Abstract
Background
The effectiveness of gemcitabine plus capecitabine compared with gemcitabine monotherapy for resected pancreatic cancer has been evaluated in the ESPAC-4 trial. We aimed to assess the cost-effectiveness of these adjuvant regimens on resected pancreatic cancer.
Methods
A Markov model was established to simulate the disease process of resected pancreatic cancer (relapse-free survival, progressive disease, and death). The efficacy and toxicity profiles were collected from the ESPAC-4 trial. Transition probabilities were calculated based on survival in each group. Cost data were calculated from the perspective of the Chinese health-care payer. The primary endpoint in the analysis was the incremental cost-effectiveness ratio (ICER), and model uncertainties were explored by one-way sensitivity analysis and probabilistic sensitivity analysis.
Results
Our results demonstrated that gemcitabine monotherapy cost $36,028.45 and yielded a survival of 1.02 quality-adjusted life year (QALY), while gemcitabine plus capecitabine cost $46,095.05 and yielded a survival of 1.23 QALY. Therefore, the incremental cost-effectiveness ratio of gemcitabine plus capecitabine vs gemcitabine monotherapy was $45,191.23 which surpassed the willingness-to-pay threshold of $29,291.42 per QALY in China.
Conclusion
The gemcitabine monotherapy regimen is more cost-effective compared with gemcitabine plus capecitabine regimen for the patients with postoperative pancreatic cancer from the Chinese societal perspective.
Introduction
Pancreatic cancer is a common and highly fatal cancer, with a poor prognosis.Citation1 In pancreatic cancer patients a 5-year survival rate is only 8%, even though there has been a gradual increase in survival for most cancers over the decades.Citation2 More than 80% of patients with pancreatic cancer are asymptomatic and exhibit unresectable advanced pancreatic cancer at diagnosis.Citation3 Only 20% of patients are eligible for initial resection.Citation4 However, after radical resection, most patients will experience recurrence within 2 years.Citation5,Citation6 Surgical resection with adjuvant chemotherapy, with either 5-fluorouracil plus folinic acid or gemcitabine, has increased the 5-year survival rate to ~20%.Citation7–Citation11 Recently, several studies indicated that adjuvant chemotherapy was an effective means for resected pancreatic patients to obtain long-term survival and it is steadily accepted as the established standard of care.Citation8,Citation10,Citation12–Citation16
Gemcitabine had been associated with significant improvement in disease-free survival (DFS) and overall survival (OS) in postoperative pancreatic patients compared with placebo cohort (median DFS: 13.4 months vs 6.9 months; median OS: 22.8 months vs 20.2 months).Citation5,Citation17 The combination of gemcitabine and capecitabine has synergistic effect on thymidylate synthase involved in normal DNA synthesis.Citation18 Moreover, previous clinical trials have demonstrated this combination produced a better tumor response with well tolerated adverse effects compared with monotherapy in patients with advanced pancreatic cancer.Citation19,Citation20
The European study group for pancreatic cancer (ESPAC-IV) trial was performed to evaluate efficacy and safety of gemcitabine plus capecitabine compared with gemcitabine monotherapy for postoperative pancreatic cancer. The results revealed that the gemcitabine plus capecitabine regimen significantly improved median overall survival (OS) and median relapse-free survival (RFS) compared with gemcitabine (28.0 months vs 25.5 months, P=0.032; 13.9 months vs 13.1 months, P=0.082). Grade 3–4 adverse events, neutropenia, white blood cell count decrease, and hand-foot syndrome were frequently reported in the gemcitabine plus capecitabine cohort (38%, 10%, 7%), whereas neutropenia, white blood cell count decrease, infection and infestations were significantly greater in the gemcitabine monotherapy cohort (24%, 8%, 7%). Thus, the combination of the gemcitabine and capecitabine regimen seemed to be a more effective option for the treatment of resected pancreatic cancer.Citation18
Even though gemcitabine plus capecitabine regimen have proven to have a better clinical response when compared with gemcitabine monotherapy, they have not been directly compared in terms of being cost effective. Taking cost-effectiveness into consideration is crucial for clinicians to make an optimal decision, as well as from a social perspective. Herein, we performed a Markov model to evaluate the cost-effectiveness of gemcitabine plus capecitabine compared with gemcitabine monotherapy for resected pancreatic cancer from the perspective of a Chinese society.
Materials and methods
Patients and regimens
The clinical data for this model was derived from the ESPAC-IV trial, a multicenter, open-label, randomized, phase III trial conducted in 92 hospitals in England, Scotland, Wales, Germany, France, and Sweden.Citation18 The inclusion criteria were patients aged 18 years or older who had undergone complete resection for pancreatic cancer.Citation18 The eligible patients were randomly assigned within 12 weeks of resection to receive 6 cycles of either 1000 mg/m2 gemcitabine alone, administered once a week for 3 of every 4 weeks cycle, or with 1660 mg/m2 oral capecitabine administered for 21 days followed by a 7 day rest per cycle.Citation18 Laboratory tests, clinical symptoms, tumor markers, chest radiographs and abdominal CT were assessed based on the protocol of ESPAC-4 trial.Citation18 The median RFS was 13.9 months in gemcitabine plus capecitabine cohort, and 13.1 months in gemcitabine monotherapy cohort The median overall survival (OS) of gemcitabine plus capecitabine and gemcitabine monotherapy was 28.0 and 25.5 months, respectively.Citation18 The other primary input clinical efficacy parameters are shown in ().
Table 1 Clinical efficacy and adverse events of gemcitabine plus capecitabine and gemcitabine monotherapy
Model structure
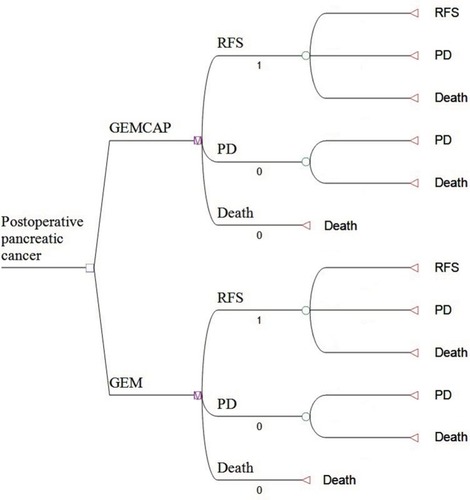
A Markov model was performed with TreeAge Pro 2011 (TreeAge Software, Inc., Williamstown, MA, USA) to simulate the disease process of resected pancreatic cancer and compare the cost-effectiveness of 2 strategies based on the ESPAC-4 trials. The decision model structure comprised 3 mutually exclusive states including RFS, progressive disease (PD), and death (). The patients could shift to a different state at the end of each cycle in the Markov model, according to the transition probabilities calculated by the 5-year RFS rate; and 5-year OS rate (), and costs and benefits were discounted to present values at 3% for 1 year.Citation21 The model cycle length was 1 month, and the time horizon was 10 years. Monthly transition probabilities of health states were calculated by the following formula: r=[1-ln(1-P1)]/t, P2= l-exp(-ru), r: instantaneous rate; P1: cumulative probability at time t (5 years), u: model cycle length, P2: Monthly transition probabilities.Citation22,Citation23
Figure 1 Markov model for postoperative pancreatic cancer.
Notes: Markov model for resected pancreatic cancer. A Markov model comprising 3 health states (relapse-free survival, PD and death) was built.
Abbreviations: GEM, gemcitabine; GEMCAP, gemcitabine plus capecitabine, PD, progressive disease; RFS, relapse-free survival.
Cost estimate
Total costs in our analysis consisted of direct medical costs and societal costs. Cost of drugs and tests were derived from the 2018 fee standards of West China Hospital, Sichuan University. The median relative dose intensity (RDI) of the RFS state drugs in gemcitabine group and gemcitabine plus capecitabine were 83%, 78%, respectively.Citation18 Direct medical costs included drugs, tests, inpatient fees and treatments for grade 3–4 AEs. The grade 3–4 AEs rates sourced from the trials were used to calculate the AE-related costs (), whereas societal costs consisted of travel fees and time costs (absenteeism fees), and travel costs were assessed at $10.20 per patient each trip to the hospital in Sichuan, China, in 2016.Citation24 Time costs were estimated at $35.73 per day based on the average monthly salary in China in 2017.Citation24 Travel costs and time costs were derived from the average length inpatient hospitalization of 2 times per month, 3 days each time and outpatient visits of 2 times per month. For the cost of PD, in patients chiefly treated with platinum-based chemotherapy regimens, a weighted cost based on FOLFIRINOX (5-FU, leucovorin, oxaliplatin, irinotecan), GEM-N (gemcitabine, nab-paclitaxel) was assumed per cycle.Citation25,Citation26 The RDIs for these treatments were assumed to be 80%.Citation27 All costs were converted to USD, at an exchange rate of $1= RMB 6.33, in March 2018.
Effectiveness estimates
Treatment effectiveness was estimated by QALYs. Utility scores of Markov states were based on the previous studies, with 0.85 for RFS state and 0.73 for PD state.Citation28,Citation29
Sensitivity analysis
One-way sensitivity analysis was performed to investigate the impact of variables on the analysis model by varying the necessary parameters within a range of ± 30%. As for probabilistic sensitivity analysis, a Monte Carlo simulation of 1,000 iterations was developed to assess the uncertainty strategies, and the results were presented as cost-effectiveness acceptability curves. According to WHO guidelines, the willingness to pay (WTP) threshold value was 3 times Gross Domestic Product per Capita (GDP) of China in 2017, which was $25,840.88/ QALY, ie $2,153.40 per quality-adjusted life month.Citation30
Results
Costs outcomes
The estimated monthly costs of the 2 treatments are briefly presented in ().
Table 2 Cost and utility scores of gemcitabine plus capecitabine and gemcitabine monotherapy
As for the cost for RFS state, the greatest cost was RDI-adjusted drugs ($1,237.01 for gemcitabine and $1,726.15 for gemcitabine plus capecitabine). The inpatient fees, test costs and total societal costs were the same in these 2 groups. Moreover, the grade 3–4 adverse effects related to cost were similar ($41.91 for gemcitabine and $55.67 for gemcitabine plus capecitabine). As for the cost of PD state, the total cost was $2,643.56 for both treatment groups. After running the Markov model to the estimated time horizon, the cumulative costs were $36,028.45 for the gemcitabine group, which was significantly lower than that of $46,095.05 for the gemcitabine plus capecitabine group ().
Table 3 Results of cost-effectiveness analysis of gemcitabine plus capecitabine and gemcitabine monotherapy
Cost-effectiveness
As shown in , according to the cost analysis and effectiveness analysis described previously, the gemcitabine monotherapy was cheaper, with a cost of $35,322.01/QALY compared with $45,191.23/QALY for the combination of gemcitabine and capecitabine. Gemcitabine plus capecitabine group provided an incremental 0.21 QALYs at an incremental cost of $10,066.60, compared with the gemcitabine group, resulting in the ICER of $47,936.19/QALY, which exceeded the WTP threshold of $25,840.88/QALY.
Sensitivity analysis
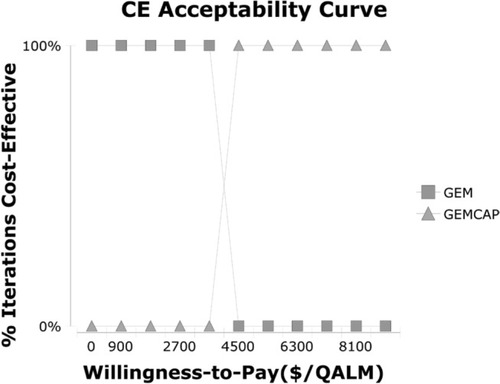
The one-way sensitivity analyses are displayed in the tornado diagram (). The cost of the PD state in gemcitabine plus capecitabine cohort and the cost of the PD state in the gemcitabine cohort played a vital role in our study. When cost of the PD state in the gemcitabine plus capecitabine cohort varied from $1,850.49 to $3,436.63, the ICER increased from $29,295.30 to $45,656.02 per QALY. If the cost of PD state in gemcitabine group changed from $1,850.49 to $3,436.63, the ICER rose from $22,593.65 to $35,989.21 per QALY. Nevertheless, the cost of test and cost of grade 3–4 AEs in these 2 strategies had a slight impact on the model. The result of the Monte Carlo simulation of 1,000 patients showed that the mean cost and effectiveness gained were: $46,300.77±741.49 and 1.23±0.02 QALY for gemcitabine plus capecitabine group, while $36,243.69±652.05 and 1.03±0.02 QALY for gemcitabine group. The probabilistic sensitivity analysis indicated nearly 100% probability of gemcitabine and 0% probability of gemcitabine plus capecitabine being a cost-effective strategy, as the WTP value was $2,153.40/QALM. ()
Figure 2 Tornado diagram of one-way sensitivity analyses.
Notes: Tornado diagrams show the influence of factors on the Markov model. The factors are listed in descending order of the influence on ICER with variation of factor values.
Abbreviations: AE, adverse event; GEM, gemcitabine; GEMCAP, gemcitabine plus capecitabine; ICER, incremental cost-effectiveness ratio; PD, progressive disease; P, transition probability; QALM, quality-adjusted life month; RFS, relapse-free survival; EV, expected value.
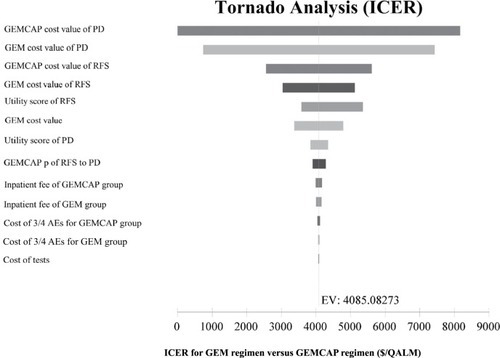
Figure 3 Cost-effectiveness acceptability curves.
Notes: The cost-effectiveness acceptability curves reflected the results of probabilistic sensitivity analysis by estimating probabilities of different treatments being considered as optimal strategies at different WTP thresholds.
Abbreviations: CE, cost effectiveness; GEM, gemcitabine; GEMCAP, gemcitabine plus capecitabine; QALM, quality adjusted life month; WTP, willingness to pay.
Discussion
Pancreatic cancer is a seriously lethal disease, and mortality rate closely coincides with incidence.Citation1 After resection, chemotherapy with fluorouracil or gemcitabine significantly prolongs OS and reduces the incidence of relapse.Citation6 However, a significant burden is placed on patients during the adjuvant therapy process for resected pancreatic cancer. An economic assessment of postoperative adjuvant regimens is vital to keep the balance between clinical benefits and health care cost, especially in developing countries such as resource-limited China.Citation31 Therefore, we established a Chinese cost-effective analysis of gemcitabine plus capecitabine vs gemcitabine alone for resected pancreatic cancer, which is the first analysis of postoperative pancreatic cancer adjuvant strategies from the efficacy and cost-effectiveness perspective.
According to our analysis, gemcitabine plus capecitabine cohort cost $2,325.25 per month which was higher than gemcitabine alone $1,822.35 for the RFS state. The chemotherapy drugs, test, and inpatient fees costs contributed most to the total costs of different treatment groups. Our one-way sensitivity analyses indicated that the key driver of the ICER of gemcitabine plus gemcitabine vs gemcitabine alone was the cost of the PD state in both cohorts. Gemcitabine plus capecitabine group offered an incremental 0.21 QALY at an incremental cost of $10,066.60, compared with the gemcitabine group, resulting in the ICER of $47,936.19/QALY. The WTP threshold is $25,840.88/QALY in our model, which is triple the per capita gross domestic product of China.Citation31 In other words, the ICER of gemcitabine plus capecitabine vs gemcitabine monotherapy dramatically surpassed the general WTP threshold in China, even though the gemcitabine plus capecitabine regimen showed better clinical response in ESPAC-4 trials. Thus, gemcitabine plus capecitabine is not an optimal cost-effective regimen for postoperative pancreatic cancer from Chinese social perspective.
So far, there has been no economic evaluation for resected pancreatic cancer to compare the standard adjuvant treatment, except in metastatic background. A study reported that paclitaxel ablumin plus gemcitabine regimen offered more 0.154 QALYs and €7082.68 than gemcitabine alone regimen. Incremental cost-utility ratio (€46,021.58) is lower than the informal threshold value of €87,330 adopted by the Italian Medicines Agency (INHS) during 2010–2013 for reimbursing oncological drugs, which means that albumin-bound paclitaxel plus gemcitabine can be considered a cost-effective regimen for metastatic pancreatic cancer by the Italian Medicines Agency.Citation32 Moreover, a pharmacological evaluation compared cost-effectiveness of gemcitabine, gemcitabine plus 5-fluorouracil, gemcitabine plus capecitabine, gemcitabine plus cisplatin, gemcitabine plus oxaliplatin, gemcitabine plus erlotinib, gemcitabine plus nab-paclitaxel, and FOLFIRINOX in the treatment of advanced pancreatic cancer, the result demonstrated that FOLFIRINOX would be the most optimal treatment for advanced pancreatic cancer as the WTP threshold of $50,000 per QALY from a Canadian public health payer threshold, and the analysis also revealed that the most cost-effective regimen relies on the societal WTP threshold.Citation33 Our previous study showed that S-1 regimen could provide the maximum societal benefits and sustainable maintenance of the national healthcare sector than gemcitabine alone or regimen combined with gemcitabine plus S-1 in advanced pancreatic cancer.Citation34
Gemcitabine plus capecitabine and gemcitabine alone both maintain a manageable toxicity profile. In terms of the grade 3–4 adverse events, neutropenia, white blood cell count decreased, and hand-foot syndrome occurred more frequently in the gemcitabine plus capecitabine cohort (38%, 10%, 7%), whereas neutropenia, white blood cell count decreased, infection and infestations were significantly greater in the gemcitabine monotherapy cohort (24%, 8%, 7%). Based on our analysis, the AEs related costs were $41.91 and $55.67 per month in gemcitabine plus capecitabine group and gemcitabine alone, respectively. And the result revealed that the AE-related costs had a minor impact on the ICER of gemcitabine plus capecitabine vs gemcitabine alone.
There were several limitations in this cost-effective analysis. The Chinese cost-effectiveness analysis developed was based on data from the ESPAC-4 trial rather than collecting data from a clinical practice. However, previous studies have discussed the suitability of using foreign clinical data with local population and concluded that the influence of such differences between local individuals and other countries’ populations on event rates should be accepted.Citation35,Citation36 Additionally, we established a sensitivity analysis to create a widely adaptable cost for a local area. Moreover, due to a lack of detailed information about quality of life, the utility value in our model was derived from published literature.
Conclusion
Overall, the current study was the first study to compare an adjuvant chemotherapy regimen in resected pancreatic cancer from a cost-effectiveness perspective. The result demonstrated that the gemcitabine monotherapy regimen is more cost-effective when compared with the gemcitabine plus capecitabine regimen for patients with postoperative pancreatic cancer from the Chinese societal perspective. Our analysis would contribute in aiding clinicians in making optimal decision for the treatment of resected pancreatic cancer patients.
Acknowledgments
The authors thank Prof David W Hutton from the department of Health Management and Policy, University of Michigan for his fruitful discussions on the study. This work was supported by the National Natural Science Foundation of China (No. 81572988) and Science & Technology Department of Sichuan Province Funding Project (No. 2016FZ0108, 18ZDYF1981).
Disclosure
The authors report no conflicts of interest in this work.
References
- KamisawaTWoodLDItoiTTakaoriKPancreatic cancerLancet201638810039738526830752
- Sato-DahlmanMWirthKYamamotoMRole of Gene Therapy in Pancreatic Cancer—A ReviewCancers2018104103
- SiegelRLMillerKDJemalACancer Statistics, 2017CA Cancer J Clin201767173028055103
- GillenSSchusterTMeyer Zum BüschenfeldeCFriessHKleeffJPreoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentagesPLoS Med201074e100026720422030
- OettleHNeuhausPHochhausAAdjuvant Chemotherapy With Gemcitabine and Long-term Outcomes Among Patients With Resected Pancreatic CancerJAMA201331014147324104372
- LiaoW-CChienK-LLinY-LAdjuvant treatments for resected pancreatic adenocarcinoma: a systematic review and network meta-analysisLancet Oncol201314111095110324035532
- NeoptolemosJESPAC-1: A Europian randomized controlled study of adjuvant chemoradiation and chemotherapy in resectable pancreatic cancerLancet20013581576158511716884
- NeoptolemosJPStockenDDFriessHA randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancerN Engl J Med2004350121200121015028824
- OettleHNeuhausPHochhausAAdjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trialJAMA2013310141473148124104372
- NeoptolemosJPStockenDDSmithCTAdjuvant 5-fluoroura-cil and folinic acid vs observation for pancreatic cancer: composite data from the ESPAC-1 and -3(v1) trialsBr J Cancer2009100224625019127260
- ValleJWPalmerDJacksonROptimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 studyJ Clin Oncol201432650451224419109
- NeoptolemosJPStockenDDBassiCAdjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trialJAMA2010304101073108120823433
- NeoptolemosJPMooreMJCoxTFEffect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trialJAMA2012308214715622782416
- UenoHKosugeTMatsuyamaYA randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic CancerBr J Cancer2009101690891519690548
- LiaoWCChienKLLinYLAdjuvant treatments for resected pancreatic adenocarcinoma: a systematic review and network meta-analysisLancet Oncol201314111095110324035532
- NeoptolemosJPCoxTFBayesian analysis unravels pancreas-cancer adjuvant therapyLancet Oncol201314111034103524035534
- OettleHPostSNeuhausPAdjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trialJAMA2007297326727717227978
- NeoptolemosJPPalmerDHGhanehPComparison of adjuvant gemcitabine and capecitabine with gemcitabine mono-therapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trialLancet2017389100731011102428129987
- CunninghamDChauIStockenDDPhase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancerJ Clin Oncol200927335513551819858379
- SultanaASmithCTCunninghamDStarlingNNeoptolemosJPGhanehPMeta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancerJ Clin Oncol200725182607261517577041
- GoldMPanel on cost-effectiveness in health and medicineMed Care199634Suppl 12197199
- CaldwellDDecision Modelling for Health Economic EvaluationBriggsASculpherMClaxtonKInt J Epidemiol2007362476477
- Aalabaf-SabaghiMBook review of Decision Modelling For Health Economic EvaluationBriggsASculpherMClaxtonKJ Epidemiol Commun Health2007619839
- ChenHDZhouJWenFCost-effectiveness analysis of apatinib treatment for chemotherapy-refractory advanced gastric cancerJ Cancer Res Clin Oncol2017143236136827798730
- ConroyTDesseigneFYchouMFOLFIRINOX versus gemcitabine for metastatic pancreatic cancerN Engl J Med2011364191817182521561347
- von HoffDDErvinTArenaFPIncreased survival in pancreatic cancer with nab-paclitaxel plus gemcitabineN Engl J Med2013369181691170324131140
- AttardCLBrownSAlloulKMooreMJCost-effectiveness of folfirinox for first-line treatment of metastatic pancreatic cancerCurr Oncol20142114151
- UesakaKBokuNFukutomiAAdjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01)Lancet20163881004124825727265347
- ZhouJZhaoRWenFCost-effectiveness analysis of treatments for metastatic pancreatic cancer based on PRODIGE and MPACT trialsTumori20162016329430027056335
- MurrayCJEvansDBAcharyaABaltussenRMDevelopment of WHO guidelines on generalized cost-effectiveness analysisHealth Econ20009323525110790702
- WuBYeMChenHShenJFCosts of trastuzumab in combination with chemotherapy for HER2-positive advanced gastric or gastroesophageal junction cancer: an economic evaluation in the Chinese contextClin Ther201234246847922325735
- LazzaroCBaroneCCaprioniFAn Italian cost-effectiveness analysis of paclitaxel albumin (nab-paclitaxel) + gemcitabine vs gemcitabine alone for metastatic pancreatic cancer patients: the APICE studyExpert Rev Pharmacoecon Outcomes Res201818443544629641931
- CoyleDKoYJCoyleKCost-Effectiveness Analysis of Systemic Therapies in Advanced Pancreatic Cancer in the Canadian Health Care SystemValue Health201720458659228408000
- ZhouJZhaoRWenFCost-effectiveness analysis of gemcitabine, S-1 and gemcitabine plus S-1 for treatment of advanced pancreatic cancer based on GEST studyMed Oncol201532412125788034
- LeeSGJeeYGChungHCCost-effectiveness analysis of adjuvant therapy for node positive breast cancer in Korea: docetaxel, doxorubicin and cyclophosphamide (TAC) versus fluorouracil, doxorubicin and cyclophosphamide (FAC)Breast Cancer Res Treat2009114358959518437555
- LiubaoPXiaominWChongqingTCost-effectiveness analysis of adjuvant therapy for operable breast cancer from a Chinese perspective: doxorubicin plus cyclophosphamide versus docetaxel plus cyclophosphamidePharmacoeconomics2009271087388619803541
