Abstract
Purpose
Our study was to evaluate the influence of positive lymph nodes ratio (PLNR) on survival for patients with pathological stage IIIA-N2 non-small cell lung cancer (NSCLC) after receiving postoperative radiotherapy (PORT).
Patients and methods
The chi-squared test was used to compare the patient baseline characteristics. Cox proportional hazard model was used to analyze the influence of different variables on overall survival (OS). X-tile model was applied to determine the cutoff values of PLNR. Kaplan–Meier method and log-rank test were used to compare survival differences. Based on different cutoff values of PLNR, Cox proportional hazard model was also used to analyze the influence factors on OS.
Results
Multivariate Cox regression analysis showed that PLNR (P=0.001) and PORT (HR=1.283; 95% CI 1.154–1.426; P<0.001) were significant independent prognostic factors for OS in patients with resected IIIA-N2 NSCLC. The X-tile model was used to screen three different cutoff values including PLNR≤20%, 20%<PLNR≤40%, PLNR>40%. Based on these different cutoff values, we found that patients with PLNR≤20% receiving PORT have a better OS (P=0.007). Further multivariable analysis showed that PORT is an independent prognostic factor of OS only for patients with PLNR≤20% (HR=1.328; 95% CI 1.139–1.549; P<0.001). Conclusion: PLNR≤20% may be a prognostic factor for patients with IIIA-N2 NSCLC receiving PORT.
Introduction
Primary bronchogenic carcinoma is one of the most common malignant tumors, and the incidence and mortality of lung cancer are on the rise in China.Citation1 Non-small cell lung cancer (NSCLC) makes up approximately 85% of all lung cancers. The choice of guiding treatment depends on the size, type and accurate staging of tumor,Citation2 as surgery, RT, chemotherapy, targeted therapy and immunotherapy can be used for the treatment of lung cancer.Citation3 Relevant studies have pointed out that a possible approach for IIIA-N2 patients might be a multimodality treatment.Citation4–Citation7 For these patients, surgical removal of cancerous swelling is the most commonly used method, with 5-year overall survival (OS) rates in the range of 7%–34%.Citation8
For patients with completely resected IIIA-N2 NSCLC, POCT has become a recognized adjuvant therapy.Citation9–Citation11 PORT is also considered as an effective means of treatment. Previous studies have pointed out that positive lymph node and LNR were confirmed to possess a significant guiding role in the prognosis of PORT with resected IIIA-N2 NSCLC.Citation12–Citation17 However, no relevant study has focused on the relationship between positive lymph nodes ratio (PLNR) and postoperative radiotherapy (PORT) in IIIA-N2 NSCLC patients. In our study, we retrospectively analyzed 3,134 patients with resected stage IIIA-N2 NSCLC either receiving or not receiving PORT to identify the subgroups who benefit from PORT. This data originated from the SEER database. According to SEER database, we analyzed the relationship between PLNR and PORT on survival time in the patients with resected IIIA-N2 NSCLC.
Patients and methods
Data source
The SEER is a National Cancer Institute program and is a comprehensive source of population-based data in the United States.Citation18 The SEER database provides detailed information regarding patient demographics, diagnosis, treatment, and survival outcomes. Using the SEER database and based on the American Joint Committee on Cancer criteria, we selected a total of 3,134 patients with pathologically resected stage IIIA-N2 NSCLC between 2004 and 2013 using the SEER*Stat 8.3.5 software. The inclusion criteria for recruiting patients were as follows: complete resection via either lobectomy or pneumonectomy, no treatment before surgery, only one primary tumor, active follow-up, available clinical information. In addition, patients with benign tumor and other ambiguous and unknown information were all excluded.
Ethics statement
Our study was constructed in accordance with the Declaration of Helsinki. We received permission to access SEER program research data with the reference number 11561-Nov2016. This study was also approved by the ethics committee of the Shandong Cancer Hospital affiliated with Shandong University. This study did not involve any personal information, and therefore informed patient consent was not required.
Statistical analysis
In this study, differences of patient baseline characteristics were analyzed using the chi-squared test. Our main endpoint was OS, which was defined as the time from diagnosis to death due to any reason. Univariate and multivariate Cox regression analyses were applied to assess the prognostic factors on OS for resected IIIA-N2 NSCLC patients receiving PORT. The X-tile model was applied to determine the cutoff values of PLNR and the Kaplan–Meier method was used to calculate OS compared by means of the log-rank test. All statistical analyses were made using Statistical Product and Service Solutions (SPSS) 22.0 software package. All statistical P-values were two-sided and P<0.05 was considered statistically significant.
Results
Patient characteristics
A total of 3,134 patients with resected stage IIIA-N2 NSCLC from the SEER database treated between 2004 and 2013 were included for analysis. Of these, 1,164 patients (37.1%) with pathological stage IIIA-N2 disease received PORT and 1970 patients (62.8%) did not receive PORT. The baseline characteristics of patients are listed in . Among patients receiving PORT, all of them were white (100%), with 51.4% patients less than 60 years of age. The PORT group was with 51% female and 49% male patients, and the ratio of patients at Grades I–II and III–IV were 46% and 54%, respectively. The adenocarcinoma was the main pathological pattern in PORT and non-PORT groups (59.4% vs 58.5%) while the proportion of T1, T2 and T3 were 30.1% vs 27.3%, 61.9% vs 65.3% and 8.4% vs 7.4%, respectively, in both groups. Our results showed that patients who received PORT were related to factors of age (P<0.001) and race (P<0.001). There were no significant differences in sex (P=0.522), grade (P=0.149), pathology (P=0.171) and T stage (P=0.141).
Table 1 Resected pathological stage IIIA-N2 NSCLC patient characteristics from SEER database
PLNR and survival
The primary focus of our study was to examine whether the PLNR was associated with PORT in patients with resected pathological IIIA-N2 NSCLC. Cox proportional hazards model was used to assess the prognostic value of baseline characteristics. Univariable analysis revealed that race, T stage, PORT and PLNR were significant prognostic factors for OS (all P<0.05). The other factors, such as age, sex, grade and pathology, did not make a significant difference to OS. In multivariable analysis, PORT (HR=1.283; 95% CI 1.154–1.426; P<0.001) and PLNR (P=0.001) were independent and significant prognostic factors for OS. Univariable and multivariable analyses of affecting factors of OS are listed in .
Table 2 Influence of different variables on OS for patients with resected pathological stage IIIA-N2 NSCLC analyzed by Cox proportional hazard model
Cutoff determination for PLNR count and survival
The cutoff values of PLNR were determined by the X-tile model. Survival curves were measured using the Kaplan– Meier and compared by long-rank test. The different cutoff values of PLNR on OS including low (PLNR≤20%), medium (20%<PLNR≤40%) and high (PLNR>40%) were produced by X-tile (). Survival curves revealed that the patients with PLNR≤20% had a better OS (P=0.007) from PORT, as measured by Kaplan–Meier (). However, the difference on OS was not found between 20%<PLNR≤40% (P=0.944) and PLNR>40% (P=0.091; ).
Figure 1 The optimal threshold of PLNR count for OS as determined by the X-tile model.
Notes: (A) X-tile plots based on PLNR. (B) OS curves based on the threshold (P<0.05). (C) The optimal cutoff point is shown by the blue (PLNR≤20%), gray (20%<PLNR<40%) and violet panels (PLNR≥40%).
Abbreviations: PLNR, positive lymph nodes ratio; OS, overall survival.
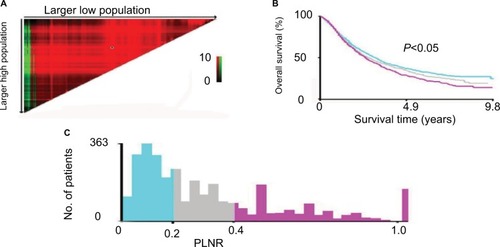
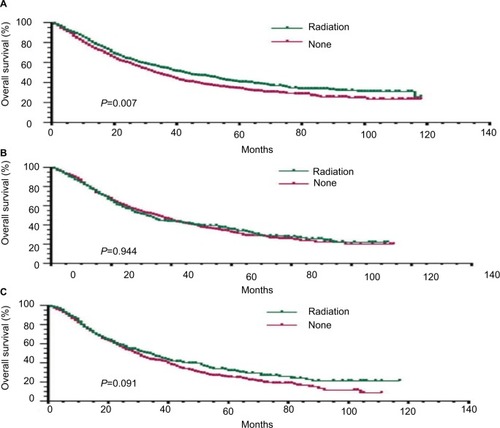
Figure 2 Prognostic survival curves according to PLNR based on different cutoff points adjusted for other variables using the Cox proportional hazard analysis in resected stage IIIA-N2 NSCLC patients receiving PORT.
Notes: The OS curves were based on the threshold. (A) OS curves for patients with PLNR≤20% receiving PORT (P=0.007). (B) OS curves for patients with 20%<PLNR<40% receiving PORT (P=0.944). (C) OS curves for patients with PLNR≥40% receiving PORT (P=0.091).
Abbreviations: PLNR, positive lymph nodes ratio; NSCLC, non-small cell lung cancer; PORT, postoperative radiotherapy; OS, overall survival.
Subgroup analysis for OS based on PLNR
Univariate and multivariate Cox regression analyses were further applied to analyze the effect of PORT on OS based on different cutoff points for PLNR. Our results showed that the patients with PLNR≤20% had a better OS (HR=1.328; 95% CI 1.139–1.549; P<0.001) after receiving PORT. However, the OS benefit was not found in patients with 20%<PLNR≤40% and PLNR>40%. Therefore, PORT was an independent prognostic factor only in patients with PLNR≤20%. The subgroup analysis for OS based on different PLNR is listed in .
Table 3 Subgroup analysis of OS based on PLNR for patients with resected pathological staged IIIA-N2 NSCLC analyzed by Cox proportional hazard model
Discussion
The value of PORT for completely resected IIIA-N2 NSCLC is controversial. A PORT meta-analysis conducted in 1998 has shown that PORT had a detrimental effect on survival.Citation19 Wisnivesky et al demonstrated that PORT was not associated with improved survival for elderly patients with completely resected stage III NSCLC (HR=1.11; 95% CI 0.97–1.27; P>0.05).Citation20 In contrast, a growing number of more research has supported the use of modern PORT for completely resected stage IIIA-N2 NSCLC.Citation21,Citation22 Recently, several studies have suggested that patients with completely resected stage IIIA-N2 NSCLC may be a result of benefits from PORT.Citation23–Citation26 Feng et al have demonstrated that PORT was an independent prognostic factor for improved locoreginal free Survival (HR=0.2, 95% CI 0.1–0.5; P=0.001) and improved OS (HR=0.4, 95% CI 0.2–0.7; P=0.001).Citation27 The Lung Adjuvant Radiotherapy Trial (LungART, NCT00410683), a randomized trial of modern PORT vs no PORT in patients with resected NSCLC, is ongoing.Citation28
Patients with stage IIIA-pN2 NSCLC are a heterogeneous group and the treatment for these patients should be individualized.Citation29 Reif et al showed that surgery alone is considered to have a more limited role in the management of stage IIIA patients.Citation30 However, many patients with locally advanced or metastatic disease lose the opportunity for surgery at the time of diagnosis.
Chemotherapy and RT have become the most important method to treat these patients. Roth et al showed that the treatment strategy using perioperative chemotherapy and surgery was more effective than surgery alone for patients with IIIA-N2 NSCLC.Citation31 Furthermore, POCT is considered a recognized treatment method for patients with resected IIIA-N2 NSCLC. In spite of this dispute, PORT was also demonstrated as an effective treatment. Feng et al showed that PORT was an independent prognostic factor for improved OS (HR=0.4, 95% CI 0.2–0.7; P=0.001); female sex (HR=0.5, 95% CI 0.3–0.7; P<0.001) and LNR>20% (HR=2.4, 95% CI 1.7–3.3; P<0.001) were the other factors for OS.Citation27 Wang et al demonstrated that in stage IIIA-pN2 NSCLC, the use of PORT demonstrated better survival results than no PORT for patients with positive LNs with n>3, but not for patients with positive LNs with n≤3.Citation17 Other studies have reported that several clinical and pathological factors, such as the number of pathologically involved lymph node stations,Citation32,Citation33 extracapsular extension,Citation34 lymph node skip status and positive LNRCitation12–Citation16 should be considered when evaluating the risks and benefits of PORT. However, so far, there are no relative reports about the relationship between PLNR and PORT in IIIA-N2 NSCLC patients. The aim of this study is to estimate the association between PORT and PLNR for patients with resected pathological stage IIIA-N2 NSCLC.
In the present study, we regard OS as our main end- point. The outcomes of multivariable analysis have shown that PLNR and the use of PORT were independent impact factors on OS in patients with resected IIIA-N2 NSCLC. We concluded that PLNR≤20% in patients with IIIA-N2 NSCLC may be a benefit from PORT. X-tile model was conducted to determine the optimal cutoff point.Citation35 Our study demonstrated that 20% and 40% were the proper cutoff PLNR values for OS with PORT patients. We found that the patients with PLNR less than 20% had a better OS rate. The patients with PLNR>20% had no significant difference on OS.
Why patients with a lower tumoral burden would benefit more from PORT with respect to patients with a higher number of pathological mediastinal lymph nodes? One plausible explanation is that a lower PLNR is associated with a better survival for patients with IIIA-N2 NSCLC than higher PLNR. Also, PLNR may reflect the body’s immune system and the tumor–host interaction. The value of the PLNR as a predictor of PORT benefits may also be a result of the interaction between the immune system and RT.Citation36 Another possible explanation may be that for patients with high PLNR, the toxicity of PORT is far greater than its benefits. In addition, the lower the PLNR, the number of lymph nodes that may be cleared or the number of negative lymph nodes is more. Studies have shown that the more the number of negative lymph nodes is cleaned, the better the prognosis of the patients.Citation37–Citation40
Limitations in this study should be noted. First, this was a retrospective study from the SEER database, and not a randomized controlled clinical trial. Second, some other variables, such as smoking history, type of surgery, involved N2 stations and number of positive nodes, affecting the prognosis, were not included in the present study. Additionally, other adjuvant therapies such as chemotherapy, targeted therapy and endocrine therapy were not included in this study. Finally, due to the constraints of the SEER database, we cannot obtain specific information about the dose and segmentation of PORT and the effects of other postoperative treatments. These issues may have some effects on the results, leading to the limitations of the study, which should be explored in future studies.
To the best of our knowledge, this study is the first to demonstrate PLNR as a prognostic factor for patients with IIIA-N2 NSCLC receiving PORT. This study showed that patients with PLNR≤20% can benefit from PORT with improved OS. The results of this study may be of help for clinicians, surgeons and radiotherapists to choose appropriate treatment. This result requires further large-scale prospective clinical study to confirm these recommendations.
Acknowledgments
This study was supported jointly by the National Natural Science Foundation of China (No. 81603348); China Postdoctoral fund (No. 21300075311104) and Shandong postdoctoral innovation special fund (No. 201602012); China Postdoctoral Special Fund (No. 2018T110696); Shandong Province key R&D Plan (No.2018GSF119014).
Disclosure
The authors report no conflicts of interest in this work.
References
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- MolinaJRYangPCassiviSDSchildSEAdjeiAANon-Small Cell Lung Cancer: Epidemiology, Risk Factors, Treatment, and SurvivorshipMayo Clin Proc200883558459418452692
- Cancer Council Australia Lung Cancer Guidelines Working PartyClinical practice guidelines for the treatment of lung cancer Available from: http://wiki.cancer.org.au/australia/Guidelines:Lung_cancerAccessed April 22, 2016
- DecaluwéHde LeynPVansteenkisteJSurgical multimodality treatment for baseline resectable stage IIIA-N2 non-small cell lung cancer. Degree of mediastinal lymph node involvement and impact on survivalEur J Cardiothorac Surg200936343343919502079
- StefaniAAlifanoMBobbioAWhich patients should be operated on after induction chemotherapy for N2 non-small cell lung cancer? Analysis of a 7-year experience in 175 patientsJ Thorac Cardiovasc Surg2010140235636320381815
- PaulSMirzaFPortJLSurvival of patients with clinical stage IIIA non-small cell lung cancer after induction therapy: age, mediastinal downstaging, and extent of pulmonary resection as independent predictorsJ Thorac Cardiovasc Surg20111411485821092990
- KappersIvan SandickJWBurgersSABelderbosJSvan ZandwijkNKlompHMSurgery after induction chemotherapy in stage IIIA-N2 non-small cell lung cancer: why pneumonectomy should be avoidedLung Cancer201068222222719664843
- MolinaJRYangPCassiviSDSchildSEAdjeiAANon-small cell lung cancer: epidemiology, risk factors, treatment, and survivorshipMayo Clin Proc200883558459418452692
- ArriagadaRBergmanBDunantAInternational Adjuvant Lung cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancerN Engl J Med200435035136014736927
- WintonTLivingstonRJohnsonDVinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancerN Engl J Med2005352252589259715972865
- DouillardJYRosellRde LenaMAdjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trialLancet Oncol20067971972716945766
- JonnalagaddaSArcinegaJSmithCWisniveskyJPValidation of the lymph node ratio as a prognostic factor in patients with N1 nonsmall cell lung cancerCancer2011117204724473121452193
- MatsugumaHOkiINakaharaRProposal of new nodal classifications for non-small-cell lung cancer based on the number and ratio of metastatic lymph nodesEur J Cardiothorac Surg2012411192421620720
- NwoguCEGromanAFaheyDNumber of lymph nodes and metastatic lymph node ratio are associated with survival in lung cancerAnn Thorac Surg20129351614162022440365
- WangCLLiYYueDSZhangLMZhangZFSunBSValue of the metastatic lymph node ratio for predicting the prognosis of non-small- cell lung cancer patientsWorld J Surg201236245546222187129
- RenaudSFalcozP-EOllandAReebJSantelmoNMassardGMediastinal downstaging after induction treatment is not a significant prognostic factor to select patients who would benefit from surgery: the clinical value of the lymph node ratioInteract Cardiovasc Thorac Surg201520222222725413781
- WangSMaZYangXChoice of postoperative radiation for stage IIIA pathologic N2 non-small cell lung cancer: impact of metastatic lymph node numberRadiat Oncol201712120729284511
- Surveillance, Epidemiology, and End Results (SEER) ProgramSEER 17 Regs Public-Use, Nov 2006 Sub (1973–2004)NCI Available from:https://www.seer.cancer.govAccessed April 2007
- PORT Meta-analysis Trialists GroupPostoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. PORT Meta-analysis Trialists GroupLancet199835291242572639690404
- WisniveskyJPHalmEABonomiMSmithCMhangoGBagiellaEPostoperative radiotherapy for elderly patients with stage III lung cancerCancer2012118184478448522331818
- CorsoCDRutterCEWilsonLDKimAWDeckerRHHusainZARe-evaluation of the role of postoperative radiotherapy and the impact of radiation dose for non-small-cell lung cancer using the National Cancer DatabaseJ Thorac Oncol201510114815525325781
- RobinsonCGPatelAPBradleyJDPostoperative radiotherapy for pathologic N2 non-small-cell lung cancer treated with adjuvant chemotherapy: a review of the National Cancer Data BaseJ Clin Oncol201533887087625667283
- LallyBEZeltermanDColasantoJMHafftyBGDetterbeckFCWilsonLDPostoperative radiotherapy for stage II or III non-small- cell lung cancer using the surveillance, epidemiology, and end results databaseJ Clin Oncol200624192998300616769986
- SawyerTEBonnerJAGouldPMEffectiveness of postoperative irradiation in stage IIIA non-small cell lung cancer according to regression tree analyses of recurrence risksAnn Thorac Surg1997645140214079386711
- MachtayMLeeJHShragerJBKaiserLRGlatsteinERisk of death from intercurrent disease is not excessively increased by modern postoperative radiotherapy for high-risk resected non-small-cell lung carcinomaJ Clin Oncol200119193912391711579111
- DouillardJYRosellRde LenaMImpact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant Navelbine International Trialist Association (ANITA) Randomized TrialInt J Radiat Oncol Biol Phys200872369570118439766
- FengWZhangQFuX-LThe emerging outcome of postoperative radiotherapy for stage IIIA(N2) non-small cell lung cancer patients: based on the three-dimensional conformal radiotherapy technique and institutional standard clinical target volumeBMC Cancer201515134825934006
- Le PéchouxCDunantAFaivre-FinnCPostoperative Radiotherapy for Pathologic N2 Non-Small-Cell Lung Cancer Treated With Adjuvant Chemotherapy: Need for Randomized EvidenceJ Clin Oncol201533262930293126215941
- AndreFGrunenwaldDPignonJPSurvival of patients with resected N2 non-small-cell lung cancer: evidence for a subclassification and implicationsJ Clin Oncol200018162981298910944131
- ReifMSocinskiMARiveraMPEvidence-based medicine in the treatment of non-small cell lung cancerClin Chest Med20002110712010763093
- RothJAFossellaFKomakiRA randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancerJ Natl Cancer Inst19948696736808158698
- MantovaniCLevraNGFilippiARPostoperative radiotherapy for patients with completely resected pathologic n2 non-small-cell lung cancer: a retrospective analysisClin Lung Cancer201314219419922885347
- MatsugumaHNakaharaRIshikawaYPostoperative radiotherapy for patients with completely resected pathological stage IIIA-N2 non- small cell lung cancer: focusing on an effect of the number of mediastinal lymph node stations involvedInteract Cardiovasc Thorac Surg20087457357718413349
- MorettiLYuDSChenHPrognostic factors for resected non- small cell lung cancer with pN2 status: implications for use of postoperative radiotherapyOncologist200914111106111519897534
- CampRLDolled-FilhartMRimmDLX-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimizationClin Cancer Res200410217252725915534099
- DemariaSFormentiSCRole of T lymphocytes in tumor response to radiotherapyFront Oncol201229522937524
- DoddoliCAragonABarlesiFDoes the extent of lymph node dissection influence outcome in patients with stage I non-small-cell lung cancer?Eur J Cardiothorac Surg200527468068515784374
- WhitsonBAGrothSSMaddausMASurgical assessment and intra- operative management of mediastinal lymph nodes in non-small cell lung cancerAnn Thorac Surg20078431059106517720443
- GajraANewmanNGambleGPKohmanLJGrazianoSLEffect of number of lymph nodes sampled on outcome in patients with stage I non-small-cell lung cancerJ Clin Oncol20032161029103412637467
- TsaiYMHuangTWHsuHHPrognostic significance of the number of removed lymph nodes at lobectomy in patients with positron emission tomography-computed tomography-negative N2 non-small cell lung cancerOnkologie20133693623549030
