the glucose-dependent energy metabolism.Citation72 This promising small-molecule inhibitor modulates both hypoxic regulators and glycolysis to prevent cancer cell growth.
Summary and future perspectives
Cancer metabolism is one of the oldest areas of research in cancer biology which pioneers from the identification of tumor suppressors and oncogenes. With explosion of research in cancer cell metabolism in the last decade, it is almost impossible to highlight all the findings in one review. In this review, we have discussed the various small molecules that were discovered in the last decade that are involved in cell metabolism, mainly glycolysis, OXPHOS, and hypoxia (). The advancement of current technologies especially in high-throughput studies allows rapid identification of compounds with selected and specific targets. The ability of these small molecules to render cell metabolism is supported by the impaired growth of tumor in vivo and/or in vitro.
Figure 1 Summary of metabolic pathways (A–C) and small molecules that modulate these pathways (green).
Abbreviation: HIF, hypoxia-inducible factor.
Rewiring metabolism in cancer cells plays a pivotal role in tumor survival, invasion, proliferation, and resistance to anticancer therapies. The use of cell metabolism small-molecule inhibitor in cancer treatment imposes several challenges. Several drugs that impair cell metabolism have demonstrated the modest effect in cancer therapy. This is most likely due to the complexity of the metabolic pathways in cancer. Many pathways and transcriptional factors associated with cancer metabolism are also involved in maintaining the physiological process of the body. For example, the normal tissues of brain, retinae, and testis use glucose as their main energy source. Impairment of glycolysis that limits the glucose supply may potentially be toxic to these tissues. Metabolic plasticity and heterogeneity in cancer cells drive tumor growth, mediate metastasis, and contribute to treatment resistance. Hence, the efficacy of cancer metabolism therapy requires careful evaluation. Inhibiting individual enzymes or blocking single pathways seldom leads to effective cancer treatment. Therefore, the combined approach of targeting cellular metabolism in conjunction with the use of chemotherapeutic drugs may provide a promising strategy to overcome therapeutic resistance and therefore aid in cancer therapy. In conclusion, the concept of cancer metabolism provides more targets for cancer treatment. Undoubtedly, like other targeted therapies being developed, therapies directed to cancer metabolism will be most effective if the orchestrations of metabolic alterations in cancer cells to support growth, proliferation, and differentiation are well understood.
Acknowledgments
We thank the Universiti Putra Malaysia (Programme Grant: Putra Grant GP-IPM/2014/9445100) for supporting this work. SHS was supported by Graduate Research Fellowship from Universiti Putra Malaysia and MyBrain program from Malaysian Ministry of Higher Education.
Disclosure
The authors report no conflicts of interest in this work.
References
- StewartBWWildCPWorld Cancer Report 2014Lyon, FranceWHO201412
- WHO [webpage on the Internet]Fact Sheets by Cancer2012 [cited June 2017[Available from: http://globocan.iarc.frAccessed June 7, 2018
- TorreLABrayFSiegelRLFerlayJLortettieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- WheelerHEMaitlandMLDolanMECoxNJRatainMJCancer pharmacogenomics: strategies and challengesNat Rev Genet2013141233423183705
- HanahanDWeinbergRAThe hallmarks of cancerCell20001001577010647931
- HanahanDWeinbergRAHallmarks of cancer: the next generationCell2011144564667421376230
- WarburgOOn the origin of cancer cells on the origin of cancerSource Sci New Ser1956123123309314
- ZuXLGuppyMCancer metabolism: facts, fantasy, and fictionBiochem Biophys Res Commun2004313345946514697210
- NunnariJSuomalainenAMitochondria: in sickness and in healthCell201214861145115922424226
- NicoteraPLeistMFerrando-MayEIntracellular ATP, a switch in the decision between apoptosis and necrosisToxicol Lett19981021038139142
- EguchiYShimizuSTsujimotoYIntracellular ATP levels determine cell death fate by apoptosis or necrosisCancer Res19975710183518409157970
- IzyumovDSAvetisyanAVPletjushkinaOY“Wages of fear”: transient threefold decrease in intracellular ATP level imposes apoptosisBiochim Biophys Acta Bioenerg200416581–2141147
- VasevaAVMarchenkoNDJiKTsirkaSEHolzmannSMollUMP53 opens the mitochondrial permeability transition pore to trigger necrosisCell201214971536154822726440
- BrownNSBicknellRHypoxia and oxidative stress in breast cancer. Oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancerBreast Cancer Res20013532332711597322
- SullivanLBChandelNSMitochondrial reactive oxygen species and cancerCancer Metab2014211725671107
- ZhaoLRenTHWangDDClinical pharmacology considerations in biologics developmentActa Pharmacol Sin201233111339134723001474
- ToMSAromatarisECCastroJRobertsMLBarrittGJRychkovGYMitochondrial uncoupler FCCP activates proton conductance but does not block store-operated Ca2+ current in liver cellsArch Biochem Biophys2010495215215820060804
- KenwoodBMWeaverJLBajwaAIdentification of a novel mitochondrial uncoupler that does not depolarize the plasma membraneMol Metab20143211412324634817
- EllinghausPHeislerIUnterschemmannKBAY 87-2243, a highly potent and selective inhibitor of hypoxia-induced gene activation has antitumor activities by inhibition of mitochondrial complex ICancer Med20132561162424403227
- SchöckelLGlasauerABasitFTargeting mitochondrial complex I using BAY 87-2243 reduces melanoma tumor growthCancer Metab2015311126500770
- KimKKAbelmanSYanoNTetrathiomolybdate inhibits mitochondrial complex IV and mediates degradation of hypoxia-inducible factor-1α in cancer cellsSci Rep201551429626469226
- OlivaCRMarkertTRossLJIdentification of small molecule inhibitors of human cytochrome c oxidase that target chemoresistant glioma cellsJ Biol Chem201629146241882419927679486
- OlivaCRNozellSEDiersAAcquisition of temozolomide chemoresistance in gliomas leads to remodeling of mitochondrial electron transport chainJ Biol Chem201028551397593976720870728
- CluntunAALukeyMJCerioneRALocasaleJWGlutamine metabolism in cancer: understanding the heterogeneityTrends Cancer20173316918028393116
- Marín-HernándezAGallardo-PérezJCRodríguez-EnríquezSEncaladaRMoreno-SánchezRSaavedraEModeling cancer glycolysisBiochim Biophys Acta Bioenerg201118076755767
- AltenbergBGreulichKOGenes of glycolysis are ubiquitously over-expressed in 24 cancer classesGenomics20048461014102015533718
- BilliardJDennisonJBBriandJQuinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cellsCancer Metab2013111924280423
- BerndtNHamiltonADSebtiSMTargeting protein prenylation for cancer therapyNat Rev Cancer2011111177579122020205
- CafforioPDammaccoFGernoneASilvestrisFStatins activate the mitochondrial pathway of apoptosis in human lymphoblasts and myeloma cellsCarcinogenesis200526588389115705602
- AbdullahMIAbedMNRichardsonAInhibition of the mevalonate pathway augments the activity of pitavastatin against ovarian cancer cellsSci Rep201771809028808351
- WoschekMKneipNJuridaKMarziIReljaBSimvastatin reduces cancerogenic potential of renal cancer cells via geranylgeranyl pyrophosphate and mevalonate pathwayNutr Cancer201668342042727042994
- PolańskiRHodgkinsonCLFusiAActivity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancerClin Cancer Res201420492693724277449
- BolaBMChadwickALMichopoulosFInhibition of monocarboxylate transporter-1 (MCT1) by AZD3965 enhances radiosensitivity by reducing lactate transportMol Cancer Ther201413122805281625281618
- DohertyJRYangCScottKENBlocking lactate export by inhibiting the myc target MCT1 disables glycolysis and glutathione synthesisCancer Res201474390892024285728
- Azevedo-SilvaJQueirósOBaltazarFThe anticancer agent 3-bromopyruvate: a simple but powerful molecule taken from the lab to the bedsideJ Bioenerg Biomembr201648434936227457582
- PedersenPL3-Bromopyruvate (3BP) a fast acting, promising, powerful, specific, and effective “small molecule” anti-cancer agent taken from labside to bedside: introduction to a special issueJ Bioenerg Biomembr20124411622382780
- ShoshanMC3-Bromopyruvate: targets and outcomesJ Bioenerg Biomembr201244171522298255
- Jardim-MessederDMoreira-PachecoF3-Bromopyruvic acid inhibits tricarboxylic acid cycle and glutaminolysis in HepG2 cellsAnticancer Res20163652233224127127128
- PaiardiniATramontiASchirchDDifferential 3-bromopyruvate inhibition of cytosolic and mitochondrial human serine hydroxymethyltransferase isoforms, key enzymes in cancer metabolic reprogrammingBiochim Biophys Acta20161864111506151727530298
- El SayedSMBaghdadiHZolalyMAlmaramhyHHAyatMDonkiJGThe promising anticancer drug 3-bromopyruvate is metabolized through glutathione conjugation which affects chemoresistance and clinical practice: an evidence-based viewMed Hypotheses2017100677728236852
- KoYHVerhoevenHALeeMJCorbinDJVoglTJPedersenPLA translational study “case report” on the small molecule “energy blocker” 3-bromopyruvate (3BP) as a potent anticancer agent: from bench side to bedsideJ Bioenerg Biomembr201244116317022328020
- IsraelsenWJVander HeidenMGPyruvate kinase: function, regulation and role in cancerSemin Cell Dev Biol201543435126277545
- DongGMaoQXiaWPKM2 and cancer: the function of PKM2 beyond glycolysis (review)Oncol Lett20161131980198626998110
- MazurekSPyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cellsInt J Biochem Cell Biol201143796998020156581
- WalshMJBrimacombeKRAnastasiouDdatabase on the InternetML265: A Potent PKM2 Activator Induces Tetramerization and Reduces Tumor Formation and Size in a Mouse Xenograft Model2010 Available from: http://www.ncbi.nlm.nih.gov/pubmed/23905203Accessed June 7, 2018
- BrimacombeKRAnastasiouDHongBSML285 affects reactive oxygen species’ inhibition of pyruvate kinase M2Probe Reports from the NIH Molecular Libraries Program50Bethesda, MDNational Center for Biotechnology Information (US)2010118
- AnastasiouDYuYIsraelsenWJPyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesisNat Chem Biol201281083984722922757
- KungCHixonJChoeSSmall molecule activation of pkm2 in cancer cells induces serine auxotrophyChem Biol20121991187119822999886
- ParnellKMFoulksJMNixRNPharmacologic activation of PKM2 slows lung tumor xenograft growthMol Cancer Ther20131281453146023720766
- KimDJParkYSKimNDA novel pyruvate kinase M2 activator compound that suppresses lung cancer cell viability under hypoxiaMol Cells201538437337925813626
- FuLZhangSZhangLSystems biology network-based discovery of a small molecule activator BL-AD008 targeting AMPK/ZIPK and inducing apoptosis in cervical cancerOncotarget20156108071808825797270
- ZadraGPhotopoulosCTyekuchevaSA novel direct activator of AMPK inhibits prostate cancer growth by blocking lipogenesisEMBO Mol Med20146451953824497570
- McDonaldPCChafeSCDedharSOvercoming hypoxia-mediated tumor progression: combinatorial approaches targeting pH regulation, angiogenesis and immune dysfunctionFront Cell Dev Biol201642727066484
- TangCYuJHypoxia-inducible factor-1 as a therapeutic target in cancerJ Gastroenterol Hepatol201328340140523173651
- CavadasMANguyenLKCheongAHypoxia-inducible factor (HIF) network: insights from mathematical modelsCell Commun Signal20131114223758895
- PengGLiuYHypoxia-inducible factors in cancer stem cells and inflammationTrends Pharmacol Sci201536637438325857287
- ShenCNettletonDJiangMKimSKPowell-CoffmanJARoles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegansJ Biol Chem200528021205802058815781453
- MajmundarAJWongWJSimonMCHypoxia-inducible factors and the response to hypoxic stressMol Cell201040229430920965423
- DenglerVLGalbraithMDEspinosaJMTranscriptional regulation by hypoxia inducible factorsCrit Rev Biochem Mol Biol2015491115
- ChoiSHChungARKangWSilencing of hypoxia-inducible factor-1β induces anti-tumor effects in hepatoma cell lines under tumor hypoxiaPLoS One201497e10330425068796
- TsaiY-PWuK-JHypoxia-regulated target genes implicated in tumor metastasisJ Biomed Sci201219110223241400
- WardCLangdonSPMullenPNew strategies for targeting the hypoxic tumour microenvironment in breast cancerCancer Treat Rev201339217117923063837
- HuFShiLMuRHypoxia-inducible factor-1α and interleukin 33 form a regulatory circuit to perpetuate the inflammation in rheumatoid arthritisPLoS One201388814
- LiGLuWWuXAdmission hypoxia-inducible factor 1α levels and in-hospital mortality in patients with acute decompensated heart failureBMC Cardiovasc Disord2015157926223692
- ShenHMakiCGPharmacologic activation of p53 by small-molecule MDM2 antagonistsCurr Pharm Des201117656056821391906
- DevineTDaiM-STargeting the ubiquitin-mediated proteasome degradation of p53 for cancer therapyCurr Pharm Des201319183248326223151129
- WelshSWilliamsRKirkpatrickLPaine-MurrietaGPowisGAntitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1alphaMol Cancer Ther20043323324415026543
- KohMYSpivak-KroizmanTVenturiniSMolecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1Mol Cancer Ther2008719010018202012
- GreenbergerLMHorakIDFilpulaDA RNA antagonist of hypoxia-inducible factor-1alpha, EZN-2968, inhibits tumor cell growthMol Cancer Ther20087113598360818974394
- BorsiEPerroneGTerragnaCHypoxia inducible factor-1 alpha as a therapeutic target in multiple myelomaOncotarget2014571779179224732040
- JeongWRapisardaAParkSRPilot trial of EZN-2968, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha (HIF-1α), in patients with refractory solid tumorsCancer Chemother Pharmacol201473234334824292632
- BanHSKimBKLeeHThe novel hypoxia-inducible factor-1α inhibitor IDF-11774 regulates cancer metabolism, thereby suppressing tumor growthCell Death Dis201786e284328569777
- TurnerNLiJYGosbyABerberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate amp-activated protein kinase and improve insulin actionDiabetes20085751414141818285556
- ProtopopovaMMadhaviBJenniferBIACS-10759: a novel OXPHOS inhibitor which selectively kill tumors with metabolic vulnerabilitiesCancer Res2015754380
- BastianAMatsuzakiSHumphriesKMAG311, a small molecule inhibitor of complex I and hypoxia-induced HIF-1α stabilizationCancer Lett201738814915727939695
- MiyaderaHShiomiKUiHAtpenins, potent and specific inhibitors of mitochondrial complex II (succinate-ubiquinone oxidoreductase)Proc Natl Acad Sci U S A2003100247347712515859
- HuangLSSunGCobessiD3-Nitropropionic acid is a suicide inhibitor of mitochondrial respiration that, upon oxidation by complex II, forms a covalent adduct with a catalytic base arginine in the active site of the enzymeJ Biol Chem200628195965597216371358
- WangLZhangXCuiGA novel agent exerts antitumor activity in breast cancer cells by targeting mitochondrial complex IIOncotarget201672211126700963
- LimSHWuLKiewLVChungLYBurgessKLeeHBRosamines targeting the cancer oxidative phosphorylation pathwayPLoS One201493e8293424622277
- XiaoDPowolnyAAMouraMBPhenethyl isothiocyanate inhibits oxidative phosphorylation to trigger reactive oxygen species-mediated death of human prostate cancer cellsJ Biol Chem201028534265582656920571029
- GohilVMShethSANilssonRNutrient-sensitized screening for drugs that shift energy metabolism from mitochondrial respiration to glycolysisNat Biotechnol201028324925520160716
- CelaOPiccoliCScrimaRBupivacaine uncouples the mitochondrial oxidative phosphorylation, inhibits respiratory chain complexes I and III and enhances ROS production: results of a study on cell culturesMitochondrion201010548749620546950
- MorrellJAOrmeJButlinRJRocheTEMayersRMKilgourEAZD7545 is a selective inhibitor of pyruvate dehydrogenase kinase 2Biochem Soc Trans200331Pt 61168117014641019
- MooreJDStaniszewskaAShawTVER-246608, a novel pan-isoform ATP competitive inhibitor of pyruvate dehydrogenase kinase, disrupts Warburg metabolism and induces context-dependent cytostasis in cancer cellsOncotarget2014524128621287625404640
- VarshneyRDwarakanathBSJainVRadiosensitization by 6-aminonicotinamide and 2-deoxy-D-glucose in human cancer cellsInt J Radiat Biol200581539740816076755
- Ganapathy-KanniappanSKunjithapathamRGeschwindJFAnticancer efficacy of the metabolic blocker 3-bromopyruvate: specific molecular targetingAnticancer Res2013331132023267123
- ClemBTelangSClemASmall-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growthMol Cancer Ther20087111012018202014
- TelangSO’NealJTapolskyGIdentification of a PFKFB3 inhibitor suitable for phase I trial testing that synergizes with the B-Raf inhibitor vemurafenibCancer Metab20142Suppl 11425215185
- LeACooperCRGouwAMInhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progressionProc Natl Acad Sci U S A201010752037204220133848
- ChenJXieJJiangZWangBWangYHuXShikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2Oncogene201130424297430621516121
- JungDWKimWHParkSHA unique small molecule inhibitor of enolase clarifies its role in fundamental biological processesACS Chem Biol2013861271128223547795
- LiXTangSWangQ-QIdentification of epigallocatechin-3-gallate as an inhibitor of phosphoglycerate mutase 1Front Pharmacol201781928149278
- SujobertPPoulainLPaubelleECo-activation of AMPK and mTORC1 induces cytotoxicity in acute myeloid leukemiaCell Rep20151191446145726004183
- KungALZabludoffSDFranceDSSmall molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathwayCancer Cell200461334315261140
- SapraPKraftPPastorinoFPotent and sustained inhibition of HIF-1α and downstream genes by a polyethyleneglycol-SN38 conjugate, EZN-2208, results in anti-angiogenic effectsAngiogenesis201114324525321452059
- TerzuoliEPuppoMRapisardaAAminoflavone, a ligand of the aryl hydrocarbon receptor (AhR), inhibits HIF-1α expression in an AhR-independent fashionCancer Res2011701768376848
- KuboTMaezawaNOsadaMKatsumuraSFunaeYImaokaSBisphenol A, an environmental endocrine-disrupting chemical, inhibits hypoxic response via degradation of hypoxia-inducible factor 1alpha (HIF-1alpha): structural requirement of bisphenol A for degradation of HIF-1alphaBiochem Biophys Res Commun200431841006101115147973
- YeoE-JChunY-SChoY-SYC-1: a potential anticancer drug targeting hypoxia-inducible factor 1J Natl Cancer Inst200395751652512671019
- JonesDTHarrisALIdentification of novel small-molecule inhibitors of hypoxia-inducible factor-1 transactivation and DNA bindingMol Cancer Ther2006592193220216985052
- NaritaTYinSGelinCFIdentification of a novel small molecule HIF-1α translation inhibitorClin Cancer Res200915196128613619789328
- Moreno-ManzanoVRodríguez-JiménezFJAceña-BonillaJLFM19G11, a new hypoxia-inducible factor (HIF) modulator, affects stem cell differentiation statusJ Biol Chem201028521333134219897487
- WelshSJWilliamsRRBirminghamANewmanDJKirkpatrickDLPowisGThe thioredoxin redox inhibitors 1-methylpropyl 2-imidazolyl disulfide and pleurotin inhibit hypoxia-induced factor 1alpha and vascular endothelial growth factor formationMol Cancer Ther20032323524312657718
- LeeKKangJEParkSKLW6, a novel HIF-1 inhibitor, promotes proteasomal degradation of HIF-1α via upregulation of VHL in a colon cancer cell lineBiochem Pharmacol201080798298920599784
- MinegishiHFukashiroSBanHSNakamuraHDiscovery of indenopyrazoles as a new class of hypoxia inducible factor (HIF)-1 inhibitorsACS Med Chem Lett20134229730124900662
- LeeSHJeeJGBaeJSLiuKHLeeYMA group of novel HIF-1α inhibitors, glyceollins, blocks HIF-1α synthesis and decreases its stability via inhibition of the PI3K/AKT/mTOR pathway and Hsp90 bindingJ Cell Physiol2015230485386225204544
- MabjeeshNJEscuinDLaValleeTM2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIFCancer Cell20033436337512726862
- KongDParkEJStephenAGEchinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activityCancer Res200565199047905516204079
- NguyenTDJinXLeeJHAbietane diterpenes from Salvia miltiorrhiza inhibit the activation of hypoxia-inducible factor-1J Nat Prod20077071093109717583950
- WallaceEMRizziJPHanGA small-molecule antagonist of HIF2α is efficacious in preclinical models of renal cell carcinomaCancer Res201676185491550027635045
- ChenWHillHChristieATargeting renal cell carcinoma with a HIF-2 antagonistNature2016539762711211727595394
