Abstract
Purpose
The aim of this study was to investigate the degree of infiltration of CD8+ tumor-infiltrating lymphocytes (TILs) including high and low density in lung sarcomatoid carcinoma (LSC) and their clinicopathological significance.
Patients and methods
The density of CD8+ TILs in paraffin-embedded tissue sections from 100 LSC patients was detected by immunohistochemical staining, and the relationship of CD8+ TILs with clinicopathological features and prognosis was analyzed.
Results
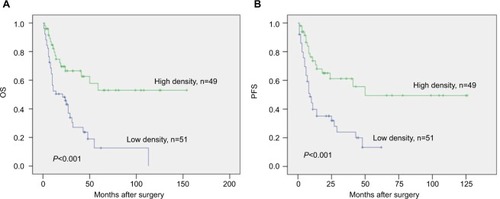
The chi-squared test showed that the degree of infiltration of CD8+ TILs was significantly correlated with the clinicopathological stage and T stage of LSC (P<0.05). The univariate analysis demonstrated that tumor size, clinicopathological stage, T stage, N stage, M stage, and CD8+ TILs are risk factors that affect prognosis of the patients (P<0.05). The mean overall survival (OS) of LSC patients with a high density of CD8+ TILs was 92.3 months, which was significantly higher than 31.2 months in patients with a low density of CD8+ TILs (P<0.05). Cox regression multivariate analysis confirmed that the density of CD8+ TILs was an independent prognostic factor for OS time of LSC patients (hazard ratio=0.455, P<0.05).
Conclusion
CD8+ TILs could be used as an effective prognostic index for LSC patients, and a high density of CD8+ TILs in tumor tissue may predict a better outcome.
Introduction
Lung cancer is a serious global public health problem. The data of the National Central Cancer Registry showed that the incidence of lung cancer and mortality rate ranks first in the year 2012 in China.Citation1 Lung sarcomatoid carcinoma (LSC) is a group of rare, poorly differentiated, non-small-cell lung cancer (NSCLC) containing sarcoma or sarcomatoid differentiation. Incidence of lung cancer accounted for 0.1%–0.4% of the total number, which is more common in older male smokers.Citation2,Citation3 Compared with other histological subtypes, LSC was more aggressive and had a higher recurrence rate.Citation4 Surgical treatment of LSC is the main therapeutic strategy.Citation5 LSC has a resistance to conventional first-line chemotherapy, and treatment effect is not ideal and has a poor prognosis.Citation6 To date, the study of LSC prognostic indicators is very rare; prior literatures revealed that PD-L1 expression was significantly correlated with a worse overall survival (OS) in LSC using immunohistochemistry (IHC).Citation7,Citation8 However, the cutoff value of PD-L1 expression remained to be unidentified in different studies due to the different clone numbers of this antibody. In addition, the molecular biomarker of LSC has been performed, including EGFR and Kras mutation, although the study showed that the associated targeted inhibitors alone may not be an effective therapeutic approach.Citation9 However, the molecular detection is too expensive to limiting its application. Therefore, in the light of a certain degree of limitation for the clinical application of these prior biomarkers, exploring the other prognostic indicators is very urgent for LSC, and today, we focus on the study of relationship between tumor-infiltrating lymphocytes (TILs) and tumor stromal and clinical outcomes.
To the best of our knowledge, tumor local immune response is mainly involved by tumor-infiltrating immune cells, and TILs are the main types of tumor-infiltrating immune cells and play a key role in immune surveillance of abnormal malignant cells.Citation10 Among the different TILs subgroups, the most valuable is the CD8+ cytotoxic T-cell (CTL), and the cytotoxicity of CD8+ T cells is one of the mechanisms of antitumor immunity.Citation11 It mediates the type 1 immune response and plays an important role in destroying the tumor.Citation12 TILs kill tumor cells and mainly rely on the effective activation of CD8+ T cells. T cells are transformed into effector cells and then play an antitumor effect.Citation13 CD8+ TILs can identify endogenous antigenic peptides presented by major histocompatibility complex (MHC) I molecules, which mediate the organism to kill the tumor cells through the immune response.Citation14 High-density infiltration of CD8+ TILs in tumor tissue is closely related to better prognosis in breast cancer, NSCLC, colorectal cancer, oral squamous cell carcinoma and hepatocellular carcinoma.Citation15–Citation19 However, the clinical significance and mechanism of CD8+ TILs in LSC have not been reported. This study was used to detect the level of CD8+ TILs in patients with LSC by IHC. The correlation between CD8+ TILs and the prognosis of LSC was evaluated by statistical analysis.
Patients and methods
Patients and tissue specimens
A total of 100 cases of paraffin-embedded LSC tissue samples were collected from the Department of Pathology, Sun Yat-sen University Cancer Center. All samples of paraffin were pretreated by standard. The retrospective analysis data of 100 patients with LSC who had pneumonectomy and/or lymphadenectomy came from Sun Yat-sen University Cancer Center, and the specimens were collected between December 2000 and June 2016 by removing the patients with neoadjuvant therapy. In all, eight of 100 cases had the synchronous metastases. All cases were diagnosed according to the WHO classification criteria in 2000, 2002 US Joint Commission and the International Joint Cancer TNM Classification System. The study was approved by the Sun Yat-sen University Cancer Center Medical Ethics Committee, and the written informed consent was obtained from all the patients who donated their specimens.
IHC
The immunohistochemical staining of CD8 protein was detected according to standard EnVision™ procedure. The paraffin-embedded tissue blocks were cut into 3 µm thick sequential sections. The slides were dried and deparaffinized in xylene, rehydrated through graded alcohol, immersed in 3% hydrogen peroxide for 10 minutes to block endogenous peroxidase activity and antigen retrieved by pressure cooking for 3 minutes in citrate buffer (pH=6). Then, the slides were incubated with 5% BSA for 15 minutes to reduce nonspecific reaction. Subsequently, the slides were incubated with the mouse monoclonal antibody anti-CD8 (ab8105, dilution 1:100; Abcam, Beijing, People’s Republic of China) for 50 minutes at 37°C. The slides were sequentially incubated with a secondary antibody (EnVision, Dako, Denmark) for 30 minutes in the incubator at 37°C and stained with 3,3-diaminobenzidine. Finally, the sections were counterstained with Mayer’s hematoxylin, dehydrated and mounted. A negative control was obtained by replacing the primary antibody with a normal rabbit IgG.
IHC evaluation
CD8 is mainly located in the cell membrane and cytoplasm. Using the high-power microscope, five visual fields with the most abundant infiltration of LSC were selected from each slice to calculate the percentage of CD8+ T lymphocytes in the total lymphocytes and take the mean value of five fields as the density of CD8+ TILs (%).Citation20 The expression of CD8 was double-blindly evaluated by two experienced pathologists. The final score was the average value of percentages that the two pathologists assessed.
Statistical analyses
Statistical analysis was performed using SPSS 20.0. The cutoff values were defined by the median.Citation21,Citation22 Correlation between density of CD8+ TILs and clinicopathological parameters in patients with LSC was analyzed using the chi-squared test. The survival analysis of LSC patients was evaluated by the Kaplan–Meier method with log-rank test. Multivariate analyses were performed using Cox proportional hazard model. All P-values were reported by two-sided analyses, and P<0.05 represented the statistical significance level. All data in our study have been recorded at the Sun Yat-sen University Cancer Center for future reference.
Results
Patients’ characteristics
The clinicopathological characteristics of LSC patients are detailed in . This LSC cohort consisted of 86 (86.0%) men and 14 (14.0%) women with mean age of 57 years. The average follow-up period was 26.2 months (median, 27.4 months; range, 1.0–129.0 months). In all, 56 (56.0%) patients were diagnosed at late stages (III and IV) and the other 44 (44.0%) patients at early stages (I and II).
Table 1 Correlations between the density of CD8+ TILs and clinicopathological characteristics in LSC
Expression of the level of CD8+TILs in LSC tissues by IHC
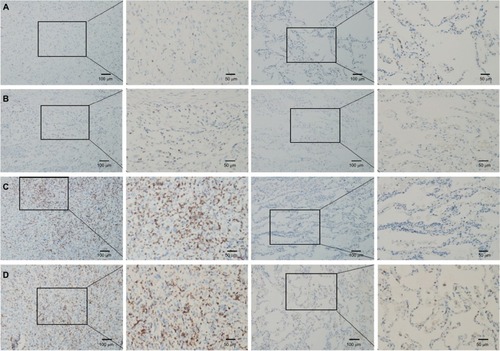
The results of IHC showed that CD8+ TILs were differentially expressed in LSC. In all, 49% (49/100) of LSC had high-density infiltration of CD8+ TILs. The variable density of CD8+ TIL infiltration of different LSC tissues and adjacent tissues was randomly selected as shown in .
Figure 1 CD8 expression in TILs for cancer and adjacent tissues.
Notes: The left panels show the cancer tissue (10×, 20×), and the right panels show the adjacent tissue (10×, 20×). (A) Patient with a low density of CD8+ TILs: female, 47 years old, T2N2M1, and stage IV. (B) Patient with a low density of CD8+ TILs: female, 65 years old, T1N2M0, and stage III. (C) Patient with a high density of CD8+ TILs: male, 62 years old, T2N0M0, and stage I. (D) Patient with a high density of CD8+ TILs: male, 58 years old, T2N0M0, and stage II.
Abbreviation: TIL, tumor-infiltrating lymphocyte.
Selection of cutoff value for different degrees of infiltration of CD8+ TILs
Statistical analysis showed that the median score of CD8+ TILs was 7.60%. Therefore, the median count (7.60%) was considered as the cutoff value for different degrees of infiltration of CD8+ TILs. In all, ≤7.60% was defined as a low-density infiltration and >7.60% as a high-density infiltration.
Association of different degrees of infiltration of CD8+TILs with LSC patients’ clinicopathological features
The chi-squared test showed that the degree of infiltration of CD8+ TILs was significantly correlated with clinical stage (P=0.015) and T stage (P=0.029), but there was no significant correlation with other clinical pathological parameters such as age, sex, lymph node metastasis, treatment and recurrence (P>0.05). All the details are given in .
Relationship between clinicopathological features, expression of CD8+ TILs and LSC patients’ survival: univariate survival analysis
The 10 clinical pathologic parameters of patients with LSC, including CD8+ TILs, were analyzed by univariate analysis. The univariate analysis demonstrated that tumor size (P=0.023), clinicopathological stage (P<0.001), T stage (P=0.018), N stage (P<0.001), M stage (P<0.001) and CD8+ TILs (P<0.001) were the risk factors of survival time. The detailed data are shown in .
Table 2 Univariate analysis of clinicopathological variables in 100 patients with LSC (log-rank test)
Survival analysis showed that the OS time and progression-free survival (PFS) time of CD8+ TILs high-density infiltration group were higher than those of low-density infiltration group. The mean survival time of patients with LSC in the high-density infiltration group was 92.3 months, which was higher than that in the low-density infiltration group (31.2 months, P<0.05; ); The mean PFS time of CD8+ TILs high-density infiltration group was 71.6 months in patients with LSC, and the mean PFS of the low-density infiltration group was 20.2 months (P<0.05; ).
Independent prognostic factors of LSC: multivariate Cox regression analysis
The significant risk factors (pT, pN, pM, CD8+ TILs) were enrolled into Cox regression model for multivariate analysis. The results showed that T stage and M stage were not independent prognostic risk factors (P>0.05), while N stage (P=0.002) and infiltration of CD8+ TILs (P=0.006) could be used as an independent prognosis factor for patients with LSC. The detailed data are given in .
Table 3 Cox multivariate analysis of CD8+ TILs and clinicopathological variables on OS
Table 4 Comparison of 3-year survival rate between low-density group and high-density groups of CD8+ TILs
Table 5 Comparison of 5-year survival rate between low-density group and high-density groups of CD8+ TILs
Relationship between the expression level of CD8+TILs in LSC and the 3-year and 5-year OS rate
Chi-squared test analysis showed that the 3-year and 5-year OS rates of CD8+ TILs low-density infiltration group was 15.7% (8/51) and 3.9% (2/51), respectively, compared with 36.7% (18/49) and 22.4% (11/49), respectively, in CD8+ TILs high-density infiltration group. The 3-year and 5-year OS rates were statistically significant between two groups (P<0.05). All the details are given in and .
Discussion
The study suggests that the patient’s immune status is an important factor for influencing the development and outcome of the tumor.Citation23 TILs are heterogeneous lymphocyte population predominantly found in tumor microenvironment with CD4+ and CD8+ T cells. In recent years, two studies reported that high- and low-density infiltration of CD8+ TILs could be used as an important indicator of prognosis of patients with NSCLC.Citation24,Citation25
In our study, we showed that the degree of infiltration of tumor CD8+ TILs was significantly correlated with the clinicopathological stage and T stage of LSC, suggesting that a high density of CD8+ TILs is significantly associated with the early stage of LSC. However, the partial or complete regression of LSC is extremely rare despite of infiltration of TILs, and the tumor remained to continue development and progression under the circumstance of presence of CD8+ TILs, which indicated that host immune response had a certain degree of role during killing tumors. Infiltration of CD8+ TILs could be inversely associated with better clinical outcomes in patients with LSC, independent of conventional predictive and prognostic factors, such as T stage and tumor metastasis status. So far, no studies have explored the prognostic value of infiltration of tumor CD8+ TILs in LSC. Our results indicated that the local immune response cells had a critical impact on development of the LSC, which is consistent with the studies of Teng et alCitation21 and Al-ShibliCitation26 who showed that CD8+ TILs could be used as independent prognostic factors in NSCLC.
In our study, Kaplan–Meier survival analysis showed that the mean OS of LSC patients with a high density of CD8+ TILs was 92.3 months, which was significantly higher than 31.2 months in patients with a low density of CD8+ TILs. The mean PFS time was 71.6 months and 20.2 months, respectively. The 3-year and 5-year survival rates of infiltration of CD8+ TILs with a high density were significantly higher than those of infiltration of CD8+ TILs with a low density. These results suggested that high-density CD8+ T lymphocytes have antitumor benefits and play an important role in improving the survival of patients with LSC. The high-density infiltration of CD8+ TILs is associated with better prognosis, which is in agreement with the findings of Al-Shibli et alCitation26 in NSCLC and Miyashita et alCitation27 in breast cancer. We found that 51% (51/100) of LSC patients harboring low-density infiltration of CD8+ TILs had the worse prognosis, and these partial patients also had not ideal response to traditional chemotherapy, thus, whether we could transfer the designed specific T cell (tumor-reactive T cells) to patients with LSC that harbor low-density infiltration for better therapeutic effect due to tumor-reactive T cells that could be increased in vitro by flow cytometry cell sorting technology.Citation28
To the best of our knowledge, CD8 is an important surface marker of cytotoxic T cells (CTL) and effective functioning of CD8+ CTL is the main antitumor effector. T cells transformed into effector cells require two stimulatory signals. T cell receptors specifically recognize and bind to pMHC (antigen peptide–MHC complex) of antigen-presenting cells (APCs), and this process provides the first signal of T-cell activation. Combining with APC surface, costimulatory molecules B7 and CD28 of T-cell surface receptor provide a second signal.Citation29 CD8+ TILs are activated to play the antitumor effect, mainly through three ways to kill tumor cells, including death receptor pathway,Citation30 degranulation pathwayCitation24 and secretion of TNF-α, IFN-γ and other cytokines.Citation31,Citation32 Therefore, we speculated that CD8+ TILs in LSC may play a role in inhibiting tumor growth through the abovementioned molecular mechanisms, furthermore increasing the survival time of patients.
Our study demonstrated that prognostic significance of the cytotoxic T-cell in LSC, which also provide evidence that CD8+ TILs have antitumor activity for LSC, and potential immunotherapeutic strategy could be applied in the clinical practice in future. In view of the complex effects of tumor microenvironment and the diversity of immune cell connections, the mechanism of CD8+ TILs in antitumor immune effects in LSC will be a meaningful attempt.
Since this was a single-center retrospective study, there were several limitations: first, the number of patients with LSC was small due to these extremely rare cases. Second, in our study, we detected the expression of CD8 IHC for infiltration of TILs by conventional tissue samples instead of tissue microarray. However, these samples are still not completely reflecting the tumor immunomicroenvironmental status. Therefore, the question of the heterogeneity of infiltration of TILs required us to determine the minimal tissue sample size for evaluation of TILs. However, to date, this question remained to be debated.Citation33 Thus, we only preliminarily investigated the correlation of different degrees of infiltration of CD8+ TILs and clinical prognosis, and we have first reported the prognostic significance of the cytotoxic T-cell in LSC. Further multi-institution investigation would be warranted to define the prognostic implication of the cytotoxic T-cell in LSC.
Conclusion
Infiltration of CD8+ TILs was closely related to the occurrence and development of LSC. CD8+ TILs could be used as an effective prognostic index for LSC patients, and high infiltration of CD8+ TILs in tumor tissue may predict a better outcome.
Acknowledgments
This work was supported by the National Key R&D Program of China (2017YFC1309000).
Disclosure
The authors report no conflicts of interest in this work.
References
- ChenWZhengRZengHZhangSThe incidence and mortality of major cancers in China, 2012Chin J Cancer20163517327484217
- UngMRouquetteIFilleronTCharacteristics and Clinical Outcomes of Sarcomatoid Carcinoma of the LungClin Lung Cancer201617539139727105684
- PelosiGSonzogniAde PasTReview article: pulmonary sarcomatoid carcinomas: a practical overviewInt J Surg Pathol201018210312019124452
- MartinLWCorreaAMOrdonezNGSarcomatoid carcinoma of the lung: a predictor of poor prognosisAnn Thorac Surg200784397398017720411
- SteuerCEBeheraMLiuYPulmonary Sarcomatoid Carcinoma: An Analysis of the National Cancer Data BaseClin Lung Cancer201718328629228043773
- VieiraTGirardNUngMEfficacy of first-line chemotherapy in patients with advanced lung sarcomatoid carcinomaJ Thorac Oncol20138121574157724389441
- YvorelVPatoirACasteilloFPD-L1 expression in pleomorphic, spindle cell and giant cell carcinoma of the lung is related to TTF-1, p40 expression and might indicate a worse prognosisPLoS One2017127e018034628671973
- ChangYLYangCYLinMWWuCTYangPCHigh co-expression of PD-L1 and HIF-1α correlates with tumour necrosis in pulmonary pleomorphic carcinomaEur J Cancer20166012513527107327
- JiangXLiuYChenCThe value of biomarkers in patients with sarcomatoid carcinoma of the lung: molecular analysis of 33 casesClin Lung Cancer201213428829622169481
- VerdegaalEMHoogstratenCSandelMHFunctional CD8+ T cells infiltrate into nonsmall cell lung carcinomaCancer Immunol Immunother200756558760016924494
- UsóMJantus-LewintreEBremnesRMAnalysis of the immune microenvironment in resected non-small cell lung cancer: the prognostic value of different T lymphocyte markersOncotarget2016733528495286127463005
- StantonSEDisisMLClinical significance of tumor-infiltrating lymphocytes in breast cancerJ Immunother Cancer201645927777769
- TamadaKShimozakiKChapovalAIModulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathwayNat Med20006328328910700230
- CaoXRegulatory T cells and immune tolerance to tumorsImmunol Res2010461–3799319763889
- MahmoudSMPaishECPoweDGTumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancerJ Clin Oncol201129151949195521483002
- KawaiOIshiiGKubotaKPredominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancerCancer200811361387139518671239
- GalonJCostesASanchez-CaboFType, density, and location of immune cells within human colorectal tumors predict clinical outcomeScience200631357951960196417008531
- FangJLiXMaDPrognostic significance of tumor infiltrating immune cells in oral squamous cell carcinomaBMC Cancer201717137528549420
- GiuşcăSEWierzbickiPMAmălineiCCăruntuIDAvădăneiERComparative analysis of CD4 and CD8 lymphocytes - evidences for different distribution in primary and secondary liver tumorsFolia Histochem Cytobiol201553327228126484587
- WangQLouWdiWWuXPrognostic value of tumor PD-L1 expression combined with CD8+ tumor infiltrating lymphocytes in high grade serous ovarian cancerInt Immunopharmacol20175271428846888
- TengFMengXWangXExpressions of CD8+TILs, PD-L1 and Foxp3+TILs in stage I NSCLC guiding adjuvant chemotherapy decisionsOncotarget2016739643186432927602763
- TokitoTAzumaKKawaharaAPredictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapyEur J Cancer20165571426771872
- PrendergastGCJaffeeEMCancer immunologists and cancer biologists: why we didn’t talk then but need to nowCancer Res20076783500350417413003
- JackuteJZemaitisMPranysDThe prognostic influence of tumor infiltrating Foxp3(+)CD4(+), CD4(+) and CD8(+) T cells in resected non-small cell lung cancerJ Inflamm20151263
- BremnesRMDonnemTBusundLTImportance of tumor infiltrating lymphocytes in non-small cell lung cancer?Ann Transl Med20164714227162792
- Al-ShibliKIDonnemTAl-SaadSPerssonMBremnesRMBusundLTPrognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancerClin Cancer Res200814165220522718698040
- MiyashitaMSasanoHTamakiKPrognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter studyBreast Cancer Res20151712426341640
- TurcotteSGrosATranETumor-reactive CD8+ T cells in metastatic gastrointestinal cancer refractory to chemotherapyClin Cancer Res201420233134324218514
- MichelFAttal-BonnefoyGManginoGMise-OmataSAcutoOCD28 as a molecular amplifier extending TCR ligation and signaling capabilitiesImmunity200115693594511754815
- BlokEJvan den BulkJDekker-EnsinkNGCombined evaluation of the FAS cell surface death receptor and CD8+ tumor infiltrating lymphocytes as a prognostic biomarker in breast cancerOncotarget201789156101562028121628
- BertrandFRochotteJColaciosCTargeting TNF alpha as a novel strategy to enhance CD8+ T cell-dependent immune response in melanoma?Oncoimmunology201651e106849526942089
- ShaoSRischEBurnerDLuLMinevBMaWIFNγ enhances cytotoxic efficiency of the cytotoxic T lymphocytes against human glioma cellsInt Immunopharmacol20174715916528410529
- SchalperKABrownJCarvajal-HausdorfDObjective measurement and clinical significance of TILs in non-small cell lung cancerJ Natl Cancer Inst20151073