Abstract
Background
Resistance to radiation therapy is still a challenge for treatment of pancreatic cancer(PC). Long non-coding RNAs (lncRNA) HOTAIR has been found to play a oncogenic role in several cancers. However, the correlation between HOTAIR and radiotherapy in PC is still unclear.
Methods
TCGA data was collected to analyze the expression of HOTAIR and its relationship with PC progression. A series of functional experiments were conducted to explore the role of HOTAIR in PC radiosensitivity and its underlying molecular mechanisms.
Results
By the analysis of the TCGA data, we found HOTAIR expression in PC tissues was significantly higher than normal tissues and associated with tumor progression. The function analysis showed HOTAIR was enriched in biological regulation and response to stimulus. And in vitro study, the expression of HOTAIR was increased in PANC-1 and AsPC-1 cells after radiation. We identified that HOTAIR knockdown could enhance radiosensitivity and influence autophagy by up-regulating ATG7 expression in PC cells. By futher rescue experiments using rapamycin, activation of autophagy could reversed the the inhibition of cell proliferation and colony formation, as well as promotion of apoptosis mediated by HOTAIR knockdown, indicating that HOTAIR knockdown promoted radiosensitivity of PC cells by regulating autophagy.
Conclusion
Our finding revealed the the regulatory role of HOTAIR in radiosensitivity and provided a a new sight to improve radiotherapy effciency in PC.
Introduction
Pancreatic cancer (PC) is a kind of highly invasive potential tumor. The survival rate of PC patients who have not performed radical resection is <2%, which is only 24% even after early radical resection.Citation1 PC patients who have not undergone radical resection are with a median survival time <6 months. In the western countries, the death caused by PC is the fourth, after lung cancer, colorectal cancer, and breast cancer.Citation2 Radioresistance is one of the main reasons for poor prognosis of PC patients. How to improve the sensitivity of PC with radiation is a clinical problem to be solved.
Long non-coding RNA (lncRNA) is of >200 nt in length, which is located in the nucleus and cytoplasm.Citation3 LncRNA has stability splice site, and it is with a specific secondary structure and expression of the space–time specialty. It can regulate gene expression in epigenetics, transcription, and post-transcription level.Citation3 Moreover, some studies indicated that lncRNA is related to cancer radioresistance, for example, lncRNA NKLA can increase laryngeal cancer cell radioresistance.Citation4 lncRNA HOTAIR is related to gynecologic cancer cell radioresistance.Citation5 lncRNA UCA1 can increase prostate cancer radioresistance.Citation6 A num ber of studiesCitation7,Citation8 about PC have demonstrated that lncRNA can promote PC genesis and development, such as lncRNA UCA1, lncRNA DUXAP10, and lncRNA HOTAIR. However, the functions of lncRNA in PC radioresistance are still unrevealed.
In addition, autophagy plays a vital role in cancer radioresistance. Cancer radiation, as a physical agent, can kill cancer cells. When tumor cells obtained high-energy radiation, their genetic material (DNA) is destroyed, and the ability of self-renewal, division, and proliferation is reduced.Citation9 Cancer cells adapted these negative factors through autophagy to remain stable.Citation10 Autophagy is a cellular metabolism with nutrient deficiency or other metabolic stress. Maintaining degradation of intracellular metabolic balance or aggregation of proteins and organelles is the main function of autophagy. Autophagy can make cancer cells survive from radiation therapy. Studies have demonstrated that inhibition of autophagy in cancer cells, such as breast cancer cells, nasopharyngeal carcinoma cells, esophageal carcinoma cells, and lung cancer cells, increases the radiation sensitivity of tumor cells.Citation10–Citation13 Studies have shown that in PC, autophagy can promote the development of the occurrence of PC.Citation14,Citation15 However, the role of autophagy in PC is rarely reported. In the process of autophagy in cancer cells, lncRNA also plays an important role. Xiao et al found that lncRNA MALAT1 can increase the drug resistance of hepatic cellular cancer (HCC) cells by regulating autophagy through HIF-2α-MALAT1-miR-216b axis.Citation16 Another research found that lncRNA HILC may regulate apoptosis and invasion of gastric cancer cells by regulating autophagy.Citation17 To sum up, lncRNA may have an effect on PC cell radioresistance through regulating autophagy and PC cells. In the present study, we intended to find the relationship between autophagy and PC cell radioresistance, which may provide theoretical foundation for the clinical radiotherapy of PC.
Materials and methods
The Cancer Genome Atlas (TCGA) analysis
The pancreatic adenocarcinoma (PAAD) genomic data were downloaded from TCGA database (https://cancergenome.nih.gov). The expression data were pretreated by Bioconductor package. A total of 179 PC cases with detailed clinical information and the corresponding HOTAIR expression data were included in this study. The PC cases were divided into two groups according to the clinical information to analyze the relationship between HOTAIR expression and clinicopathological features. Kaplan–Meier method was used to draw the survival curves compared with the log-rank test.
Function analysis
The function enrichment analysis was conducted on the high and low HOTAIR expression in PC cases. The biological process category was used in the analysis. Statistical significance was considered as P<0.05.
Cell culture and transfection
The human PC cell lines PANC-1 and AsPC-1 cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in DMEM (Sigma-Aldrich Co., St Louis, MO, USA) with 10% FBS at 37ºc in humidified air with 5% CO2. All the vector plasmids such as pcDNA3.1-HOTAIR, si-HOTAIR, and negative control were chemically synthesized by Genechem Co. Ltd. (Shanghai, China). Cell transfection was performed using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA).
Radiation treatment of cells
Cells were irradiated by Varian Clinac 23EX with 6-MV X-ray at 400 cGy/min dose rate. Cells were exposed to different doses of irradiation treatment (0, 2, 4, 6, and 8 Gy). After 24–96 hours, the cells were used for other analysis. For time-course experiment, the cells received 4 Gy and then were collected for quantitative real-time PCR (qRT-PCR) analysis every 2 hours for 24 hours after irradiation.
qRT-PCR
Total RNA from cells was extracted by using TRIzol regent (Thermo Fisher Scientific). First-strand complementary DNA (cDNA) was synthesized using random primers using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). qRT-PCR was performed by SYBR Premix Ex Taq II (Takara, Dalian, China). The relative expression of HOTAIR was calculated by the 2−ΔΔCT method and normalized with GAPDH as the internal control. The primers used were as follows: HOTAIR, 5′-CAGTGGGGAACTCTGACTCG-3′ forward and 5′-GTGCCTGGTGCTCTCTTACC-3′ reverse; GADPH, 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ forward, 5′-AGCCTTCTCCATGGTGGTGAGAC-3′ reverse.
Proliferation assay
Cells were inoculated into a 96-well plate and transfected with si-HOTAIR or si-NC and then exposed to 4 Gy dose X-ray. At 0, 24, 48, 72, and 96 hours after radiation, 20 µL MTT reagent (Sigma-Aldrich Co.) was added into each well and incubated for another 4 hours at 37°C in the dark. The medium was removed and 150 µL of dimethyl sulfoxide was added to each well for another 15 minutes. The optical density (OD) value was measured at 490 nm with a multi-functional microplate reader (BioTek, Winooski, VT, USA).
Flow cytometric analysis of apoptosis
PANC-1 and AsPC-1 cells were inoculated into a six-well plate and transfected with si-HOTAIR or si-NC for 24 hours. Then the cells were treated with a 4 Gy dose X-ray. At 24 hours after irradiation treatment, annexin-V, fluorescein isothiocyanate, and propidium iodide apoptosis detection kit was used to measure cell apoptosis rate. Each experiment was conducted at least three times.
Colony formation assay
PANC-1 and AsPC-1 cells (1,000 cells/plate) transfected with si-HOTAIR or si-NC were seeded in six-well plates. At 24 hours after incubation, the cells were treated with different doses (0, 2, 4, 6, and 8 Gy) of X-ray irradiation at a dose rate of 3.5 Gy/min and seeded into 60 mm dishes until visible colonies appeared. The colonies were then stained with crystal violet (Santa Cruz Biotechnology Inc., Dallas TX, USA) and were counted.
Autophagy-related detection
The autophagy flux detection was the same as that in our previous study.Citation18,Citation19
Statistical analysis
Data analyses were performed using the SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). The results are presented as mean ± SD; statistical analyses were performed using Student’s t-test or analysis of variance. P<0.05 was considered statistically significant.
Results
Expression profile and function analysis of HOTAIR in PC
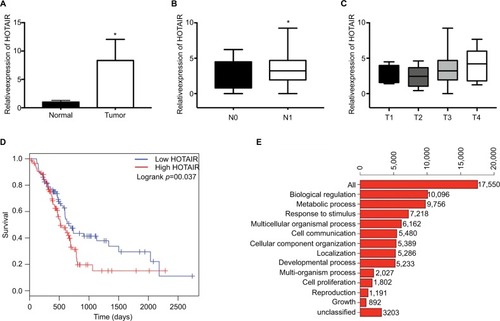
To explore the expression profile of lncRNA HOTAIR in PC tissues, we analyzed the PAAD genomic data which can be downloaded from TCGA database (https://cancergenome.nih.gov). By analysis of the RNA-seq data, we found that HOTAIR was significantly upregulated in PC tissue samples than normal pancreatic tissues, where the raw data were obtained from GTEx (). To analyze the relationship between HOTAIR expression and clinicopathological features, the results showed that HOTAIR expression was correlated with tumor T stage and lymph node metastasis. The expression of HOTAIR was significantly higher in lymph node metastasis group () and was correlated with T stage (). In addition, Kaplan–Meier analysis of 179 PC patients presented that PC patients with high HOTAIR expression has a poor prognosis than patients with relatively low HOTAIR expression (). Function analysis of biological process for HOTAIR in PC showed that biological regulation and metabolic response to stimulus were the most enriched processes (). Taken together, these results indicated that HOTAIR plays an important role in PC progression.
Figure 1 The expression of HOTAIR in PC samples and function analysis.
Notes: (A) The expression of HOTAIR in 179 PC samples from TCGA database, compared with normal pancreatic tissues from GTEx. (B) The expression profile of HOTAIR in N0 (negative lymph node metastasis) samples or in N1 (lymph node metastasis) samples. (C) The expression profile of HOTAIR in T1-T4 samples. (D) Kaplan– Meier analysis of 179 PC patients in low HOTAIR samples or high HOTAIR samples. (E) Function analysis of HOTAIR in PC samples. *P<0.05.
Abbreviations: GTEx, genotype-tissue expression; HOTAIR, HOX transcript antisense RNA; PC, pancreatic cancer.
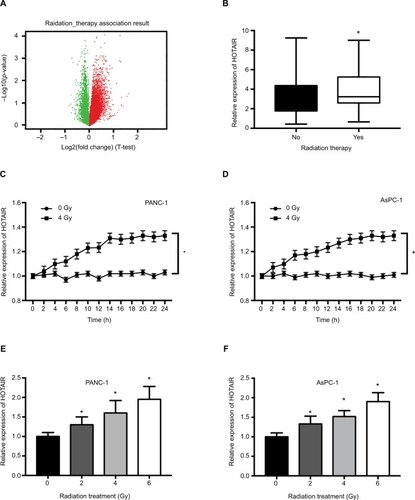
HOTAIR was upregulated after radiotherapy in PC samples and cells
Growing evidence has shown that lncRNA can participate in the process of radiosensitivity. To explore the role of HOTAIR in radiotherapy, we performed RNA-seq analysis in PC samples which had received radiation therapy. There are 119 PC patients who had received radiotherapy and 44 PC patients without radiotherapy. Differential expression analysis revealed a set of differentially expressed genes, in which red points represented upregulated genes and green points represented downregulated genes (). The analysis revealed HOTAIR was one of the significant upregulated gene expression (). In in vitro experiments, we detected whether radiation treatment could influence the expression of HOTAIR in PC cells. When treated with 4 Gy X-ray in PANC-1 and AsPC-1 cells, the expression of HOTAIR was significantly increased after 24 hours (). Additionally, HOTAIR expression was measured in PANC-1 and AsPC-1 cells after different doses (0, 2, 4, 6 Gy) of radiation treatment for 24 hours (). Taken together, these results indicated that the expression of HOTAIR in PC cells can be modulated by radiation treatment in time- and dose-dependent manners.
Figure 2 The effect of radiation on HOTAIR expression in PC samples and cells.
Notes: (A) Volcano plots reporting the adjusted P-value and the log2 fold change of gene expression profile. Red points represented upregulated genes and green points represented downregulated genes. (B) HOTAIR expression was analyzed in 119 PC patients who received radiotherapy and 44 PC patients without radiotherapy. (C, D) HOTAIR expression was detected in PANC-1 and AsPC-1 cells every 2 hours after 0 or 4 Gy of radiation treatment. (E, F) HOTAIR expression was detected in PANC-1 and AsPC-1 cells after different doses (0, 2, 4, 6 Gy) of radiation treatment for 24 hours. *P<0.05.
Abbreviations: HOTAIR, HOX transcript antisense RNA; PC, pancreatic cancer.
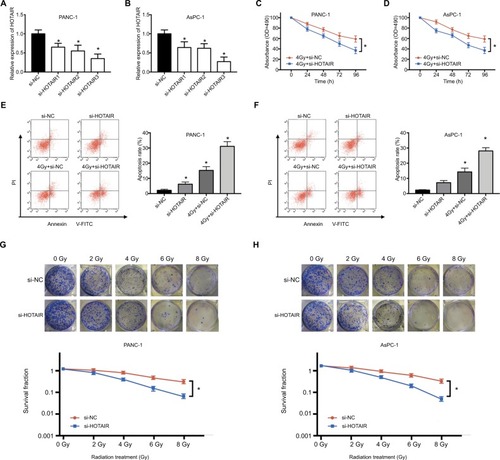
HOTAIR knockdown enhances radiosensitivity of PC cells
According to the upregulation of HOTAIR in response to radiation treatment, we next explored whether HOTAIR could affect the radiosensitivity in PC cells. First, we used three different siRNAs to knock down HOTAIR expression in PC cells. The si-HOTAIR3 has the highest transfection efficiency (). Consequently, we used si-HOTAIR3 in the follow-up experiments. PANC-1 and AsPC-1 cells were exposed to 4 Gy radiation treatment transfected with si-NC or si-HOTAIR. The results indicated that knockdown of HOTAIR could further inhibit the proliferation of PC cells compared with only radiation treatment (). Additionally, knockdown of HOTAIR or radiation therapy could induce apoptosis of PC cells. Combination of si-HOTAIR and radiation therapy significantly increased the apoptosis rates of PANC-1 and AsPC-1 cells compared to si-HOTAIR or 4 Gy+ si-HOTAIR treatment (). Moreover, after exposed to various doses of X-ray, colony formation assay was performed in PC cells transfected with si-NC or si-HOTAIR. The results showed that the PC cell knockdown of HOTAIR were more sensitive to X-ray irradiation than si-NC group (). These results indicated that HOTAIR knockdown can enhance radiosensitivity of PC cells.
Figure 3 The effect of HOTAIR knockdown on radiosensitivity in PC cells.
Notes: (A, B) The transfection efficiency of si-HOTAIR1, si-HOTAIR2, and si-HOTAIR3 was performed by qRT-PCR in PANC-1 and AsPC-1 cells. (C, D) MTT assay was performed to evaluate cell viability at 24, 48, 72, and 96 hours after PC cells transfected with si-HOTAIR or si-NC exposed to 4 Gy radiation. (E, F) Apoptosis rate was detected in PC cells transfected with si-HOTAIR or si-NC exposed to 4 Gy radiation after 24 hours. (G, H) Colony formation assay was performed in PC cells transfected with si-NC or si-HOTAIR exposed to various doses of X-ray. *P<0.05.
Abbreviations: NC, negative control; si-, small interfering; qRT-PCR, quantitative real-time PCR; HOTAIR, HOX transcript antisense RNA; PC, pancreatic cancer.
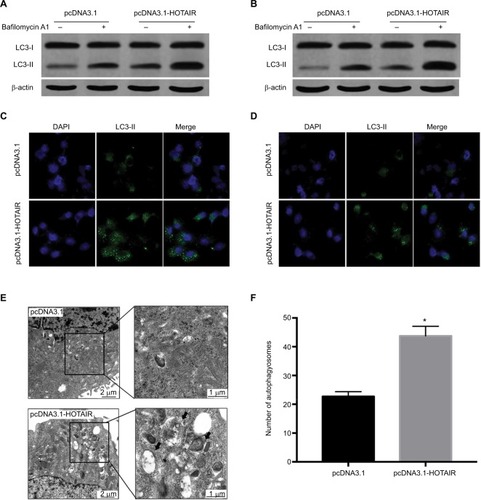
HOTAIR can promote autophagy of PC cells
Growing evidence has demonstrated that autophagy is involved in radiosensitivity in cancer cells. And several studies have found that lncRNA can participate in the regulation of autophagy. We hypothesized that HOTAIR affected radiosensitivity through regulating autophagy in PC cells. To probe for the relationship between HOTAIR and autophagy, we detected the autophagy flux level when PC cells were overexpression of HOTAIR and knockdown of HOTAIR. The results showed that the ratio of LC3-II/LC3-I was increased. Because autophagy is a dynamic process, both induction of autophagosome formation and inhibition of autophagosomes degradation can lead to the accumulation of LC3-II. So we used bafilomycin to inhibit the degradation of LC3-II. The result showed that accumulation of LC3-II was enhanced in treated with bafilomycin and HOTAIR overexpression cells, which suggests that HOTAIR promoted the autophagosome formation (). Then we utilized the LC3-fused GFP construct (pGFP-LC3) to investigate the distribution of autophagic marker protein LC3. We found that the GFP-LC3 puncta increased in the HOTAIR overexpression group (). In addition, we observed the formation of autophagosome and autophagolysosome in PC cells transfected with pcDNA3.1-HOTAIR by the TEM method (). HOTAIR markedly increased the number of autophagosomes (). These results support that HOTAIR can promote autophagy flux of PC cells.
Figure 4 The effect of HOTAIR on autophagy in PC cells.
Notes: (A, B) The expressions of LC3-I and LC3-II were detected by Western blot when PC cells were transfected with pcDNA3.1 or pcDNA3.1-HOTAIR and treated with bafilomycin to detect autophagy flux. (C, D) Using pGFP-LC3 to detect the LC3 expression when PC cells were transfected with pcDNA3.1 or pcDNA3.1-HOTAIR under fluorescence microscope. (E) Transmission electron microscopy showed autophagosome formation was detected in PC cells transfected with pcDNA3.1-HOTAIR. (F) The number of autophagosomes was counted in pcDNA3.1 and pcDNA3.1-HOTAIR group; *P<0.05.
Abbreviations: LC3, microtubule-associated protein 1 light chain 3; HOTAIR, HOX transcript antisense RNA; PC, pancreatic cancer.
HOTAIR promotes autophagy by upregulating Atg7 expression
Autophagy is a dynamic and continuous catabolic process, which involves multiple stages. Many autophagy- related genes (ATG) take part in the progression of each stage.
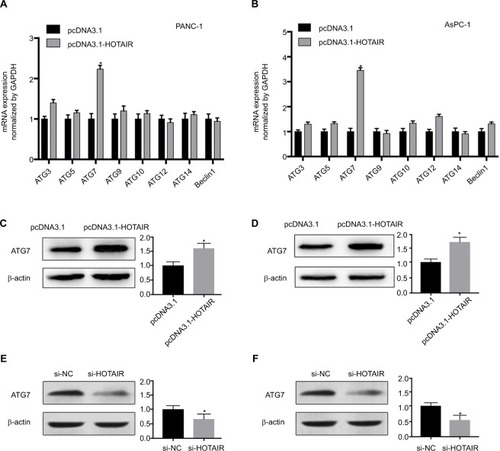
To explore the molecular mechanism by which HOTAIR promotes autophagy in PC cells, we used qRT-PCR to detect the main autophagy-related gene expression change in PC cells, which overexpressed HOTAIR. Among autophagy-related genes of ATG3, ATG5, ATG7, ATG9, ATG10, ATG12, ATG14, and Beclin1, the expression of ATG7 was markedly increased by HOTAIR at mRNA level (). In addition, the protein level of ATG7 was also upregulated in our immunoblot assay (). On the contrary, knockdown of HOTAIR decreased ATG7 expression (). ATG7 is a key regulator of the autophagosome formation and responsible for vesicle progression.Citation20 These data revealed that HOTAIR promoted autophagy by targeting Atg7.
Figure 5 HOTAIR promotes autophagy by upregulating ATG7 expression.
Notes: (A, B) The mRNA expression of several autophagy-related genes in PANC-1 and AsPC-1 cells transfected with pcDNA3.1-HOTAIR were measured by real-time PCR. (C, D) ATG7 protein from the PANC-1 and AsPC-1cells was detected by Western blotting, in which cells were transfected with pcDNA3.1-HOTAIR compared with negative control plasmid. (C, D) ATG7 protein from the PANC-1 and AsPC-1 cells was detected by Western blotting, in which cells were transfected with si-HOTAIR compared with negative control plasmid. *P<0.05.
Abbreviations: ATG7, autophagy-related gene 7; HOTAIR, HOX transcript antisense RNA.
HOTAIR affects radiosensitivity of PC cells by regulating autophagy
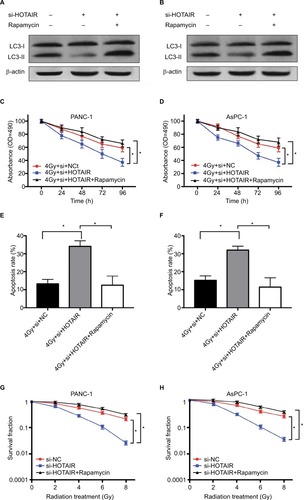
Based on the results of HOTAIR regulating autophagy in PC cells, we further validated this regulatory axis between HOTAIR, autophagy, and radiosensitivity. The result showed that rapamycin treatment restored the autophagy levels in HOTAIR knockdown cells (). PANC-1 and AsPC-1 cells were exposed to 4 Gy radiation transfected with si-NC or si-HOTAIR or combined si-HOTAIR and rapamycin (an activator of autophagy). MTT assay results showed that HOTAIR knockdown markedly decreased cell proliferation but rapamycin significantly reversed this effect in PC cells (). In addition, rapamycin-mediated activation of autophagy in part reversed the apoptosis effect of si-HOTAIR (). Knockdown of HOTAIR notably reduced colony formation rates in PANC-1 and AsPC-1 cells, which was reversed by rapamycin (). These results indicated that HOTAIR knockdown promoted radio-sensitivity of PC cells by regulating autophagy.
Figure 6 HOTAIR affects radiosensitivity of PC cells by regulating autophagy.
Notes: (A, B) The effect of rapamycin treatment on autophagy levels in HOTAIR knockdown cells. (C, D) PANC-1 and AsPC-1 cells were transfected with si-NC or si-HOTAIR or combined si-HOTAIR and rapamycin. Cell proliferation was detected at the indicated times after 4 Gy of radiation treatment. (E, F) Cell apoptosis was tested by flow cytometry at 24 hours after radiotherapy. (G, H) Colony formation assay was performed in PC cells with different doses of radiation treatment. *P<0.05.
Abbreviations: HOTAIR, HOX transcript antisense RNA; PC, pancreatic cancer.
Discussion
Accumulated evidence has identified that lncRNA can affect a number of biological processes of cancer cells, such as proliferation, migration, metastasis, apoptosis, and so on.Citation21,Citation22 And more and more lncRNAs have been found to be aber-rantly expressed in PC cells and involved in various steps of PC progression. Many lncRNAs have the potential value in diagnosis and prognosis in PC.Citation23 By next-generation sequenc ing method, Müller et alCitation24 have found that 43 lncRNAs were abnormally expressed in PC tissues. In addition, Wang et alCitation25 aimed to probe for the potential biomarkers in 7,419 lncRNAs by microarray, in which chip platform was designed for screening lncRNA. The results showed that HOTTIP-005, XLOC_006390, and RP11-567G11.1 were significantly increased in PC tissue. HOTAIR was a lncRNA transcribed from the antisense strand of the HOXC gene locus and has been demonstrated to be overexpressed in various carcinomas.Citation26 In the present study, we identified HOTAIR expression profile in PC RNA-seq data from TCGA database. The advantage of TCGA database is its large sample size and detailed clinical parameter information. Therefore, the result is more persuasive. Our analysis result revealed that HOTAIR was upregulated in PC tissues and significantly related to prognosis. Besides, the function analysis showed that the abnormal expression of HOTAIR was associated with biological regulation and metabolic response to stimulus.
Radiotherapy is one of the main treatment approaches for PC patients.Citation27 However, radioresistance is still the main limitation for the radiation treatment. Several lncRNAs have been found to play an important role in radiosensitivity.Citation28–Citation30 The expression of lncRNA PVT1 has been identified to be increased after radiation in non-small-cell lung cancer (NSCLC) tissues. And PVT1 knockdown enhanced radio-sensitivity of NSCLC cells by sponging miR-195. Shen et alCitation30 demonstrated that LincRNA-p21 knockdown enhances radiosensitivity of hypoxic tumor cells through HIF-1/Akt/ mTOR/P70S6K pathway. Yang et al found that ectopic expression of lncRNA GACAT3 can enhance the effect of radiotherapy in A549 cells.Citation28 These evidence revealed that lncRNA can influence radiosensitivity of cancer cells. From the result of our function analysis, we suspected that HOTAIR may participate in the biological regulation and response to stimulus in PC cells. In in vitro study, our results showed that HOTAIR knockdown enhances radiosensitivity of PC cells. This finding indicated that HOTAIR maybe a potential target for improving the efficiency of radiotherapy.
Next, our aim is to research the mechanism of HOTAIR regulating radiosensitivity in PC cells. A growing number of studies have found that autophagy is related to radiosensitivity.Citation31 Autophagy is a conserved lysosome-mediated and dynamic process that can generate energy and nutrients for maintaining cellular homeostasis.Citation32 It is widely accepted that autophagy plays a protective role in cancer cells during stressors.Citation33 Not only that, many studies have identified that autophagy can protect cells from IR-induced cellular damage and enhance radioresistance.Citation34,Citation35 Inhibition of autophagy is important for the improvement of efficacy of radiotherapy in cancer. Recently, some studies found that lncRNA could activate autophagy by regulating Atg gene expression in cancer cells.Citation19,Citation36 Based on these findings, we suspected that whether HOATIR as a lncRNA could influence radiosensitivity by regulating autophagy in PC cells. Our study found that the ratio of LC3-II/LC3-I and the formation of autophagosome were increased when HOTAIR was overexpressed, indicating that HOTAIR can promote autophagy in PC cells. Furthermore, we utilized the activator of autophagy in the rescue experiments. Our results showed that rapamycin could reverse the inhibition of cell proliferation and colony formation, as well as promotion of apoptosis in vitro mediated by HOTAIR knockdown. It revealed that HOTAIR knockdown promoted radiosensitivity of PC cells by regulating autophagy. Our finding was consistent with a previous study about non-coding RNA and radiotherapy, for example, Peng et al found that inhibition of miR-23b increases autophagy to promote radioresistance.Citation37 However, the detail molecular mechanism about this phenomenon should be further explored.
Conclusion
In conclusion, we identified that HOTAIR plays a key role in PC progression and it is correlated with radiotherapy through the analysis of the TCGA database. From in vitro approaches, we found that HOTAIR expression can be upregulated by radiation. And our study showed that HOTAIR knockdown can enhance radiosensitivity via regulating autophagy in PC cells. Our investigation of the relationship between HOTAIR and radiotherapy provided a novel highlight for lncRNA in cancer therapy.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number 81402537 to Chunli Wu); and we also thank the Key Laboratory of Diagnostic Imaging and Interventional Radiology of Liaoning Province for the support.
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalASiegelRXuJWardECancer statistics, 2010CA Cancer J Clin201060527730020610543
- AmbeCMMahipalAFulpJChenLMalafaMPEffect of Met-formin Use on Survival in Resectable Pancreatic Cancer: A Single-Institution Experience and Review of the LiteraturePLoS One2016113e015163226967162
- UlitskyIBartelDPlincRNAs: genomics, evolution, and mechanismsCell20131541264623827673
- YangTLiSLiuJYinDYangXTangQlncRNA-NKILA/NF-κB feedback loop modulates laryngeal cancer cell proliferation, invasion, and radioresistanceCancer Med2018752048206329573243
- LiJWangJZhongYHOTAIR: a key regulator in gynecologic cancersCancer Cell Int2017176528649178
- Fotouhi GhiamATaebSHuangXLong non-coding RNA uro-thelial carcinoma associated 1 (UCA1) mediates radiation response in prostate cancerOncotarget2017834668468927902466
- ZhangMZhaoYZhangYLncRNA UCA1 promotes migration and invasion in pancreatic cancer cells via the Hippo pathwayBiochim Biophys Acta201818645 Pt A17701782
- WangLDongPWangWHuangMTianBGemcitabine treatment causes resistance and malignancy of pancreatic cancer stem-like cells via induction of lncRNA HOTAIRExp Ther Med20171454773478029201179
- BaskarRLeeKAYeoRYeohKWCancer and radiation therapy: current advances and future directionsInt J Med Sci20129319319922408567
- WangYYinWZhuXBlocked autophagy enhances radiosensitivity of nasopharyngeal carcinoma cell line CNE-2 in vitroActa Otolaryngol2014134110511024256039
- ChenYLiXGuoLCombining radiation with autophagy inhibition enhances suppression of tumor growth and angiogenesis in esophageal cancerMol Med Rep20151221645165225891159
- HanMWLeeJCChoiJYAutophagy inhibition can overcome radioresistance in breast cancer cells through suppression of TAK1 activationAnticancer Res20143431449145524596393
- SchaafMBJuttenBKeulersTGCanonical autophagy does not contribute to cellular radioresistanceRadiother Oncol2015114340641225779723
- IovannaJLAutophagy contributes to the initiation of pancreatic cancerMed Sci2017333335339
- NewMvan AckerTLongJSSakamakiJIRyanKMToozeSAMolecular Pathways Controlling Autophagy in Pancreatic CancerFront Oncol201772828316954
- XiaoHTangKLiuPLncRNA MALAT1 functions as a competing endogenous RNA to regulate ZEB2 expression by sponging miR-200s in clear cell kidney carcinomaOncotarget2015635380053801526461224
- ZhaoYGuoQChenJHuJWangSSunYRole of long non-coding RNA HULC in cell proliferation, apoptosis and tumor metastasis of gastric cancer: a clinical and in vitro investigationOncol Rep201431135836424247585
- QiangLWuCMingMViolletBHeYYYyHAutophagy controls p38 activation to promote cell survival under genotoxic stressJ Biol Chem201328831603161123212914
- YangLZhangXLiHLiuJThe long noncoding RNA HOTAIR activates autophagy by upregulating ATG3 and ATG7 in hepatocellular carcinomaMol Biosyst20161282605261227301338
- WeidbergHShvetsEElazarZBiogenesis and cargo selectivity of autophagosomesAnnu Rev Biochem20118012515621548784
- MorlandoMFaticaAAlteration of Epigenetic Regulation by Long Noncoding RNAs in CancerInt J Mol Sci2018192570
- BhanASoleimaniMMandalSSLong Noncoding RNA and Cancer: A New ParadigmCancer Res201777153965398128701486
- PrevidiMCCarotenutoPZitoDPandolfoRBraconiCNoncoding RNAs as novel biomarkers in pancreatic cancer: what do we know?Future Oncol201713544345327841659
- MüllerSRaulefsSBrunsPNext-generation sequencing reveals novel differentially regulated mRNAs, lncRNAs, miRNAs, sdRNAs and a piRNA in pancreatic cancerMol Cancer2015149425910082
- WangYLiZZhengSExpression profile of long non-coding RNAs in pancreatic cancer and their clinical significance as biomarkersOncotarget2015634356843569826447755
- MiaoZDingJChenBYangYChenYHOTAIR overexpression correlated with worse survival in patients with solid tumorsMinerva Med2016107639240027333150
- ChinVNagrialASjoquistKChemotherapy and radiotherapy for advanced pancreatic cancerCochrane Database Syst Rev20183CD01104429557103
- YangXZhangWChengSQYangRLHigh expression of lncRNA GACAT3 inhibits invasion and metastasis of non-small cell lung cancer to enhance the effect of radiotherapyEur Rev Med Pharmacol Sci20182251315132229565489
- WuDLiYZhangHHuXKnockdown of Lncrna PVT1 Enhances Radiosensitivity in Non-Small Cell Lung Cancer by Sponging Mir-195Cell Physiol Biochem20174262453246628848163
- ShenYLiuYSunTYangWLincRNA-p21 knockdown enhances radiosensitivity of hypoxic tumor cells by reducing autophagy through HIF-1/Akt/mTOR/P70S6K pathwayExp Cell Res2017358218819828689810
- XinYJiangFYangCRole of autophagy in regulating the radiosensi-tivity of tumor cellsJ Cancer Res Clin Oncol2017143112147215728786037
- LevyJMMTowersCGThorburnATargeting autophagy in cancerNat Rev Cancer201717952854228751651
- GuoJYXiaBWhiteEPromotion A-MediatedtumorAutophagy-mediated tumor promotionCell201315561216121924315093
- TamSYWuVWLawHKInfluence of autophagy on the efficacy of radiotherapyRadiat Oncol20171215728320471
- ApelAHerrISchwarzHRodemannHPMayerABlocked autophagy sensitizes resistant carcinoma cells to radiation therapyCancer Res20086851485149418316613
- XiongHNiZHeJLncRNA HULC triggers autophagy via stabilizing Sirt1 and attenuates the chemosensitivity of HCC cellsOncogene201736253528354028166203
- WangPZhangJZhangLMicroRNA 23b regulates autophagy associated with radioresistance of pancreatic cancer cellsGastroen-terology2013145511331143
