Abstract
Background
The association between vitamin D receptor gene Bsm I (rs1544410) polymorphism and prostate cancer (PCa) risk has been investigated by numerous previous studies, which yielded inconsistent results. We conducted this meta-analysis to derive a relatively precise description of this association.
Methods
All studies published up to December 2017 were identified via a systematic search of PubMed, Embase, and China National Knowledge Infrastructure databases. Pooled odds ratios (ORs) with their 95% confidence intervals (CIs) were estimated to describe the strength of the relationship between Bsm I and PCa risk.
Results
In this meta-analysis, 27 studies with 9,993 cases and 9,345 controls were included. The pooled results revealed that Bsm I polymorphism was not associated with PCa risk in the overall analysis. Moreover, no significant relationship was found in the subgroup analyses by ethnicities, genotyping methods, Hardy–Weinberg equilibrium status, and Gleason score. In the stratified analysis by the source of controls and clinical stages, controls of benign prostatic hyperplasia (BPH) seemed to be in the particular groups in which the association of PCa risk with Bsm I polymorphism was significant (Bb vs. bb: OR=0.643, 95% CI=0.436–0.949, p=0.026; BB/Bb vs. bb: OR=0.627, 95% CI=0.411–0.954, p=0.029; B vs. b: OR=0.715, 95% CI=0.530–0.965, p=0.029).
Conclusion
Our results suggest that Bsm I polymorphism is weakly associated with PCa risk, and hence, it cannot be considered as a predictor of the occurrence and development of PCa in clinical practice. Future studies with a larger number of samples are needed to verify our results.
Introduction
According to a recent report published in the CA: A Cancer Journal for Clinicians in January, 164,690 new prostate cancer (PCa) cases and 29,430 PCa-related deaths were estimated in Americans in 2015.Citation1 PCa has risen to the first place among new cancer cases, and become the second leading cause of cancer-related deaths in males.Citation1 To make matters worse, the global prevalence rate of PCa is rising rapidly. It is forecasted that by 2030, the number of newly diagnosed PCa cases and deaths will rise up to more than 1.8 million and 0.5 million, respectively.Citation2 Existing evidence suggests that PCa risk might increase due to multiple factors, including aging, genetic factors, pathological changes, diet, hormonal level, as well as ethnicity and environment.Citation3 However, the pathophysiological mechanism of PCa remains largely unclear.
In a laboratory investigation, prostate cell division and growth was reported to be affected by vitamin D.Citation4 Thus, low plasma levels of vitamin D were hypothesized to be one of the important contributors to PCa.Citation4 The clinical trial also found that pre-diagnostic serum levels of vitamin D >85 nmol/L may improve survival in men with PCa.Citation5 The action of vitamin D is mediated by vitamin D receptor (VDR).Citation6 1,25-Dihydroxy vitamin D3 (1,25(OH)2D3), which is one of the active forms of vitamin D, would combine with VDR to form a heterodimer complex. Subsequently, the complex binds to vitamin D response element inducing reduced transcriptional levels of many genes which then stimulates tumor cell growth and differentiation.Citation7,Citation8
In recent years, the association between PCa risk and some single-nucleotide polymorphisms of VDR gene has become the focus of research attention.Citation9 We also conducted a meta-analysis on Taq I and Fok I polymorphisms and their relationships with PCa risk.Citation7 Bsm I polymorphism (rs1544410) is one of the most frequently researched variants. It is a restriction site located in intron 8 of VDR gene, which does not affect the amino acid sequence during VDR protein expression.Citation10 However, mutations in the intron region might be able to lower the stability of mRNA and affect the mRNA levels. Numerous research has revealed that Bsm I mutation might play a significant role in the development or progression of PCa.Citation11–Citation14 However, some other studies do not support this association.Citation15–Citation18 These results are inconsistent and worth further exploration. In addition, previous meta-analysesCitation10,Citation19–Citation22 seemed to be out of date due to availability of new data.Citation3,Citation9,Citation14,Citation23,Citation24 Therefore, we performed a new meta-analysis with the aim of obtaining more accurate and updated results.
Methods
Literature retrieval strategy
PubMed, Embase, and China National Knowledge Infrastructure (CNKI) electronic databases were searched for eligible studies published till December 2017. The terms “VDR/vitamin D receptor”, “prostate cancer/tumor/carcinoma”, and “polymorphism/mutation/variant” were used for searching titles or abstracts. Full search expressions were “vitamin D receptor [Title/Abstract] AND ((polymorphism [Title/Abstract] OR mutation [Title/Abstract]) OR variant [Title/Abstract]) AND prostate cancer [Title/Abstract]” for PubMed, “‘vitamin d receptor’:ab,ti AND ‘polymorphism’:ab,ti AND ‘prostate cancer’:ab,ti” for Embase, and “vitamin D receptor AND polymorphism AND prostate cancer” in Chinese for CNKI. In addition, we read the original or review reports carefully and searched manually for more eligible literature based on their references.
Study selection
Candidate studies were evaluated by two authors independently (Lei Wang and Jian Liu) for the following inclusion criteria: (1) studies in nonfamilial case–control or nested case–control design conducted on human beings; (2) studies that assessed the relationship between Bsm I polymorphism and risk or progression of PCa; (3) studies in which the distribution frequency of genotype and allelic profile of participants could be acquired or calculated; (4) studies in which no significant difference was reported between cases and controls in the aspect of baseline characters; (5) studies that scored more than 5 points on the Newcastle–Ottawa Scale (NOS).
Data extraction
Two investigators (Lei Wang and Jian Liu) collected the following information independently: first author’s name, publication year, population information, genotyping methods, the number of participants, genotype and allelic profile, as well as the source of controls. Cases and controls were classified into different subgroups by ethnicity, source of controls, and genotyping method, respectively. The subjects were also divided into group with Gleason score <7 and group with Gleason score ≥7 by pathological grade, and localized group and aggressive group by clinical stages, respectively. Any controversial content was discussed and evaluated by a third reviewer (Yansheng Zhao) to reach an agreement on all the items.
Statistical analyses
The heterogeneity was evaluated by using χ2-test based on Cochran’s Q-test and I2 statistics. If I2>50% and p<0.05, the heterogeneity between studies was significant and the random-effects model was used to combine the values from single studies;Citation25 otherwise, in the absence of heterogeneity, the fixed-effects model was chosen. The pooled odds ratios (ORs), together with 95% confidence intervals (CIs), were calculated to assess the strength of the relationship. The statistical significance of ORs was determined with Z-test. Five genetic comparison models were calculated in our analysis, including homozygote model (BB vs. bb), heterozygous model (Bb vs. bb), dominant model (BB vs. Bb/bb), recessive model (BB/Bb vs. bb), and allele genetic model (B vs. b allele). Begg’s funnel plot and Egger’s linear regression were used to evaluate the potential publication bias. Sensitivity analysis was performed to evaluate the stability of pooled results. Moreover, the Hardy–Weinberg equilibrium (HWE) status of controls was recalculated with the goodness-of-fit χ2-test; p<0.05 indicated that the genotype frequency of controls was not consistent with HWE.
For each outcome, we also conducted subgroup analyses by ethnicity, the source of controls, genotyping method, and clinical stages. p-values were two-sided, and p<0.05 was considered statistically significant. All analyses were done using the STATA package version 12.0 (Stata Corp, College Station, TX, USA).
Results
Characteristics of studies
A total of 87 studies were identified to be potentially related to the topic through our search strategy. Following our inclusion criteria, 27 studiesCitation3,Citation9,Citation11–Citation17,Citation23,Citation24,Citation26–Citation41 published between the years 1998 and 2017 were finally included to evaluate the association (). As shown in , out of the 27 studies, 25 explored the relationship of PCa risk with Bsm I, and nine were about the association between PCa progression and Bsm I. The number of participants in the case group and control group varied from 28 to 1,034, and 30 to 1,566, respectively. For all studies, except five, the genotype distribution frequency of Bsm I polymorphism in the control groups conformed to the HWE. All the studies scored more than 5 on the NOS, and were considered to be of high quality ().
Table 1 Characteristics and quality assessment of the studies included in this meta-analysis
Heterogeneity
Obvious heterogeneity between the studies was found in overall analysis for some genetic comparison models (Bb vs. bb: p=0.000, I2=59.2%; BB/Bb vs. bb: p=0.000, I2=65.9%; and B vs. b: p=0.000, I2=65.8%) (–). Thus, the random-effects model was chosen for data analysis in these comparison models. Meanwhile, in the recessive model, no heterogeneity was detected (BB vs. Bb/bb: p=0.285, I2=12.5%), and the fixed-effects model was used. Similar results were found in the subgroup analyses.
Pooled results in terms of PCa risk with Bsm I polymorphism
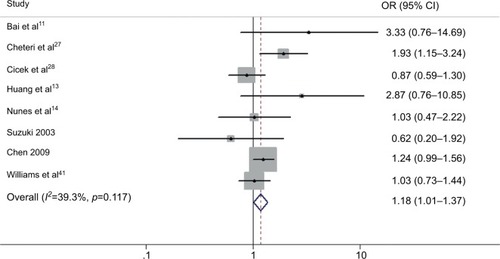
The results of the overall analysis obtained by pooling all the 25 studies are shown in and . These results indicate that Bsm I mutation does not increase the risk of PCa under different comparison models (BB vs. bb: OR=0.977, 95% CI=0.889–1.074, p=0.634; Bb vs. bb: OR=0.940, 95% CI=0.825–1.072, p=0.357; BB/Bb vs. bb: OR=0.951, 95% CI=0.832–1.087, p=0.462; BB vs. Bb/bb: OR=1.002, 95% CI=0.923–1.087, p=0.963; B vs. b: OR=0.969, 95% CI=0.883–1.065, p=0.516) ().
Table 2 Results of the association between Bsm I polymorphism and PCa risk in the whole population
Figure 2 Forest plots to estimate the association of VDR Bsm I polymorphism with PCa in the overall analysis. (A) Homozygote model (BB vs. bb). (B) Recessive model (BB vs. Bb/bb).
Abbreviations: PCa, prostate cancer; OR, odds ratio; CI, confidence interval.
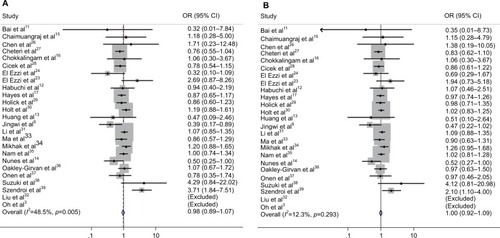
In the subgroup analyses conducted for a more detailed evaluation of the relationship, the results did not reveal any association by different ethnicities (), different genotyping methods (), or different HWE statuses of control groups (results not shown).
Table 3 Results of the association between Bsm I polymorphism and PCa risk by different ethnicities
Table 4 Results of the association between Bsm I polymorphism and PCa risk by different genotyping methods
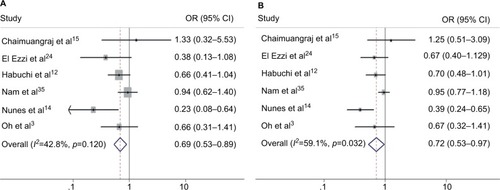
As shown in and , in the stratified analysis by the source of control groups, the PCa risk was significantly increased in patients with bb genotype or b genotype specifically in the subgroup of benign prostatic hyperplasia (BPH) controls (Bb vs. bb: OR=0.689, 95% CI=0.534–0.890, p=0.004; BB/Bb vs. bb: OR=0.627, 95% CI=0.411–0.954, p=0.029; B vs. b: OR=0.715, 95% CI=0.530–0.965, p=0.029). However, the results for the other two control groups revealed no significant association ().
Table 5 Results of the association between Bsm I polymorphism and PCa risk by different sources of controls
Figure 3 Forest plots to estimate the association of VDR Bsm I polymorphism with PCa in the subgroup of BPH controls. (A) Heterozygote model (Bb vs. bb). (B) Allelic frequency model (B vs. b).
Abbreviations: PCa, prostate cancer; BPH, benign prostatic hyperplasia; OR, odds ratio; CI, confidence interval.
Pooled results in terms of Bsm I polymorphism with PCa progression
Stratified analyses, according to the clinical stages and Gleason score of patients, were also performed. As shown in , the pooled results for the patients with Gleason score <7 and Gleason score ≥7 did not reveal any relationship between the Bsm I variant and PCa risk in various genetic models compared to controls. Similarly, the subgroup of PCa cases with localized stage and aggressive stage showed no association.
Table 6 Results of the association between Bsm I polymorphism and PCa risk by different tumor stages
In the inter-patient comparisons by different clinical stages and Gleason score statuses, a weak influence of Bsm I polymorphism on PCa progression was detected in patients with Gleason score ≥7 compared to the group with Gleason score <7 (BB/Bb vs. bb: OR=1.176, 95% CI=1.008–1.373, p=0.04). However, no effect of Bsm I polymorphism on the clinical stages was detected ( and ).
Publication bias and sensitivity analysis
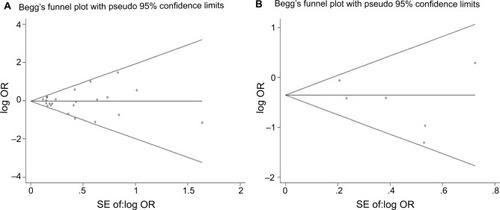
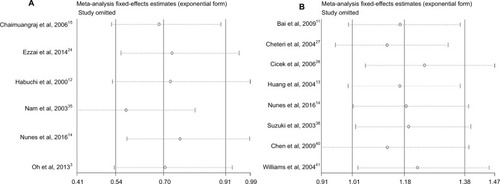
The funnel plots for publication bias analysis did not show any significant asymmetry in the overall analysis (). Moreover, Begg’s and Egger’s tests also revealed no publication bias in overall analysis as well as subgroup analyses (–). Sensitivity analysis for the positive results suggested that no obvious change in the pooled results was detected by omitting each individual study for the subgroup analysis of BPH controls, while the results were unstable in the comparison of PCa cases in terms of Gleason scores ().
Discussion
Polymorphisms of VDR gene and their relationships with PCa susceptibility have drawn a lot of attention in recent years. Bsm I polymorphism is one of the “star biomarkers”. Even though Bsm I polymorphism is located in the noncoding regions of VDR gene, it is frequently considered to be associated with PCa risk by numerous studies.Citation9,Citation11,Citation12,Citation39 Meanwhile, some studies support the opposite conclusion.Citation15,Citation16,Citation30,Citation34 Five meta-analyses conducted by Yin et al,Citation19 Zhang et al,Citation20 Guo et al,Citation21 Xu et al,Citation22 and Liu et al,Citation10 including 14, 19, 19, 15, and 6 primary studies, respectively, also yielded conflicting results. Moreover, some new data were reported.Citation3,Citation9,Citation14,Citation23,Citation24 Therefore, a new meta-analysis is necessary to clarify this issue. In the present study, data of 27 independent studies including 9,993 cases and 9,345 controls, which is higher compared to the previous meta-analyses, were pooled. Therefore, our updated results will be more convincing and stringent.
According to our results, no association between PCa risk and Bsm I polymorphism was detected in the overall population, which was similar to the results reported by Guo et al,Citation21 Liu et al,Citation10 and Xu et al,Citation22 but different from the other two meta-analyses.Citation19,Citation20 As we mentioned above, the results of previous meta-analyses might be suspect due to outdated data or inclusion of incomplete studies. Ethnicity might be an important biological factor for the genetic difference.Citation42 The genotype frequency distribution of Bsm I was found to be different between Asians, Caucasians and Africans, but in each subgroup by ethnicity, no association was found. In addition, subgroup analyses by the genotyping method and HWE status both revealed no influence of Bsm I on PCa risk, suggesting that these two variables would not change the negative result of the overall analysis either.
An interesting finding was that according to the results of the subgroup analysis by different sources of controls, Bsm I mutation increased the risk of PCa in BPH controls in the heterozygote model, recessive model, and allele model. Moreover, this result was proved to be robust by sensitivity analysis, and the heterogeneity was found to be acceptable as well. Based on this result, for individuals with BPH, the bb genotype or b might increase the risk of PCa, however, this result was suspicious and difficult to explain. Age was reported to be a risk factor for the relationship between Bsm I mutation and PCa risk.Citation26,Citation35 We intended to perform a subgroup meta-analysis by age, but the age classification in the included studies was too ambiguous to be pooled.
Similar to overall analysis, subgroup analyses by clinical stage and Gleason score revealed no relationship between PCa risk and Bsm I. Moreover, we conducted inter-patient analysis to assess the relationship of Bsm I polymorphism with PCa progression by comparing cases with aggressive stage and Gleason score ≥7 to cases with localized stage and Gleason score <7, respectively. Almost all the results were negative, except for the comparison between cases with Gleason score ≥7 and <7 in the recessive model. However, the only positive result was not stable in sensitivity analysis. Therefore, we have ignored the weak relationship.
Regrettably, we failed to perform a subgroup analysis by vitamin D intake, because only two primary studies have a detailed description of the effect of plasma vitamin D levels on the association between Bsm I and PCa risk. Ma et al reported that in patients with low levels of 25-D, which is one of the vitamin D metabolites, the PCa risk would be significantly increased by carrying bb genotype.Citation33 Meanwhile, in the group with high levels of 25-D, the relationship was not significant. Similar results were reported by Ahn et al in 2009.Citation18 These studies suggest that plasma levels of 25-D might influence our pooled result, and a stratified analysis by vitamin D intake or 25-D levels is warranted in the future.
Significant heterogeneity between studies was detected in both overall analysis and subgroup analyses under multiple comparison models. We noted that the BB genotype in the Asian group was quite rare but very commonly detected in Caucasians and Africans. It may contribute to this heterogeneity. However, no obvious publication bias was found and the sensitivity analysis supported the stability of our results. Overall, the present analysis was credible and statistically valid for the studied population.
However, some limitations of our meta-analysis should be acknowledged. First of all, some reports with a small number of cases and controls were included in our analysis, which increases the statistical power but introduces potential bias and heterogeneity as well. Second, our pooled outcomes were based on the initial results of the included studies, which were not adjusted by patient characteristics and other potential factors, such as age, gender, smoking, alcohol, sunshine, vitamin D intake, and so on. Therefore, a more precise analysis is required, in which the results should be adjusted by some related parameters. Besides, heterogeneity was obviously detected in some pooled results, which cannot be eliminated by subgroup analyses.
In conclusion, the present pooled analysis might be the largest one so far to evaluate the relationship between PCa susceptibility and Bsm I polymorphism of VDR gene. No increased risk of PCa was detected to be associated with Bsm I mutant in the overall analysis, and similarly in different subgroup analyses by race, genotyping methods, HWE status of controls, and clinical stage and Gleason score of cases. The association between PCa progression and Bsm I was also negative. Individuals with BPH, carrying bb genotype and b, seemed to have an increased risk of PCa. More large-scale and well-designed studies are needed in future to demonstrate the weak influence of Bsm I mutant on PCa risk and progression.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statisticsCA Cancer J Clin201868173029313949
- WangKWuGLiJRole of vitamin D receptor gene Cdx2 and Apa1 polymorphisms in prostate cancer susceptibility: a meta-analysisBMC Cancer201616167427553621
- OhJJByunSSLeeSEGenetic variations in VDR associated with prostate cancer risk and progression in a Korean populationGene20145331869324120391
- GalunskaBGerovaDKosevPSerum 25-hydroxy vitamin D levels in Bulgarian patients with prostate cancer: a pilot studyClin Lab2015613–432933525975000
- BrandstedtJAlmquistMManjerJVitamin D, PTH, and calcium in relation to survival following prostate cancerCancer Causes Control201627566977727023469
- ChakrabortiCKVitamin D as a promising anticancer agentIndian J Pharmacol201143211312021572642
- KangSZhaoYLiuJAssociation of vitamin D receptor Fok I polymorphism with the risk of prostate cancer: a meta-analysisOncotarget2016747778787788927788484
- TayebMTClarkCHaitesNEVitamin D receptor, HER-2 polymorphisms and risk of prostate cancer in men with benign prostate hyperplasiaSaudi Med J200425444745115083213
- JingwiEYAbbasMRicks-SantiLVitamin D receptor genetic polymorphisms are associated with PSA level, Gleason score and prostate cancer risk in African-American menAnticancer Res20153531549155825750310
- LiuSCaiHChengWAssociation of VDR polymorphisms (Taq I and Bsm I) with prostate cancer: a new meta-analysisJ Int Med Res201745131028222630
- BaiYYuYYuBAssociation of vitamin D receptor polymorphisms with the risk of prostate cancer in the Han population of Southern ChinaBMC Med Genet20091012519961572
- HabuchiTSuzukiTSasakiRAssociation of vitamin D receptor gene polymorphism with prostate cancer and benign prostatic hyperplasia in a Japanese populationCancer Res200060230530810667581
- HuangSPChouYHWayne ChangWSAssociation between vitamin D receptor polymorphisms and prostate cancer risk in a Taiwanese populationCancer Lett20042071697715050735
- NunesSBde Matos OliveiraFNevesAFAssociation of vitamin D receptor variants with clinical parameters in prostate cancerSpringerplus20165364
- ChaimuangrajSThammachotiROngphiphadhanakulBLack of association of VDR polymorphisms with Thai prostate cancer as compared with benign prostate hyperplasia and controlsAsian Pac J Cancer Prev20067113613916629532
- ChokkalingamAPMcGlynnKAGaoYTVitamin D receptor gene polymorphisms, insulin-like growth factors, and prostate cancer risk: a population-based case-control study in ChinaCancer Res200161114333433611389055
- HayesVMSeveriGPadillaEJGenetic variants in the vitamin D receptor gene and prostate cancer riskCancer Epidemiol Biomarkers Prev200514499799915824177
- AhnJAlbanesDBerndtSIVitamin D-related genes, serum vitamin D concentrations and prostate cancer riskCarcinogenesis200930576977619255064
- YinMWeiSWeiQVitamin D receptor genetic polymorphisms and prostate cancer risk: a meta-analysis of 36 published studiesInt J Clin Exp Med20092215917519684888
- ZhangQShanYGenetic polymorphisms of vitamin D receptor and the risk of prostate cancer: a meta-analysisJ BUON201318496196924344024
- GuoZWenJKanQLack of association between vitamin D receptor gene Fok I and Bsm I polymorphisms and prostate cancer risk: an updated meta-analysis involving 21,756 subjectsTumour Biol20133453189320023807674
- XuYHeBPanYSystematic review and meta-analysis on vitamin D receptor polymorphisms and cancer riskTumour Biol20143554153416924408013
- El EzziAABakerMTZaidanWRAssociation of polymorphisms in the VDR, CYP17 and SRD5A2 genes and prostate cancer among Lebanese menAsian Pac J Cancer Prev20171819310028240015
- El EzziAAZaidanWREl-SaidiMAAssociation of benign prostate hyperplasia with polymorphisms in VDR, CYP17, and SRD5A2 genes among Lebanese menAsian Pac J Cancer Prev20141531255126224606449
- BohningDMeta-analysis: a unifying meta-likelihood approach framing unobserved heterogeneity, study covariates, publication bias, and study qualityMethods Inf Med200544112713515778804
- ChenZQDengLSLinWJThe association of vitamin D receptor genotypes and risk of prostate cancerChin J Lab Med Clin Sci2001226062
- CheteriMBStanfordJLFriedrichsenDMVitamin D receptor gene polymorphisms and prostate cancer riskProstate200459440941815065089
- CicekMSLiuXSchumacherFRVitamin D receptor genotypes/haplotypes and prostate cancer riskCancer Epidemiol Biomarkers Prev200615122549255217164384
- HolickCNStanfordJLKwonEMComprehensive association analysis of the vitamin D pathway genes, VDR, CYP27B1, and CYP24A1, in prostate cancerCancer Epidemiol Biomarkers Prev200716101990199917932346
- HoltSKKwonEMPetersUVitamin D pathway gene variants and prostate cancer riskCancer Epidemiol Biomarkers Prev20091861929193319454612
- LiHStampferMJHollisJBA prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancerPLoS Med200743e10317388667
- LiuJHLiWHWangQJVitamin D receptor gene Bsm I polymorphism and the suscepbility to prostate cancer in Northern Chinese Han populationNatl J Androl200396413416
- MaJStampferMJGannPHVitamin D receptor polymorphisms, circulating vitamin D metabolites, and risk of prostate cancer in United States physiciansCancer Epidemiol Biomarkers Prev1998753853909610787
- MikhakBHunterDJSpiegelmanDVitamin D receptor (VDR) gene polymorphisms and haplotypes, interactions with plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D, and prostate cancer riskProstate200767991192317440943
- NamRKZhangWWTrachtenbergJComprehensive assessment of candidate genes and serological markers for the detection of prostate cancerCancer Epidemiol Biomarkers Prev200312121429143714693733
- Oakley-GirvanIFeldmanDEccleshallTRRisk of early-onset prostate cancer in relation to germ line polymorphisms of the vitamin D receptorCancer Epidemiol Biomarkers Prev20041381325133015298953
- OnenIHEkmekciAErogluMAssociation of genetic polymorphisms in vitamin D receptor gene and susceptibility to sporadic prostate cancerExp Biol Med (Maywood)2008233121608161418849534
- SuzukiKMatsuiHOhtakeNVitamin D receptor gene polymorphism in familial prostate cancer in a Japanese populationInt J Urol200310526126612694466
- SzendroiASpeerGTabakAThe role of vitamin D, estrogen, calcium sensing receptor genotypes and serum calcium in the pathogenesis of prostate cancerCan J Urol20111835710571621703046
- ChenLDavey SmithGEvansDMGenetic variants in the vitamin d receptor are associated with advanced prostate cancer at diagnosis: findings from the prostate testing for cancer and treatment study and a systematic reviewCancer Epidemiol Biomarkers Prev200918112874288119861519
- WilliamsHPowellIJLandSJVitamin D receptor gene polymorphisms and disease free survival after radical prostatectomyProstate200461326727515368470
- YaoSHongCCBanderaEVDemographic, lifestyle, and genetic determinants of circulating concentrations of 25-hydroxyvitamin D and vitamin D-binding protein in African American and European American womenAm J Clin Nutr201710561362137128424184