Abstract
Introduction
Currently in papillary thyroid cancer (PTC), the correlation between lymph node positivity (LN+) and patient’s age at diagnosis is still inconclusive. The aim of this study was to investigate whether younger PTC patients had higher LN+ rates.
Patients and methods
From the 1998–2013 Surveillance, Epidemiology, and End Results database, we analyzed PTC patients with at least 1 LN examined. The patients were divided into 5 groups by age separately for each T stage: ≤30; 31–40; 41–50; 51–60; >60 years.
Results
A total of 46,077 PTC patients were identified, including 8,386 (18.2%) patients aged ≤30 years, 10,971 (23.8%) patients aged 31–40 years, 11,646 (25.3%) patients aged 41–50 years, 8,596 (18.7%) patients aged 51–60 years, and 6,478 (14.1%) patients aged >60 years. In each T stage, LN+ rates were inversely associated with age at diagnosis, which was validated by multivariate logistic regression analysis (p<0.001). In addition, the subset of patients 30 or younger had the highest lymph node ratio compared with other subsets (p<0.001).
Conclusion
We identified that younger PTC patients have an increased predisposition for LN+ regardless of T stage. This finding could help surgeons to select the optimal treatment for younger PTC patients.
Introduction
Due to advanced diagnostic level and periodic health screening, thyroid cancer has become the cancer with one of the highest incidences in America.Citation1,Citation2 Younger patients with papillary thyroid cancer (PTC) contribute a large proportion to this increasing trend.Citation3 Despite the rate of survival for PTC being favorable, with 98% survival rate for the next 5 years,Citation1 several studies demonstrated that the risk of recurrence ranges from 5% to 21% in PTC.Citation4,Citation5
Multiple risk factors were well known to be associated with recurrence and/or survival of PTC patients, including age at diagnosis, T stage, N stage, massive extrathyroid extension, BRAF mutation, and histological subtypes.Citation6–Citation9 For example, several reports identified that the number of positive lymph node (PLN) appears to predict the rate of recurrence in PTC.Citation10,Citation11 It is generally agreed that the LN+ status is also affected by the patient’s individual clinicopathologic characteristics in malignancies.
Patients’ LN+ status was affected by the patients’ age at diagnosis, which was demonstrated in carcinoma of the rectum, breast, and in melanomas.Citation12–Citation14 In 2016 in the analysis of 56,074 patients, Meyer et alCitation12 had pointed out that young age at diagnosis was associated with increased LN+ rates in early-stage rectal cancer. Similarly, Chao et alCitation13 also showed that the incidence of sentinel LN+ declined with increasing age at diagnosis for melanoma. This pattern was also seen in the study of early-stage breast cancers by Anders et al.Citation14
However, the correlation between LN+ and patient’s age at diagnosis in PTC is still inconclusive. While several studies claimed that young age at diagnosis could be an independent predictor of LN+,Citation15–Citation19 one study showed that older PTC patients were more likely to exhibit higher LN+ rates,Citation20 and another study did not find a correlation between age and LN+.Citation21 Therefore, based on a large population data-set, we aimed to investigate whether younger PTC patients were associated with higher LN+ rates.
Patients and methods
Patients
All the PTC patients who were diagnosed between 1988 and 2013 from the Surveillance, Epidemiology, and End Results (SEER) database were analyzed in current study. The SEER database is a national collaboration program set up by the National Cancer Institute of United States. It covers up to 26% of America’s population, using 17 population-based cancer registries (totaling approximately 3 million cases).
Patients diagnosed with PTC were identified using histopathology codes of the International Classification of Diseases for Oncology, 3rd edition: 8050, 8260–8263, 8340–8344, 8450. Pathologically staged patients who underwent thyroidectomy with 1 or more LNs surgically examined and no evidence of distant metastasis were included. Patients who received radiotherapy prior to surgery or whose thyroid malignancy was not their first primary cancer were excluded.
Statistical analyses
Our analyses included demographic characteristics (race, sex, age at diagnosis), tumor characteristics (T stage, LN+ status), and type of surgery. According to the SEER extent of disease extension codes for cases from 1988 to 2003, and the American Joint Committee on Cancer–derived T stage for cases from 2004 to 2008, T stage was categorized as T1, T2, T3, and T4. In addition, T1 group of the PTC patients was classified into T1a and T1b, due to different histopathologic characteristics among them.Citation22,Citation23 Age was divided as a categorical variable using 10-year intervals, except for the group of ages ≤30 and >60 years, because of the relatively smaller number of cases. The primary analysis of the study was LN+ status. It was illustrated by LN+ rates, number of PLN and lymph node ratio (LNR). The LNR was calculated as the ratio of number of PLN divided by the total number of lymph nodes examined. Trends in the mean number of PLN and LNR by age were evaluated using quantile regression models. Trends in LN+ rates with age were evaluated with Cochran Armitage trend tests.
All data were analyzed by the SPSS version 21.0 (IBM Corporation, Armonk, NY, USA) and the R software version 3.13 (http://www.r-project.org/). A p-value <0.05 was considered to be statistically significant, and all the tests were 2-sided.
Ethical approval
SEER data are deidentified before release and do not contain any personally identifying information. As the data is publicly available, no ethical approval is required.
Results
Clinicopathologic characteristics of patients
A total of 46,077 nonmetastatic PTC patients who received surgery between 1988 and 2013 were included in this study. The clinicopathologic characteristics of PTC were separated by age at diagnosis (). Overall, the mean age at diagnosis was 44.3±14.5 years. A total of 8,386 (18.2%) patients’ age at diagnosis were 30 years or younger, 10,971 (23.8%) patients were diagnosed between the age of 31 and 40 years, 11,646 (25.3%) patients between the age of 41 and 50 years, 8,596 (18.7%) patients between the age of 51 and 60 years, and 6,478 (14.1%) patients’ age at diagnosis was more than 60 years. More than 78.2% patients were female. The majority of the patients was white (83.9%). A total of 20,850 (45.3%) patients had at least 1 PLN. The greatest T-stage proportion was T1a (29.2%).
Table 1 Characteristic of PTC patients stratified by age at diagnosis
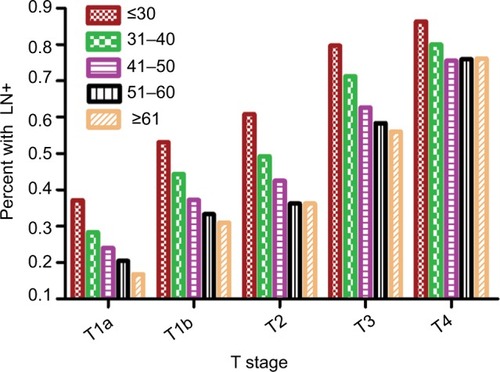
The LN+ rate was 24.8%, 40.8%, 47.5%, 65.9%, and 78.7% for T1a, T1b, T2, T3, and T4 stage patients, respectively. As shown in the , the mean number of PLN and LNR increased by T stage with each age at diagnosis. With the increasing age at diagnosis, the LN+ rate and mean number of PLN and LNR all decreased with each T stage (all p<0.001).
Table 2 The LN+ rate and mean number of PLN and LNR stratified by T stage and age
As shown in , as the T stage increases the LN+ rates also increased. Additionally, there is a reverse association between age at diagnosis and LN+ rates with each T stage (all p<0.001).
Multivariable analysis of LN+
The association between age at diagnosis and LN+ was identified by logistic analysis. As shown in the , younger patients (age at diagnosis ≤30 years) had higher correlation with higher LN+ rates at each T stage. We used multivariate logistic regression model to evaluate whether the association between the patient’s age at diagnosis and the LN+ rates was independent of other risk factors. Patient age at diagnosis, sex, race, and surgery types were included into the adjusted model. According to the multivariate analysis, the age at diagnosis still correlated with the LN+ rates ().
Table 3 The association of age and LN+ by logistical analysis
Discussion
In the current study, a total of 46,077 PTC patients from the SEER database were analyzed. We first identified that within each T stage, younger patients have an increased risk of LN+. The results were validated in multivariable analyses which including sex, race, and surgery types.
Thyroid cancer is unique among malignant tumors, because age is a vital prognostic factor in its staging systems.Citation24,Citation25 Our study showed that younger age at diagnosis is significantly associated with higher LN+ rates. In 2016, Zhang et alCitation26 also made a similar finding by comparing 3 different age groups, but they ignored the influence of T stages.Citation26 While some studies had reported that more advanced T stage is an important predictive factor for LN+, it is influenced (to some extent) by the presence of the total tumor load.Citation27,Citation28 In our study, we were able to show the inverse association between age at diagnosis and LN+, regardless of patient’s T stage.
Interestingly, we also found that younger patients are associated with a higher PLN and LNR when compared to older patients at the same T stage. Several studies highlighted that LNR could be an independent predictor for the recurrence for PTC.Citation29–Citation31 In our analysis, the subset of patients aged 30 or younger had the highest LNR, compared with other subsets (p<0.001). This finding also supports the notion that locoregional LN recurrence rate is higher for younger patients.Citation32,Citation33
Recurrence of PTC carries not only a higher surgical complication, but also psychological repercussions for the patient. Therefore, this present study could be interpreted as an implication for a change in surgical management of younger PTC patients. For instance, the results showed that LN+ rate of younger patients in T1a group reached up to 37.3%; the rate of older patients in T1a group was 16.8% relatively, which was consistent with the findings of Zhang et al.Citation26 They came up with the opinion that the treatment strategy of younger PTC patients could be more aggressive, with the consideration of long life span and recurrent risk.Citation26 Ito et alCitation34 also pointed out that younger PTC patients (<40 years of age) have a higher progression risk during observation without immediate surgery, compared with older patients. Although the presence or absence of cervical LN+ does not affect mortality in younger PTC patients, locoregional LN recurrence would inevitably result in reoperation. Indeed, the rate of injuring the recurrent nerve, parathyroid glands, or their circulation during reoperation is much higher than initial surgery.Citation35,Citation36 With the increased risk associated with reoperations, the quality of life of these patients may be affected to some extent. Therefore, we should pay more attention to the patient’s age. The very young age may not be the indicator to narrow surgical scope. On the contrary, adequate primary surgery and prophylactic/therapeutic LN dissection should be routinely performed for younger PTC patients, due to high LN+ rates.
The reasons why the probability of LN+ is higher in the younger group compared to the older patients had been demonstrated in other cancers. In 2012, Chang et alCitation37 reported that early-onset (≤40 years of age) colorectal adenocarcinoma patients were much more vulnerable to positive circumferential margins, due to signet ring cell differentiation, venous invasion, and perineural invasion. A study of melanoma suggested that the mitotic rate plays an important role in younger patients.Citation38 With respect to PTC, Moses et alCitation39 found multiple genetic alterations in younger patients. For instance, RET/PTC1 rearrangements, 1 key somatic genetic alteration in PTC development, were more common in childhood PTC.Citation40 These findings may lend more support to the hypothesis that thyroid follicular cells in the young are more susceptible to promoting DNA damage than in the old.Citation39,Citation41 As a result, young PTC patients could be more likely to develop nonthyroid second primary cancers after treatment with radioisotope therapy.Citation42 Moreover, there is also data showing that the incidence of capsule infiltration and vascular invasion is higher among younger patients.Citation18,Citation43–Citation45 These aggressive histological features are thought to be associated with high rates of LN+.Citation45
This study has several potential limitations. First, the SEER database collected information on large numbers of patients from 17 population-based cancer registries. Some clinicopathologic information may be miscoded or missed during registration process. For instance, patients’ BRAF gene mutations were not provided in the current dataset. Second, SEER database did not capture the preoperative data, such as neck ultrasonography and computed tomography. Therefore, we could not analyze the discrepancy between prophylactic LN dissection and therapeutic LN dissection, radical neck dissection, and modified radical neck dissection.
Conclusion
In conclusion, our study demonstrated that younger PTC patients have an increased predisposition for LN+, across every T stage. Younger PTC patients could need more standardized treatment compared to older PTC patients with the same TNM stage. This finding could be useful for surgeons when selecting the optimal treatment for young PTC patients.
Acknowledgments
The authors thank all medical personnel of the Department of Head, Neck and Breast Surgery, The First Affiliated Hospital of University of Science and Technology of China, Anhui Provincial Cancer Hospital, Hefei, People’s Republic of China, for their technical assistance.
Author contributions
Shengying Wang conceived and designed the study. Jing Wang and Shengying Wang developed the methodology. Collection and assembly of data was done by Jing Wang and Jianjun Liu. Data analysis and interpretation was by Jing Wang, Jianjun Liu. All authors were involved with the writing, review, and/or revision of the manuscript. Admin istrative, technical, or material support was provided by Jianjun Liu. The study was supervised by Shengying Wang. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2016CA Cancer J Clin201666173026742998
- KitaharaCMSosaJAThe changing incidence of thyroid cancerNat Rev Endocrinol2016121164665327418023
- AltekruseSDasAChoHPetkovVYuMDo US thyroid cancer incidence rates increase with socioeconomic status among people with health insurance? An observational study using SEER population-based dataBMJ Open2015512e009843
- LiuFHKuoSFHsuehCChaoTCLinJDPostoperative recurrence of papillary thyroid carcinoma with lymph node metastasisJ Surg Oncol2015112214915426175314
- GrantCSRecurrence of papillary thyroid cancer after optimized surgeryGland Surg201541526225713780
- ItoYHigashiyamaTTakamuraYRisk factors for recurrence to the lymph node in papillary thyroid carcinoma patients without pre-operatively detectable lateral node metastasis: validity of prophylactic modified radical neck dissectionWorld J Surg200731112085209117885787
- Lino-SilvaLSDominguez-MalagonHRCaro-SanchezCHSalcedo-HernandezRAThyroid gland papillary carcinomas with “micropapillary pattern,” a recently recognized poor prognostic finding: clinicopathologic and survival analysis of 7 casesHum Pathol201243101596160022425190
- XingMBRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implicationsEndocr Rev200728774276217940185
- AkslenLAMykingAOSalvesenHVarhaugJEPrognostic impact of EGF-receptor in papillary thyroid carcinomaBr J Cancer19936848088128398712
- LeboulleuxSRubinoCBaudinEPrognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid capsule at initial diagnosisJ Clin Endocrinol Metab200590105723572916030160
- SugitaniIKasaiNFujimotoYYanagisawaAA novel classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the followup periodSurgery2004135213914814739848
- MeyerJECohenSJRuthKJSigurdsonERHallMJYoung age increases risk of lymph node positivity in early-stage rectal cancerJ Natl Cancer Inst20161081djv284
- ChaoCMartinRNRossMICorrelation between prognostic factors and increasing age in melanomaAnn Surg Oncol200411325926414993020
- AndersCKHsuDSBroadwaterGYoung age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expressionJ Clin Oncol200826203324333018612148
- PatronVHitierMBedfertCPredictive factors for lateral occult lymph node metastasis in papillary thyroid carcinomaEur Arch Otorhinolaryngol201327072095210023238703
- ZhangLWeiWJJiQHRisk factors for neck nodal metastasis in papillary thyroid microcarcinoma: a study of 1,066 patientsJ Clin Endocrinol Metab20129741250125722319042
- MalandrinoPPellegritiGAttardMPapillary thyroid micro-carcinomas: a comparative study of the characteristics and risk factors at presentation in two cancer registriesJ Clin Endocrinol Metab20139841427143423482606
- SojakJSicakMKalisASlastanMPapillary thyroid carcinoma: analysis of the central compartment’s lymph nodes metastasesActa Medica (Hradec Kralove)2017601445028591551
- SemradTJSemradAMFarwellDGChenYCressRInitial treatment patterns in younger adult patients with differentiated thyroid cancer in CaliforniaThyroid201525550951325744759
- ShindoMWuJCParkEETanzellaFThe importance of central compartment elective lymph node excision in the staging and treatment of papillary thyroid cancerArch Otolaryngol Head Neck Surg2006132665065416785411
- VasileiadisIKaratzasTVasileiadisDClinical and pathological characteristics of incidental and nonincidental papillary thyroid micro-carcinoma in 339 patientsHead Neck201436456457023780707
- RossiRRotiETrasforiniGDifferentiated thyroid cancers 11–20 mm in diameter have clinical and histopathologic characteristics suggesting higher aggressiveness than those ≤10 mmThyroid200818330931518341377
- AndersonKJYoungwirthLMScheriRPT1a versus T1b differentiated thyroid cancers: do we need to make the distinction?Thyroid20162681046105227266722
- HaymartMRUnderstanding the relationship between age and thyroid cancerOncologist200914321622119270027
- EdgeSBComptonCCThe American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNMAnn Surg Oncol20101761471147420180029
- ZhangLYangJSunQRisk factors for lymph node metastasis in papillary thyroid microcarcinoma: older patients with fewer lymph node metastasesEur J Surg Oncol201642101478148227475736
- WangPWangYMiaoCDefining a new tumor dimension in staging of papillary thyroid carcinomaAnn Surg Oncol20172461551155628078481
- LimYSLeeJCLeeYSLateral cervical lymph node metastases from papillary thyroid carcinoma: predictive factors of nodal metastasisSurgery2011150111612121507446
- RyuISSongCIChoiSHRohJLNamSYKimSYLymph node ratio of the central compartment is a significant predictor for locoregional recurrence after prophylactic central neck dissection in patients with thyroid papillary carcinomaAnn Surg Oncol201421127728324006096
- ZhengCMJiYBSongCMGeMHTaeKNumber of metastatic lymph nodes and ratio of metastatic lymph nodes to total number of retrieved lymph nodes are risk factors for recurrence in patients with clinically node negative papillary thyroid carcinomaClin Exp Otorhinolaryngol2018111586429032663
- SchneiderDFMazehHChenHSippelRSLymph node ratio predicts recurrence in papillary thyroid cancerOncologist201318215716223345543
- ItoYMiyauchiAKiharaMTakamuraYKobayashiKMiyaARelationship between prognosis of papillary thyroid carcinoma patient and age: a retrospective single-institution studyEndocr J201259539940522374240
- KruijffSPetersenJFChenPPatterns of structural recurrence in papillary thyroid cancerWorld J Surg201438365365924149717
- ItoYMiyauchiAKiharaMHigashiyamaTKobayashiKMiyaAPatient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observationThyroid2014241273424001104
- KimMKMandelSHBalochZMorbidity following central compartment reoperation for recurrent or persistent thyroid cancerArch Otolaryngol Head Neck Surg2004130101214121615492172
- ChaoTCJengLBLinJDChenMFReoperative thyroid surgeryWorld J Surg19972166446479230664
- ChangDTPaiRKRybickiLAClinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic featuresMod Pathol20122581128113922481281
- SondakVKTaylorJMSabelMSMitotic rate and younger age are predictors of sentinel lymph node positivity: lessons learned from the generation of a probabilistic modelAnn Surg Oncol200411324725814993019
- MosesWWengJKhanafsharEDuhQYClarkOHKebebewEMultiple genetic alterations in papillary thyroid cancer are associated with younger age at presentationJ Surg Res2010160217918319765726
- NikiforovYERET/PTC rearrangement in thyroid tumorsEndocr Pathol200213131612114746
- RomeiCCiampiREliseiRA comprehensive overview of the role of the RET proto-oncogene in thyroid carcinomaNat Rev Endocrinol201612419220226868437
- BrownAPChenJHitchcockYJSzaboAShrieveDCTwardJDThe risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancerJ Clin Endocrinol Metab200893250451518029468
- MiccoliPMinutoMNUgoliniCPapillary thyroid cancer: pathological parameters as prognostic factors in different classes of ageOtolaryngol Head Neck Surg2008138220020318241716
- ParkHSJungCKLeeSHClinicopathologic characteristics and surgical outcomes of elderly patients with thyroid cancerJpn J Clin Oncol201444111045105125205673
- MeteOAsaSLPathological definition and clinical significance of vascular invasion in thyroid carcinomas of follicular epithelial derivationMod Pathol201124111545155221804527