Abstract
Neoadjuvant therapy (NAT) has been used increasingly in patients with locally advanced or early-stage breast cancer. However, the accurate evaluation and prediction of response to NAT remain the great challenge. Biomarkers could prove useful to identify responders or nonresponders, or even to distinguish between early and delayed responses. These biomarkers could include markers from the tumor itself, such as versatile proteins, genes, and ribonucleic acids, various biological factors or peripheral blood cells, and clinical and pathological features. Possible predictive markers could also include multiple features from functional imaging, such as standard uptake values in positron emission tomography, apparent diffusion coefficient in magnetic resonance, or radiomics imaging biomarkers. In addition, cells that indirectly present the immune status of tumor cells and/or their host could also potentially be used as biomarkers, eg, tumor-infiltrating lymphocytes, tumor-associated macrophages, and myeloid-derived suppressor cells. Though numerous biomarkers have been widely investigated, only estrogen and/or progesterone receptors and human epidermal growth factor receptor have been proven to be reliable biomarkers to predict the response to NAT. They are the only biomarkers recommended in several international guidelines. The other aforementioned biomarkers warrant further validation studies. Some multigene profiling assays that are commercially available, eg, Oncotype DX and MammaPrint, should be used with caution when extrapolated to NAT settings. A panel of combined multilevel biomarkers might be able to predict the response to NAT more robustly than individual biomarkers. To establish such a panel and its prediction model, reliable methods and extensive clinical validation are warranted.
Introduction
Breast cancer accounts for ~30% of female malignant tumors both in USACitation1 and China.Citation2 Treatment of breast cancer includes locoregional resection, with or without radiotherapy as well as systemic therapies such as chemotherapy, endocrine therapy, biological targeting agents, and a combination of the above. The need for the selection of local and systemic therapies depends mainly on various clinical, pathological, and molecular features. Markers are served as surrogates of these features for establishing prognostics and predicting outcomes.Citation3 Prognostic factors may help select patients most likely to benefit from adjuvant therapy, while predictive factors can be valuable to predict the most efficacious therapy or measure response to therapy early in the course of treatment.Citation4,Citation5
Neoadjuvant therapy (NAT), which corresponds to the administration of systemic anticancer agents prior to local treatment, has been recommended as a general approach in locally advanced-stage diseases.Citation6 Though NAT of breast cancer has been shown to be effective with higher local recurrence after breast-conserving therapy, the distant recurrence, breast cancer mortality, and death by any cause in patients with NAT were not statistically different from those with adjuvant therapy.Citation7 The advantages of NAT for breast cancer include decreasing the tumor size, improving outcomes of radical or more conservative surgical interventions and early evaluation of clinical efficacy,Citation8 and serving as an excellent research platform to test new predictive biomarkers by tumor and/or blood sampling prior to and during systemic therapy.Citation6 However, tailored therapies based on clinical responses to standard NAT are still not well established because of the highly heterogeneous nature of breast cancer, which presents various subtypes at the molecular, histopathological, and clinical levels.Citation9 Robust predictive biomarkersCitation9,Citation10 and reliable measures of clinical benefit from biomarker-derived personalized therapy remain limited.Citation6 This work offers an overview of the literature related to biomarkers that may predict the response to NAT for breast cancer. These biomarkers provide clinical, biological, and imaging information.
Clinical characteristics
Clinical characteristics usually include clinical and demographic features, such as family history, menstrual status, patient’s age, mammographic breast density, and racial disparity. Family history that includes cases of early onset or bilateral breast cancers and multiple cases of breast or ovarian cancers can be a strong predictor of hereditary breast cancer.Citation11 Menstrual status has become a pivotal consideration while selecting optimal endocrinal treatment strategies.Citation6,Citation12,Citation13 Patient’s age is an important prognostic factor for patients positive for hormone receptors, but for patients positive for human epidermal growth factor receptor 2 (HER2+) or triple-negative breast cancer (TNBC), age is not an independent prognostic factor.Citation14 Moreover, mammographic breast density might be associated with response to NAT and a low mammographic density could predict improved pathological complete remission (pCR).Citation15 In addition, the response to NAT displays racial disparity, wherein HER2+ metastatic breast cancer presented worse clinical outcomes when comparing Caucasian with African-American patients.Citation16 Whether these clinical and demographic features can robustly predict the response to NAT warrants further investigation. This article does not contain any individual participants that required informed consent. This article does not contain any studies with human participants or animals performed by any of the authors.
Pathological features
Pathological features of breast cancer may include the histological tumor type and grade; tumor-node-metastasis (TNM) stage; tumor estrogen receptor (ER), progesterone receptor (PR), and HER2 status; Ki-67 index; mitotic counts; and necrosis. According to the status of ER, PR, HER2, and Ki-67, breast cancer was divided into four different molecular subtypes, such as luminal A, luminal B, HER2+, and TNBC.Citation6 Each subtype might have different treatment strategies, and the predictive factors may not be the same. For example, the best treatment regimen for breast cancer with luminal A subtype, which is clinically acceptable, is endocrine therapy alone without chemotherapy,Citation17 whereas for luminal B subtype, the optimal combination of chemotherapy and endocrine therapy is necessary. Recently, the PAMELA study showed that breast cancer patients with the HER2+ subtype are more likely to benefit from the dual HER2 blockade treatment.Citation18 TNBC itself represents a heterogeneous group with varying treatment sensitivities and prognoses. Various systemic therapies including those with cytotoxic and molecularly targeted agents improve the response rate of NAT for breast cancer.Citation19
Classical morphological parameters, such as lobular histotype and presence of inflammation, predict the response to NAT, particularly in luminal B and HER2+ subgroups.Citation20 The pattern of central necrosis and fibrosis has been found to affect the prognosis and prediction of breast cancer. In a pooled data analysis, the outcome in the presence of central necrosis and fibrosis was worse than those in their absence, regardless of tumor grade. In contrast, in the absence of central necrosis and fibrosis, patients with grade 3 breast cancer tumors had poorer outcome than those with grade 1–2 tumors.Citation21 Mitosis counting and Ki-67 index, which are proliferation markers of tumor cells, have proven effective, practical, easily assessable, inexpensive, and highly reproducible prognostic factors and predictors. Higher mitotic counts were associated with the presence of lymph node metastasis.Citation22 In an NAT cohort study, histological grade was not found to be significantly correlated with Ki-67, even though, in approximately two-thirds of the cases, Ki-67 decreased after NAT.Citation23 Some of these pathological features have proven to be predictive, eg, ER and HER2. However, their predictive response to NAT warrants further clinical validation.
Pathological complete response (pCR), which was defined as the absence of tumor cells in the surgical specimen, both at the primary tumor site and at regional lymph nodes,Citation24–Citation26 has been used as an endpoint in numerous trials of neoadjuvant systemic therapy for breast cancer, and molecular subtypes have been independently associated with pCR rate.Citation27 In the Collaborative Trials in Neoadjuvant Breast Cancer (CTneoBC) pooled analysis,Citation25 which assessed the relationship between pCR and long-term outcome, the absence of residual invasive cancer in the breast and axillary nodes with the presence or absence of in situ cancer provided a better association with improved outcomes than eradication of invasive tumor from the breast alone. Complete remission both in breast and lymph nodes, especially in patients with TNBC and in those with HER2-positive breast cancer, has been proposed as a surrogate endpoint for the prediction of long-term clinical benefit, such as disease-free survival, event-free survival (EFS), and overall survival (OS).Citation25,Citation28,Citation29 Some pathological features, such as smaller tumor size, absence of axillary lymph nodes,Citation4,Citation30 and histological grade 3,Citation31–Citation33 are more likely to achieve pCR.
Circulating biomarkers
Circulating markers include soluble cells, molecules, and exosomal nucleic acids.Citation34 They might be useful for diagnosis, prognosis, and real-time therapy monitoring with lower costs and higher compliance than tumor biopsy, due to their minimal invasiveness.Citation34 This section addresses circulating tumor cells (CTCs),Citation35 circulating endothelial cells (CECs),Citation36 and the circulating tumor DNA (ctDNA), which are rare populations in the peripheral circulation and potential biomarkers. Although myeloid-derived suppressor cells (MDSCs) and various lymphocyte subpopulations are also present in the peripheral blood, they are discussed in the “Immunological biomarkers” section.
CTCs
The presence of disseminated tumor cells in the bone marrow, as well as detection and persistence of CTC, enables the identification of patients with especially poor prognosis. In patients with metastatic breast cancer, the presence and enumeration of CTC in the peripheral blood are associated with reduced survival.Citation37–Citation39 Elevated CTC levels in the early course of treatment were found to be the early independent marker, predictive of poor survival,Citation40 while molecular profiling of CTC offers superior prognostic information for assessing the risk of recurrence and superior predictive judgment of therapeutic regimens.Citation41 In adjuvantCitation42–Citation44 or neoadjuvantCitation45,Citation46 settings, CTCs could also be prognostic factors, which suggests that they could potentially be used as a monitoring tool during follow-up and a selection criterion of patients for secondary treatment intervention. For inflammatory breast cancer treated with NAT, CTC detection at the baseline was associated with 3-year shorter disease-free survival and OS. This suggests that CTC could be used for prediction and stratification.Citation47 Recently, a meta-analysis with individual patient data from 21 studies showed that CTC was detectable in 25.2% of patients before NAT and the number of CTCs could negatively affect the OS, distant disease-free survival, and locoregional relapse-free interval, which suggest that the CTC count is an independent and quantitative prognostic factor for the treatment of breast cancer with NAT.Citation48 The challenges associated with CTC detection and characterization include its scarcity, difficult enrichment, and limited blood volume for analysis.Citation49 A reliable method for detecting CTCs warrants further improvement,Citation50 and their value for predicting response to NAT needs to be validated in clinical trials.
ctDNA
Compared with healthy people, cancer patients usually have higher levels of circulating cell-free DNA (cfDNA).Citation51 The ctDNA harbors DNA mutations, epigenetic alterations, and other forms of tumor-specific abnormalities.Citation52 It is released into the bloodstream both from tumor tissue and from lysed CTCs following apoptosis and/or necrosis of tumor cells.Citation51,Citation53 ctDNA can be detected in various solid malignancies, and its levels are associated with the disease stage.
Various genetic and epigenetic events including DNA strand integrity, gene amplifications, gene mutations, DNA methylation, and microsatellite abnormalities might occur during the initiation and progression of breast cancer. Studies on circulating DNA in plasma and serum of patients with breast cancer are well summarized.Citation54 These studies have suggested that analyses of circulating DNA might provide prognostic and predictive information for the diagnosis and treatment of breast cancer.Citation54 With the RNA sequencing, high levels of HER2 and low levels of ESR1 (ER 1) were shown to be associated with higher pCR for breast cancer.Citation55 However, the role of cfDNA or ctDNA in the prediction of the response to NAT has not been demonstrated. Analysis of cfDNA is performed using technologies such as quantitative polymerase chain reaction (qPCR); beads, emulsion, amplification, and magnetics (BEAMing); pyrophosphorolysis-activated polymerization (PAP); combined bisulfite restriction analysis (COBRA); and microarray and next-generation sequencing among others. Each of these techniques presents various advantages and limitations.Citation54
CECs
Endothelial cells can enter circulation as CECs when sloughing off vessel walls and as endothelial progenitor cells (EPCs) when mobilized from the bone marrow.Citation36 Both CECs and their populations, which can be enumerated by flow cytomery,Citation56–Citation58 could potentially serve as biomarkers for the prognosis and prediction of various solid tumors.Citation36 CECs and EPCs appear to change dynamically during the course of chemotherapy of patients with breast cancer. A decrease in CECs and an increase in EPCs could serve as surrogate markers of angiogenesis in antiangiogenesis treatments combined with chemotherapy.Citation59 For patients with metastatic breast cancer, orally administered S-1 could suppress CECs, as measured using the CellSearch system.Citation60 In adjuvant settings, CEC numbers could predict higher Nottingham prognostic index scores and correlated positively with tumor invasiveness and size, which might reflect total tumor vascular volume.Citation61 In neoadjuvant settings, 35 patients with operable breast cancer received NAT with a regimen based on anthracycline and/or taxane. The number of baseline CEC, in particular CD34+ CEC, and the percentage of CD34+ cells were associated with the rate of pCR of NAT, which suggests that CEC could potentially be used as a predictive biomarker for NAT.Citation62 However, the extent and the significance of the contribution of CECs and EPCs for breast cancer growth are still not well defined.Citation63
CTCs, CECs and their subsets, and ctDNA are “magic tools” of liquid biopsy, promising new biomarkers in oncology, with potential clinical applications for the monitoring and comprehensive molecular profiling of breast cancer.Citation64 Great challenges remain in the interpretation of the data and their optimal utilization in improving patient treatment and outcomes.Citation65
Biochemical markers
A variety of biochemical biomarkers, including proteins, enzymes, DNA, and RNA, could be used as predictive biomarkers for detecting breast cancer and monitoring patient’s treatments. Glycosylated proteins such as carcinoembryonic antigen (CEA), cancer antigen 15-3 (CA15-3), and cancer antigen 125 (CA125) are biochemical markers, whereas ER, PR, HER2, and Ki-67 are biomolecular markers.
CA15-3 and CEA, the biomarkers most frequently used in clinical practice, are serum-based glycosylated tumor markers that have been approved by the US Food and Drug Administration (FDA) for monitoring breast cancer.Citation66,Citation67 Several studies have shown that elevated levels of serum CA15-3 and CEA may be used for detection, prognostic estimation, and treatment response prediction in patients with breast cancer.Citation68–Citation70 Moazzezy et alCitation69 reported that the serum levels of CA15-3 and CEA were independent of the stage of the breast cancer. A study by Uehara et alCitation67 found significant differences in the expression level of CA15-3 across different stages of breast cancer.Citation67 Samy et alCitation71 showed that preoperative serum levels of CEA in breast cancer patients were significantly higher than those in the control group. A meta-analysis that included 13 case–control studies showed that both CA15-3 and CEA showed potential as biomarkers for breast cancer monitoring and were strongly associated with the clinical stage.Citation72 In addition, tumor marker CA125 and malignant tumor-specific growth factor were shown to be differentially expressed in breast cancer patients compared to the control subjects. However, in the breast cancer guideline from American Society of Clinical Oncology (ASCO),Citation73 CEA, CA15-3, CA27.29, lactate dehydrogenase, and others were not recommended for the screening, diagnosis, staging, or routine surveillance of breast cancer patients after primary therapy, mainly because of their limited sensitivity and specificity, even though they could be minimally complementary to other clinical information.Citation74
In addition, some emerging novel biomarkers such as noncoding RNA including microRNAs and long noncoding RNAs have been widely investigated for the diagnosis, prediction, and prognosis of breast cancer.Citation75,Citation76 Their detection, quantification, and molecular characterization have provided new avenues for studying the metastatic process and have provided new perspectives in terms of the early detection and prediction of breast cancer. A number of studiesCitation77–Citation82 have investigated the predictive and/or prognostic value of circulating RNA in patients with breast cancer, but these results were inconclusive partially because of the limited patient number in the published studies. A number of issues regarding the normalization and standardization of sample collection, as well as measuring methods, warrant further improvement. In addition, the predictive response value of these noncoding RNA needs to be validated in a wide clinical setting.
Multigene/multiprotein profiling
Multigene profiling assays have been developed that might improve the prediction of outcomes compared to standard clinical and pathological markers. Biomarker assays such as Oncotype DX, MammaPrint, EndoPredict, PAM50, and Breast Cancer Index (BCI) could prove useful for specific subgroups of breast cancer.Citation10 For example, if a patient has an ER/PR-positive HER2-negative breast cancer, 21-gene recurrence score (Oncotype DX; Genomic Health, Redwood City, CA, USA) can be used to guide decisions on adjuvant systemic chemotherapy.Citation10,Citation83 This multigene model showed 10-year recurrence rates among low-, intermediate-, and high-risk groups of 6.8, 14.3, and 30.5%, respectively.Citation83 It is valuable to predict the probability of late recurrence and the magnitude of chemotherapy benefit, which could spare unnecessary adjuvant chemotherapy.Citation84–Citation87
MammaPrint, developed by the Netherlands Cancer Institute group, considers 70 genes related to early risk of metastasis, including tumor invasion-, metastasis-, interstitial invasion-, and angiogenesis-related genes.Citation88,Citation89 According to gene expression levels, the good- and poor-prognosis signatures showed significantly different risks of 10-year distant metastasis (85 and 51%) and OS (95 and 55%).Citation89 Adjuvant chemotherapy could significantly reduce the rate of 10-year distant metastasis for breast cancer patients with poor-prognosis signature.Citation90 Recently, the prospective MINDACT studyCitation91 confirmed the clinical utility of MammaPrint. Patients with early-stage breast cancer who are at a high clinical risk and low genomic risk for recurrence, based on the 70-gene signature, might not benefit from adjuvant chemotherapy, and 46% of breast cancer patients with a high clinical risk could be spared from chemotherapy.Citation91 MammaPrint has been recommended to determine the prognosis and guide decision making for selecting patients who could benefit from adjuvant chemotherapy for invasive breast cancers that are lymph node negative or 1–3 lymph node positive.Citation92
The Prosigna test (previously known as PAM50; NanoString Technologies, Seattle, WA, USA)Citation93,Citation94 measures the expression of 50 genes at the mRNA level, the EndoPredict testCitation95 detects the expression levels of 11 genes (Sividon Diagnostics GmbH, Cologne, Germany), and the BCI (bioTheranostics, San Diego, CA, USA) test measures the expression of 11 genes.Citation96 In combination with clinical and pathological factors, Prosigna, EndoPredict, and BCI have been recommended for predicting the outcome and aid decision making concerning adjuvant therapy in ER/PR-positive HER2-negative patients with negative or 1–3 positive lymph nodes.Citation92
Although published guidelines give conflicting recommendations regarding breast cancer biomarkers,Citation92 various multigene panels could improve risk discrimination relative to clinicopathological factors and the prediction of clinical outcome. Furthermore, they could identify patients who might safely avoid adjuvant chemotherapy, thereby saving costs, and could promote the progress of personalized clinical decision making. It has been recommended that TNM classification of breast cancer, described in the “Imaging biomarkers” section, is complemented with some multigene panels, such as Oncotype DX.Citation33 However, the efficacy of multigene panels in predicting relapse rates in HR-positive breast cancer beyond 5 years remains to be investigated. Multigene panels were only validated in adjuvant settings; hence, they should be used with caution when extrapolating to a NAT context.
Immunological biomarkers
The immune system plays a pivotal role in maintaining tissue homeostasis by continuous immunosurveillance and the initiation of inflammatory reactions.Citation97 Cancer, as it transforms from normal tissues, induces innate immune responses to eliminate incipient tumor cells via immunoediting.Citation98,Citation99 Tumor microenvironment within tumor regions, such as non-transformed elements, include immune cells or molecules, blood vessels, fibroblast, mesenchymal cells, adipocyte, and extracellular matrix, among others.Citation100 Immune cells that contribute to tumor immunoediting include tumor-infiltrating lymphocytes (TILs), tumor-associated macrophages (TAMs), regulatory T cells (Tregs), type II natural killer T (NKT) cells, and MDSCs.Citation101 Strategies are rapidly emerging that target various immune cells and tumor microenvironment to improve cancer treatment.Citation102 Therefore, both tumor immunological status and microenvironment could potentially be used as biomarkers to predict response to anticancer therapy.
TILs, as immune cells that infiltrate tumor tissue, are present in various solid tumors including breast cancer.Citation103 TILs, classified as either stromal or intratumoral, are usually detected in hematoxylin and eosin-stained histological slides.Citation104 Many studies showed a positive association between higher TIL numbers and more favorable prognosis or better response to treatment.Citation105 The presence of TILs at the time of diagnosis provides prognostic and potentially predictive values, particularly in cases of triple-negative and HER2 breast cancer patients.Citation103–Citation107 In adjuvant settings, TILs might serve as predictive biomarkers for the benefit from cytotoxic chemotherapy,Citation108,Citation109 while, in the neoadjuvant context, the presence of TILs has been found to be associated with increased rates of pCR.Citation107 In a recent pooled analysis, the increased TIL concentration could predict a better response to NAT for all the molecular subtypes of breast cancer and a better survival for HER2+ and TNBC subgroups.Citation110 TILs might also play an important role in immunomodulatory effects of chemotherapy. For example, chemotherapy might relieve immunosuppression and reset the attenuated functional immunity that was triggered by chemotherapy-induced cell death, thus possibly contributing to the elimination of the remaining primary viable tumor cells and distant micrometastatic disease.Citation103 Nevertheless, a standardized methodology to measure TILs is needed to improve the consistency and reproducibility of future studies.Citation104 In summary, understanding and exploiting the immunobiology of breast cancer, especially the importance of TILs in a breast cancer immune microenvironment, remain a great challenge.Citation103
TAMs are prominent components of tumor microenvironment in breast cancer.Citation111,Citation112 Macrophages are usually polarized into classically activated (M1) and alternatively activated (M2) macrophages.Citation113 TAMs demonstrate high plasticity in response to various signals and participate in the immune responses that control the tumor microenvironment.Citation111,Citation112 They are versatile and have been involved in the regulation of tumor growth, angiogenesis, invasion, metastasis, immunosuppression, and chemotherapeutic resistance.Citation100,Citation111,Citation112 TAMs could also be used as important prognostic or predictive factors.Citation100 High density of TAMs as a predictor of patient outcome after chemotherapy for various tumors has been well summarized.Citation112 A recent meta-analysis of 16 studies with 4,541 patients showed that a high density of TAMs was related to worse OS and disease-free survival.Citation114 High infiltration of TAMs correlated significantly with many clinicopathological features, such as age, tumor size, histological grade, ER/PR status, and vascular invasion.Citation114 Because patient-to-patient variability in cancer progression complicates clinical treatment decisions, a multivariate predictive in vitro model, using monocyte-derived macrophages and related biomolecules, has been established, which allows the scoring of patients for invasion risk. This might prove useful to inform oncologists and patients of invasive/metastatic risk.Citation115 Nevertheless, this predictive model should be validated for application to current breast cancer patients with long-term clinical outcomes in the context of a more robust dataset. Hopefully, TAMs will provide tools to tailor the use of local and systemic therapies in a personalized medicine approach, including chemotherapy, hormonal therapy, anti-angiogenesis, and immunotherapy. TAM-focused therapeutic strategies might potentially complement and synergize with other cancer therapies.Citation112 However, the biomarker potential of TAMs and the approach to target macrophages in cancer warrant further investigation.
MDSCs affect the local tumor microenvironment by suppressing host immune responsesCitation116,Citation117 and by playing an important role in tumor formation and progression.Citation101 Available treatment strategies that target MDSCs include inhibition of MDSC development, expansion, and function, as well as differentiation, depletion, or destruction of MDSCs.Citation101 Several studies have indicated that the number of MDSCs in the peripheral blood of some cancer patients correlated with the clinical stage and metastatic tumor burden.Citation101 In a case cohort study, myeloid cells were found to be significantly expanded in the tumor microenvironment of breast cancer patients.Citation118 Circulating MDSCs have been found to be significantly higher in breast cancer patients compared to healthy volunteers, with the number of MDSCs correlating significantly with the clinical stage.Citation119 In the setting of neo-adjuvant chemotherapy for breast cancer, peripheral blood levels of granulocytic MDSCs increased significantly in all molecular subtypes during doxorubicin and cyclophosphamide therapy. Granulocytic MDSC levels were quantitatively lower in patients with pCR than in patients with no pCR.Citation120 At present, the major problem in using MDSCs as a prognostic or predictive biomarker and their utilization in cancer research lie in the difficulty of reliably defining MDSCs themselves.
Lymphocyte subpopulations participate in distinct components of the immune response in various conditions, including solid tumors and leukemia.Citation121 The total number of lymphocytes and the composition of lymphocyte subpopulations can be measured by flow cytometry.Citation122,Citation123 These lymphocyte subpopulations could be potentially used as predictive and prognostic biomarkers for many tumors, including breast cancer. The absolute number of regulatory T cells, characterized by the expression of CD4+CD25+CD127–, has been found to be higher in breast cancer patients compared to healthy controls, with numbers higher in patients with stage III or IV breast cancer than in those with stage I or II.Citation124 The density of regulatory T cells that infiltrate intratumor before chemotherapy has been described as the strongest predictor for survival.Citation125 For breast cancer, different lymphocyte sub-populations might respond differently to radiotherapy with or without chemotherapy. For example, the numbers of CD4(+) T cells and regulatory T cells increased during the treatment course of radiotherapy, while those of NK cells and B cells decreased. All numbers returned to normal within 6 months after radiation treatment.Citation126 Elevated numbers of circulating CD8+CD28– suppressor T cells have been associated with shorter progression-free survival and OS for metastatic breast cancer.Citation127,Citation128 Depressed circulating anti-HER2 CD4-positive (CD4+) T-helper type 1 (Th1) response correlated with higher risk of recurrence in patients with completely treated HER2-positive invasive breast cancerCitation129 and has been related to pathological response following NAT in HER2-positive breast cancer,Citation130 which suggested that benefit from trastuzumab therapy may be restricted to immune-enriched tumors. Though several studies suggest that lymphocyte subpopulations in tumor or peripheral blood could potentially be used as bio-markers for breast cancer prediction and prognosis, it should be validated in prospective studies recruiting higher numbers of patients. Additionally, standardization of the measurement of each lymphocytic subpopulation should be improved.
Imaging biomarkers
Various breast imaging examinations are assessed prior to NAT to evaluate the extent of disease and axillary lymphadenopathy, as well as to screen the contralateral breast. Typically, patients undergo physical examination and breast imaging, such as mammography and/or ultrasonography (US), that have been proposed to evaluate response to NAT,Citation131 but they were only moderately useful for predicting residual pathological tumor size after NAT.Citation132 Physical examination, which has an accuracy of 57%, might overestimate the amount of residual disease due to the presence of firm fibroglandular tissue and post-therapy fibrosis.Citation133 The diagnostic accuracy of mammography and US for determining the pCR rate of NAT was measured to be 74 and 79%, respectively.Citation134 Breast ultrasound has been more accurate than mammography in predicting residual tumor size.Citation135 When both imaging modalities demonstrated no residual disease, the likelihood of pCR was found to be 80%.Citation135 The advantages and disadvantages of mammography and US have been well reviewed.Citation133,Citation135–Citation137 In this section, the potential of molecular imaging such as positron emission tomography (PET)–computed tomography (CT), magnetic resonance (MR), and radiomics as biomarkers for breast cancer is discussed.
PET–CT imaging biomarker
Fluorine 18 fluorodeoxyglucose (F18-FDG) is the most commonly used molecular imaging tracer for imaging tumor metabolism by PET–CT. Imaging with PET–CT can be used, optionally, to determine the systemic stage of breast for cancer patients with stage III or IV of the disease or with disease failure after antitumor therapy.Citation6 Several studies have been performed to evaluate the potential of F18-FDG PET–CT imaging to predict breast cancer pCR after NAT. In a cohort study with locally advanced breast cancer that received NAT, the standardized uptake value (SUV) in responding tumors F18-FDG decreased significantly just after the first course of chemotherapy. The SUV of all responders decreased <55% of their baseline, with a sensitivity of 100% and a specificity of 85%.Citation138 Histopathological response could be predicted with an accuracy of 88–91% after the first or second cycle of NAT.Citation138 Early changes in SUV after a course of NAT was a good independent biomarker to predict pathological response.Citation139 Similarly, for patients with metastatic breast cancer, longitudinal PET might allow the prediction of response to treatment after the first cycle of chemotherapy.Citation140 Three meta-analyses studying F18-FDG PET–CT for the evaluation of response to NAT showed a pooled sensitivity and specificity of 80–85 and 66–79%, respectively.Citation133 Based on the available literature, F18-FDG PET–CT imaging could potentially be used as an early selection marker of chemosensitive patients. It could help identify ineffective chemotherapy and direct patients to either alternative therapy or surgery.Citation133 The use of new tracers for PET–CT, such as fluorothymidine,Citation141 radiolabeled essential amino acid,Citation11,Citation142 C-choline,Citation143 and fluciclovine,Citation144 has been investigated in a number of case-cohort studies to predict the response of breast cancer patients to NAT. Prospective, response-guided clinical trials are needed to validate these possible advantages.
MR imaging biomarker
MR, a powerful, versatile, and precise imaging technique, is the most sensitive modality for breast cancer detectionCitation145,Citation146 and the most accurate imaging technique for assessing tumor response to NAT.Citation147–Citation150
Dynamic contrast-enhanced (DCE) MR, the backbone of the breast MR imaging protocol, has an excellent sensitivity and a good specificity for breast cancer detection.Citation145,Citation151 Pharmacokinetic analysis of DCE perfusion MR imaging has demonstrated that the volume transfer constant (Ktrans), which describes the trans-endothelial transport of imaging contrast agent from blood vessels into the extravascular–extracellular space by diffusion,Citation131 is a promising parameter for the early identification of treatment response.Citation152,Citation153 Ktrans changes during the course of NAT and may predict pathological response and long-term survival.Citation154,Citation155 Due to quantification and reproducibility, it has been used widely in the clinical practice for breast cancer and standardized techniques and methodological analyses of DCE MR imaging quantification have been recommended.Citation131,Citation156
Diffusion-weighted (DW) MR imaging can be used to complement DCE MR imaging to evaluate tumor response to NAT. Compared with normal tissue, most invasive breast cancers have lower apparent diffusion coefficient (ADC) values.Citation157 Anticancer agents usually result in cell membrane damage and reduce the number of viable malignant cells. Therefore, ADC values tend to increase more significantly in pathological responders than in nonresponders after chemotherapy.Citation158,Citation159 Similar to Ktrans, ADC value may change after only one cycle of chemotherapy and before the tumor size is affected. A meta-analysis,Citation160 with 34 studies covering 1,932 breast cancer patients, reported the sensitivity and specificity of DW MR to predict the pCR of 0.93 and 0.82, respectively. In a recent meta-analysis of 57 published studies, >80% of studies used the DCE MR technique and most studies used a 1.5 T magnet strength to evaluate pCR of the patients who received NAT.Citation150 The pCR rate ranged from 4.1 to 54.9% with a median of 16.8%. Sensitivity and specificity (correct detection of residual tumor) of DCE MR were reported to be 0.64 and 0.92, respectively, while those of DW MR were 0.93 and 0.85, respectively. The accuracy rate, calculated from Youden’s index, of DCE MR and DW MR was similar.Citation150
DCE MR had greater accuracy than US and mammography and PET–CT or DW MR had high sensitivity, and DCE MR had high specificity.Citation150 Proton MR spectroscopy allows the evaluation of total choline (tCho) peak as a biomarker of breast cancer.Citation161 The resonance peak of tCho might decrease or disappear completely in locally advanced breast cancer patients undergoing chemotherapy.Citation162 In breast cancer patients undergoing NAT, tCho decreasedCitation163 more significantly within 24 hours of initial treatment in responders than in nonresponders. Levels of tChoCitation164 reduced even more after 1–2 cycles of chemotherapy. In addition, diffusion tensor imaging (DTI), an extension of standard DW with diffusion encoding, can measure the full diffusion tensor and characterize the motion of water.Citation165 In a case cohort, DTI was used to monitor the changes in volume and diffusion tensor parameters were proven to predict response to NAT as accurately as DCE.Citation165,Citation166
Most of the MR imaging studiesCitation149,Citation150,Citation165–Citation167 were performed on relatively small cohorts, which might limit the statistical power and warrant further validation and refinement in large prospective research projects. In addition, color ultrasonic studies might provide more valuable information when combined with MR functional imaging such as DW, DCE, spectroscopy MR, and some other multimodal imaging, for example, PET–CT with various tracers. Both the molecular subtype of breast cancer and the antitumor regimen might influence the accuracy of breast MR detection of residual lesions.Citation147 In addition, both DW MR and DTI MR are research tools with major limitations, such as relatively long acquisition times; frequent low quality spectra; and difficulty in standardization, quantification of tCho tissue concentration, and fat suppression.Citation133,Citation163–Citation165
Radiomics biomarker
Radiomics is a bridge between medical imaging and personalized medicine via high-throughput data mining. It enables image features to be quantified and used for cancer diagnosis and treatment, which could improve diagnostics, prognostics, and predictive accuracies.Citation168 With quantitative computer-aided radiomic techniques, the tumor and its surrounding parenchyma could present thousands of imaging features including size, shape, margin morphology, enhancement texture, and kinetic assessment. These features could help identify the molecular subtypes of breast cancer.Citation169 Radiomics features provide a novel imaging biomarker for estimating intratumoral heterogeneity.Citation170 Intratumoral texture features have the potential to serve as a valuable imaging biomarker to enhance the prediction of breast cancer prognosis.Citation171 Metabolic radiomic patterns in breast cancer have been associated with the propensity of pCR after NAT and risk of recurrence,Citation172 which suggests that they could serve as biomarkers with predictive and prognostic abilities for the personalized management of breast cancer. For patients with invasive breast cancer, the radiomics signature on MR has proved to be an independent biomarker for the estimation of disease-free survival.Citation173
Challenges and future directions
Response evaluation criteria of NAT and clinical validation
For the majority of solid cancers, changes in tumor burden are usually evaluated using validated and consistent criteria, such as response evaluation criteria in solid tumors (RECIST),Citation174 immune-related response RECIST,Citation175 and PET response criteria in solid tumors (PERCIST).Citation176 The objective of NAT might be slightly different from the adjuvant or palliative contexts. Therefore, these response evaluation criteria should be used with caution when extrapolating to neoadjuvant settings. Although the use of various proteins and molecules such as miRNAs and exosomes as novel biomarkers has opened new opportunities for cancer diagnosis and monitoring, we are now facing several challenges including documentation of test reproducibility in multiple clinical trials, standardization of data acquisition, data analysis, reporting of results, and demonstration of strong correlations between test and clinical outcomes. In addition, these biomarkers need to be widely validated in well-designed, biomarker-guided, prospective trials before their final clinical application. In the era of precision medicine and big data, a closer integration of breast biology, immunology, imaging, and related biomedical fields is necessary. In addition, the creation of large integrated and shareable databases of clinical, molecular, and imaging biomarkers could promote advancement in the field to continue guiding breast cancer care and research.Citation177
Combined multimodality biomarker approaches
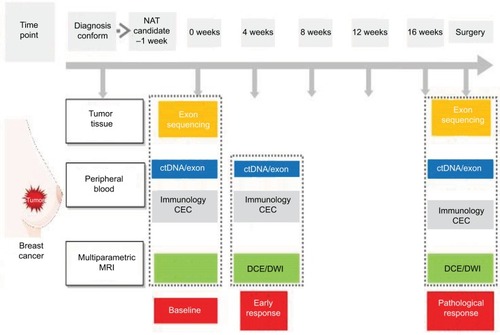
Combining quantitative imaging features with multigene assays could provide a promising mean for image-based phenotyping to assess the risk of breast cancer recurrence.Citation178 At present, >1,300 trials have been registered to investigate potential biomarkers for breast cancer. We have also initiated a clinical trial (clinicaltrials.gov identifier: NCT03242551) to investigate the potential value of combining biomarkers from breast tumor, peripheral blood, and imaging (). Merging these clinical characteristics, imaging phenotypes, and genomic data may lead to improved clinical outcome predictors. Reliable, quantitative prognostic or predictive models with multiple parameters of breast cancer could prove useful for precision medicine and for deciding on a patient treatment strategy. Radiogenomics, which integrates imaging characteristics with gene expression patterns, gene mutations, and other genome-related characteristics, will facilitate a deeper understanding of tumor biology and will enable the capturing of intrinsic tumor heterogeneity.Citation9.
Figure 1 The schematic representation of a clinical trial merging biomarkers from tumor, blood, and imaging.
Note: This clinical trial (NCT03242551), ie, BINC-B, aims to use a combination of multiple biomarkers to improve their ability to predict the response to neoadjuvant chemotherapy of patients with early-stage breast cancer.
Abbreviations: BINC-B, Biomarkers Investigating Neoadjuvant Chemotherapy for Breast cancer; CEC, circulating endothelial cell; ctDNA, circulating tumor DNA; DCE, dynamic contrast enhanced; DWI, diffusion-weighted imaging; MRI, magnetic resonance imaging; NAT, neoadjuvant therapy.
Predictive biomarkers for the molecular target therapy or immunotherapy
Both molecular target agents and immune checkpoint inhibitors are emerging as an important solution for recurrent or metastatic breast cancer, and some of these agents are increasingly used in the adjuvant or neoadjuvant setting. Most of the novel agents target one or more molecular pathways that affect the tumor growth and/or its microenvironments. For the early-stage breast cancer, some patients might be resistant to the aromatase inhibitor such as anastrozole and the cyclin-dependent kinase (CDK) 4/6 inhibitors, eg, palbociclib, which could be an active antiproliferative agent.Citation180 The combination of an mTOR pathway inhibitor such as everolimus with chemotherapy could be used in the NAT of TNBC.Citation181 The combination of immunotherapy agent and HER2 inhibitors is promising in advanced breast cancer patients, but the efficacy of immune agents in NAT or in an adjuvant setting has not been shown.Citation182 Though these new treatment agents are increasingly available and widely used, the response and survival benefit as well as the reliable biomarkers that might stratify response to NAT need to be further validated.
Conclusion
Despite massive efforts toward the development of novel biomarkers for breast cancer, only three, ie, ER, PR, and HER2, biomarkers are recommended as mandatory in international guidelines.Citation6,Citation10,Citation12,Citation92 Reliable biomarkers currently used in clinical practice include ER and PR for predicting the benefit from endocrine therapy and HER2 for predicting the benefit from anti-HER2 therapy. A few clinical features, such as age and menstrual status, are useful to make clinical decisions and could predict the risk of failure after anticancer therapy and guide the selection of treatment strategies. Some multi-gene assays, for example, Oncotype DX, MammaPrint, and Prosigna, could be recommended for specific subgroups of early-stage breast cancer patients. A few imaging biomarkers, such as SUV in PET–CT and Ktrans and ADC in MR, as well as their changes during the course of NAT, could be used to identify patients not benefiting from the treatment and to direct them to continue or change the delivery of NAT. Although biochemical biomarkers from peripheral blood and/or tumor tissue appear promising, there is still a lack of consensus in practice guidelines to guide the NAT of breast cancer. In addition, the amount of circulating cancer biomarkers and their profiles are growing almost exponentially, which might prove useful in discriminating among different molecular cancer subtypes. A reliable technical and clinical quality of biomarker assays needs to be established. Standardized reporting, interpretation of results, and assessment of interlaboratory variation should be carried out.Citation183 The clinical utility of biomarker-derived NAT for breast cancer still requires further evidence in prospective multicenter clinical trials, particularly in the context of big sharable databases.
Acknowledgments
We would like to acknowledge Editage Inc. for careful English proofreading. This work was funded by the Sanming Project of Medicine in Shenzhen (nos SZSM201612023 and SYLY201724) and Shenzhen Clinical research funding (2018A01008).
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2017CA: A Cancer Journal for Clinicians201767173028055103
- ChenWZhengRBaadePDCancer statistics in China, 2015CA: A Cancer Journal for Clinicians201666211513226808342
- PeartOBreast intervention and breast cancer treatment optionsRadiol Technol2015865quiz 559–562535M555825995404
- CianfroccaMGoldsteinLJPrognostic and Predictive Factors in Early-Stage Breast CancerOncologist20049660661615561805
- SawyersCLThe cancer biomarker problemNature2008452718754855218385728
- KaufmannMvon MinckwitzGMamounasEPRecommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancerAnnals of Surgical Oncology20121951508151622193884
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG)Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trialsLancet Oncol2018191273929242041
- RubovszkyGHorváthZRecent Advances in the Neoadjuvant Treatment of Breast CancerJ Breast Cancer201720211913128690648
- KaufmannMPusztaiLUse of standard markers and incorporation of molecular markers into breast cancer therapy: Consensus recommendations from an International Expert PanelCancer201111781575158221472705
- HarrisLNIsmailaNMcshaneLMUse of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women With Early-Stage Invasive Breast Cancer: American Society of Clinical Oncology Clinical Practice GuidelineJ Clin Oncol201634101134115026858339
- BrandtALorenzo BermejoJSundquistJHemminkiKBreast cancer risk in women who fulfill high-risk criteria: at what age should surveillance start?Breast Cancer Res Treat2010121113314119641988
- CoatesASWinerEPGoldhirschATailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015Ann Oncol20152681533154625939896
- CuriglianoGBursteinHJP WinerEDe-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017Ann Oncol20172881700171228838210
- LoiblSJackischCLedererBOutcome after neoadjuvant chemotherapy in young breast cancer patients: a pooled analysis of individual patient data from eight prospectively randomized controlled trialsBreast Cancer Res Treat2015152237738726109347
- ElsamanySAlzahraniAAbozeedWNMammographic breast density: Predictive value for pathological response to neoadjuvant chemotherapy in breast cancer patientsBreast201524557658126071795
- RugoHSBrufskyAMUlcickas YoodMRacial disparities in treatment patterns and clinical outcomes in patients with HER2-positive metastatic breast cancerBreast Cancer Res Treat2013141346147024062208
- GaoJJSwainSMLuminal A Breast Cancer and Molecular Assays: A ReviewOncologist201823555656529472313
- Llombart-CussacACortésJParéLHER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): an open-label, single-group, multicentre, phase 2 trialLancet Oncol201718454555428238593
- LoiblSO’ShaughnessyJUntchMAddition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trialLancet Oncol201819449750929501363
- BalmativolaDMarchiòCMauleMPathological non-response to chemotherapy in a neoadjuvant setting of breast cancer: an inter-institutional studyBreast Cancer Res Treat2014148351152325395316
- MaioranoEReganMMVialeGPrognostic and predictive impact of central necrosis and fibrosis in early breast cancer: Results from two International Breast Cancer Study Group randomized trials of chemoendocrine adjuvant therapyBreast Cancer Res Treat2010121121121819280340
- BuhmeidaAAl-MaghrabiJMerdadAPrognostic value of mitotic counts in breast cancer of Saudi Arabian patientsAnticancer Res20113119710321273586
- PetricMMartinezSAcevedoFCorrelation between Ki67 and Histological Grade in Breast Cancer Patients Treated with Preoperative ChemotherapyAsian Pacific Journal of Cancer Prevention201515231027710280
- MacchiaGGambacortaMAMasciocchiCTime to surgery and pathologic complete response after neoadjuvant chemoradiation in rectal cancer: A population study on 2094 patientsClin Transl Radiat Oncol2017481429594202
- CortazarPZhangLUntchMPathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysisLancet2014384993816417224529560
- CortazarPGeyerCEPathological Complete Response in Neoadjuvant Treatment of Breast CancerAnn Surg Oncol20152251441144625727556
- HoussamiNMacaskillPvon MinckwitzGMarinovichMLMamounasEMeta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapyEur J Cancer201248183342335422766518
- von MinckwitzGUntchMBlohmerJUDefinition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypesJ Clin Oncol201230151796180422508812
- MazouniCPeintingerFWan-KauSResidual Ductal Carcinoma In Situ in Patients With Complete Eradication of Invasive Breast Cancer After Neoadjuvant Chemotherapy Does Not Adversely Affect Patient OutcomeJournal of Clinical Oncology200725192650265517602071
- FungFCornacchiSDVanniyasingamTPredictors of 5-year local, regional, and distant recurrent events in a population-based cohort of breast cancer patientsAm J Surg2017213241842527424042
- RakhaEAEl-SayedMEMenonSGreenARLeeAHSEllisIOHistologic grading is an independent prognostic factor in invasive lobular carcinoma of the breastBreast Cancer Res Treat2008111112112717929165
- Alvarado-CabreroIAlderete-VázquezGQuintal-RamírezMPatiñoMRuízEIncidence of pathologic complete response in women treated with preoperative chemotherapy for locally advanced breast cancer: correlation of histology, hormone receptor status, Her2/Neu, and gross pathologic findingsAnn Diagn Pathol200913315115719433292
- GiulianoAEConnollyJLEdgeSBBreast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manualCA: A Cancer Journal for Clinicians201767429030328294295
- RavelliAReubenJMLanzaFBreast cancer circulating biomarkers: advantages, drawbacks, and new insightsTumor Biology20153696653666526307395
- BielčikováZJakabováAPinkasMZemanováMKološtováKBobekVCirculating tumor cells: what we know, what do we want to know about them and are they ready to be used in clinics?Am J Transl Res2017962807282328670371
- ZhouFZhouYDongJTanWCirculating endothelial cells and their subsets: novel biomarkers for cancerBiomark Med2017118665676
- CristofanilliMBuddGTEllisMJCirculating Tumor Cells, Disease Progression, and Survival in Metastatic Breast CancerN Engl J Med Overseas Ed20043518781791
- BidardFCVincent-SalomonASigal-ZafraniBPrognosis of women with stage IV breast cancer depends on detection of circulating tumor cells rather than disseminated tumor cellsAnn Oncol200819349650018187488
- BuddGTCristofanilliMEllisMJCirculating tumor cells versus imaging--predicting overall survival in metastatic breast cancerClin Cancer Res200612216403640917085652
- PiergaJYHajageDBachelotTHigh independent prognostic and predictive value of circulating tumor cells compared with serum tumor markers in a large prospective trial in first-line chemotherapy for metastatic breast cancer patientsAnn Oncol201223361862421642515
- TewesMAktasBWeltAMolecular profiling and predictive value of circulating tumor cells in patients with metastatic breast cancer: an option for monitoring response to breast cancer related therapiesBreast Cancer Res Treat2009115358159018679793
- FalckA-KBendahlP-OIngvarCAnalysis of and prognostic information from disseminated tumour cells in bone marrow in primary breast cancer: a prospective observational studyBMC Cancer201212140322963449
- DomschkeCDielIJEnglertSPrognostic Value of Disseminated Tumor Cells in the Bone Marrow of Patients with Operable Primary Breast Cancer: A Long-term Follow-up StudyAnn Surg Oncol20132061865187123263703
- HartkopfADWallwienerMFehmTNDisseminated tumor cells from the bone marrow of patients with nonmetastatic primary breast cancer are predictive of locoregional relapseAnn Oncol20152661155116025791636
- HallCKrishnamurthySLodhiADisseminated tumor cells predict survival after neoadjuvant therapy in primary breast cancerCancer2012118234234821717428
- MathiesenRRBorgenERenolenAPersistence of disseminated tumor cells after neoadjuvant treatment for locally advanced breast cancer predicts poor survivalBreast Cancer Research2012144R11722889108
- PiergaJYBidardFCAutretACirculating tumour cells and pathological complete response: independent prognostic factors in inflammatory breast cancer in a pooled analysis of two multicentre phase II trials (BEVERLY-1 and -2) of neoadjuvant chemotherapy combined with bevacizumabAnna Oncol2017281103109
- BidardFCMichielsSRiethdorfSCirculating Tumor Cells in Breast Cancer Patients Treated by Neoadjuvant Chemotherapy: A Meta-analysisJ Natl Cancer Inst2018110656056729659933
- IgnatiadisMDawsonSJCirculating tumor cells and circulating tumor DNA for precision medicine: dream or reality?Ann Oncol201425122304231325336116
- JueckstockJRackBFriedlTWPDetection of circulating tumor cells using manually performed immunocytochemistry (MICC) does not correlate with outcome in patients with early breast cancer – Results of the German SUCCESS-A- trialBMC Cancer201616140127387743
- GormallyECabouxEVineisPHainautPCirculating free DNA in plasma or serum as biomarker of carcinogenesis: Practical aspects and biological significanceMutation Research/Reviews in Mutation Research20076352–3105117
- CaiCGuoZCaoYZhangWChenYA dual biomarker detection platform for quantitating circulating tumor DNA (ctDNA)Nanotheranostics201821122029291160
- JahrSHentzeHEnglischSDNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cellsCancer Res20016141659166511245480
- SchwarzenbachHPantelKCirculating DNA as biomarker in breast cancerBreast Cancer Research201517113626453190
- FumagalliDVenetDIgnatiadisMRNA Sequencing to Predict Response to Neoadjuvant Anti-HER2 Therapy: A Secondary Analysis of the NeoALTTO Randomized Clinical TrialJAMA Oncol201732227234
- JacquesNVimondNConfortiRQuantification of circulating mature endothelial cells using a whole blood four-color flow cytometric assayJ Immunol Methods2008337213214318662694
- WoywodtABlannADKirschTIsolation and enumeration of circulating endothelial cells by immunomagnetic isolation: proposal of a definition and a consensus protocolJ Thromb Haemost20064367167716460450
- ZhouFZhouYYangMWenJDongJTanWOptimized multipara-metric flow cytometric analysis of circulating endothelial cells and their subpopulations in peripheral blood of patients with solid tumors: a technical analysisCancer Management and Research20181044746429563835
- KuoY-HLinC-HShauW-YDynamics of circulating endothelial cells and endothelial progenitor cells in breast cancer patients receiving cytotoxic chemotherapyBMC Cancer201212162023268621
- TsujiWIshiguroHTanakaSTakeuchiMUenoTToiMOrally administered S-1 suppresses circulating endothelial cell counts in metastatic breast cancer patientsInt J Clin Oncol201419345245923739924
- GoonPKLipGYStonelakePSBlannADCirculating endothelial cells and circulating progenitor cells in breast cancer: relationship to endothelial damage/dysfunction/apoptosis, clinicopathologic factors, and the Nottingham Prognostic IndexNeoplasia200911877177919649207
- AliAMUenoTTanakaSDetermining circulating endothelial cells using CellSearch system during preoperative systemic chemotherapy in breast cancer patientsEur J Cancer201147152265227221737256
- BotelhoMCAlvesHEndothelial Progenitor Cells in Breast CancerInt J Immunother Cancer Res201621226878074
- GingrasISalgadoRIgnatiadisMLiquid biopsy: will it be the ‘magic tool’ for monitoring response of solid tumors to anticancer therapies?Curr Opin Oncol201527656056726335664
- LowesLBratmanSDittamoreRCirculating Tumor Cells (CTC) and Cell-Free DNA (cfDNA) Workshop 2016: Scientific Opportunities and Logistics for Cancer Clinical Trial IncorporationInt J Mol Sci20161791505
- O’HanlonDMKerinMJKentPMaherDGrimesHGivenHFAn evaluation of preoperative CA 15-3 measurement in primary breast carcinomaBr J Cancer1995716128812917779725
- UeharaMKinoshitaTHojoTAkashi-TanakaSIwamotoEFukutomiTLong-term prognostic study of carcinoembryonic antigen (CEA) and carbohydrate antigen 15-3 (CA 15-3) in breast cancerInt J Clin Oncol200813544745118946756
- ParkBWOhJWKimJHPreoperative CA 15-3 and CEA serum levels as predictor for breast cancer outcomesAnn Oncol200819467568118037623
- MoazzezyNFarahanyTZOloomiMBouzariSRelationship between preoperative serum CA 15-3 and CEA levels and clinicopathological parameters in breast cancerAsian Pac J Cancer Prev20141541685168824641390
- AtoumMNimerNAbdeldayemSNasrHRelationships among serum CA15-3 tumor marker, TNM staging, and estrogen and progesterone receptor expression in benign and malignant breast lesionsAsian Pac J Cancer Prev201213385786022631661
- SamyNRagabHMEl MaksoudNAShaalanMPrognostic significance of serum Her2/neu, BCL2, CA15-3 and CEA in breast cancer patients: A short follow-upCancer Biomarkers201062637220571232
- FuYLiHAssessing Clinical Significance of Serum CA15-3 and Carcinoembryonic Antigen (CEA) Levels in Breast Cancer Patients: A Meta-AnalysisMed Sci Monit2016223154316227596019
- HarrisLFritscheHMennelRAmerican Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancerJ Clin Oncol200725335287531217954709
- HarrisLFritscheHMennelRAmerican Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancerJ Clin Oncol200725335287531217954709
- NassarFJNasrRTalhoukRMicroRNAs as biomarkers for early breast cancer diagnosis, prognosis and therapy predictionPharmacol Ther2017172344927916656
- AmorimMSaltaSHenriqueRJerónimoCDecoding the usefulness of non-coding RNAs as breast cancer markersJ Transl Med201614126527629831
- IorioMVCasaliniPTagliabueEMénardSCroceCMMicroRNA profiling as a tool to understand prognosis, therapy response and resistance in breast cancerEur J Cancer200844182753275919022662
- MarkouAYousefGMStathopoulosEGeorgouliasVLianidouEPrognostic Significance of Metastasis-Related MicroRNAs in Early Breast Cancer Patients with a Long Follow-upClin Chem201460119720524132943
- D’AiutoFCallariMDugoMmiR-30e* is an independent subtype-specific prognostic marker in breast cancerBr J Cancer2015113229029826057454
- BaileySTWesterlingTBrownMLoss of Estrogen-Regulated microRNA Expression Increases HER2 Signaling and Is Prognostic of Poor Outcome in Luminal Breast CancerCancer Res201575243644525388283
- GodinhoMFESieuwertsAMLookMPRelevance of BCAR4 in tamoxifen resistance and tumour aggressiveness of human breast cancerBr J Cancer201010381284129120859285
- ChenY-MLiuYWeiH-YLvK-ZFuPLinc-ROR induces epithelial-mesenchymal transition and contributes to drug resistance and invasion of breast cancer cellsTumor Biology2016378108611087026883251
- PaikSShakSTangGA Multigene Assay to Predict Recurrence of Tamoxifen-Treated, Node-Negative Breast CancerN Engl J Med Overseas Ed20043512728172826
- PaikSTangGShakSGene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancerJ Clin Oncol200624233726373416720680
- SparanoJAGrayRJMakowerDFProspective Validation of a 21-Gene Expression Assay in Breast CancerN Engl J Med Overseas Ed20153732120052014
- AlbainKSBarlowWEShakSPrognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trialLancet Oncol2010111556520005174
- GluzONitzUAChristgenMWest German Study Group Phase III PlanB Trial: First Prospective Outcome Data for the 21-Gene Recurrence Score Assay and Concordance of Prognostic Markers by Central and Local Pathology AssessmentJ Clin Oncol201634202341234926926676
- van ‘t VeerLJDaiHvan de VijverMJGene expression profiling predicts clinical outcome of breast cancerNature2002415687153053611823860
- van de VijverMJHeYDvan ‘t VeerLJA Gene-Expression Signature as a Predictor of Survival in Breast CancerN Engl J Med Overseas Ed20023472519992009
- EstevaFJSahinAACristofanilliMPrognostic Role of a Multigene Reverse Transcriptase-PCR Assay in Patients with Node-Negative Breast Cancer Not Receiving Adjuvant Systemic TherapyClinical Cancer Research20051193315331915867229
- CardosoFvan’t VeerLJBogaertsJ70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast CancerN Engl J Med Overseas Ed20163758717729
- DuffyMJHarbeckNNapMClinical use of biomarkers in breast cancer: Updated guidelines from the European Group on Tumor Markers (EGTM)Eur J Cancer20177528429828259011
- GnantMFilipitsMGreilRPredicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy aloneAnn Oncol201425233934524347518
- ParkerJSMullinsMCheangMCSupervised risk predictor of breast cancer based on intrinsic subtypesJ Clin Oncol20092781160116719204204
- FilipitsMRudasMJakeszRA New Molecular Predictor of Distant Recurrence in ER-Positive, HER2-Negative Breast Cancer Adds Independent Information to Conventional Clinical Risk FactorsClinical Cancer Research201117186012602021807638
- ZhangYSchnabelCASchroederBEBreast Cancer Index Identifies Early-Stage Estrogen Receptor-Positive Breast Cancer Patients at Risk for Early- and Late-Distant RecurrenceClinical Cancer Research201319154196420523757354
- DemariaSPikarskyEKarinMCancer and inflammation: promise for biologic therapyJ Immunother201033433535120386472
- MohmeMRiethdorfSPantelKCirculating and disseminated tumour cells — mechanisms of immune surveillance and escapeNat Rev Clin Oncol201714315516727644321
- MittalDGubinMMSchreiberRDSmythMJNew insights into cancer immunoediting and its three component phases—elimination, equilibrium and escapeCurr Opin Immunol201427162524531241
- ChoiJGyamfiJJangHKooJSThe role of tumor-associated macrophage in breast cancer biologyHistol Histopathol201711916
- AnaniWShurinMRTargeting Myeloid-Derived Suppressor Cells in CancerAdv Exp Med Biol2017103610512829275468
- WargoJAReubenACooperZAOhKSSullivanRJImmune Effects of Chemotherapy, Radiation, and Targeted Therapy and Opportunities for Combination With ImmunotherapySemin Oncol201542460161626320064
- SavasPSalgadoRDenkertCClinical relevance of host immunity in breast cancer: from TILs to the clinicNat Rev Clin Oncol201613422824126667975
- SalgadoRDenkertCDemariaSThe evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014Ann Oncol201526225927125214542
- SimonRMPaikSHayesDFUse of Archived Specimens in Evaluation of Prognostic and Predictive BiomarkersJNCI Journal of the National Cancer Institute2009101211446145219815849
- SeoANLeeHJKimEJTumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancerBr J Cancer2013109102705271324129232
- SalgadoRDenkertCCampbellCTumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and TrastuzumabJAMA Oncol20151444845426181252
- AliHRProvenzanoEDawsonS-JAssociation between CD8+ T-cell infiltration and breast cancer survival in 12 439 patientsAnnals of Oncology20142581536154324915873
- LoiSSirtaineNPietteFPrognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98J Clin Oncol201331786086723341518
- DenkertCvon MinckwitzGDarb-EsfahaniSTumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapyLancet Oncol2018191405029233559
- LewisCEPollardJWDistinct Role of Macrophages in Different Tumor MicroenvironmentsCancer Res200666260561216423985
- MantovaniAMarchesiFMalesciALaghiLAllavenaPTumour-associated macrophages as treatment targets in oncologyNat Rev Clin Oncol201714739941628117416
- BiswasSKAllavenaPMantovaniATumor-associated macrophages: functional diversity, clinical significance, and open questionsSemin Immunopathol201335558560023657835
- ZhaoXQuJSunYPrognostic significance of tumor-associated macrophages in breast cancer: a meta-analysis of the literatureOncotarget2017818305763058628427165
- ParkK-YLiGPlattMOMonocyte-derived macrophage assisted breast cancer cell invasion as a personalized, predictive metric to score metastatic riskSci Rep2015511385526349896
- GabrilovichDINagarajSMyeloid-derived suppressor cells as regulators of the immune systemNat Rev Immunol20099316217419197294
- CondamineTGabrilovichDIMolecular mechanisms regulating myeloid-derived suppressor cell differentiation and functionTrends Immunol2011321192521067974
- ToorSMSyed KhajaASEl SalhatHMyeloid cells in circulation and tumor microenvironment of breast cancer patientsCancer Immunology, Immunotherapy201766675376428283696
- Diaz-MonteroCMSalemMLNishimuraMIGarrett-MayerEColeDJMonteroAJIncreased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapyCancer Immunology, Immunotherapy2009581495918446337
- WesolowskiRDugganMCStiffACirculating myeloid-derived suppressor cells increase in patients undergoing neo-adjuvant chemotherapy for breast cancerCancer Immunology, Immunotherapy201766111437144728688082
- LebienTWTedderTFB lymphocytes: how they develop and functionBlood200811251570158018725575
- BrownJRWimberlyHLanninDRNixonCRimmDLBossuytVMultiplexed Quantitative Analysis of CD3, CD8, and CD20 Predicts Response to Neoadjuvant Chemotherapy in Breast CancerClinical Cancer Research201420235995600525255793
- MehrRSternberg-SimonMMichaeliMPickmanYModels and methods for analysis of lymphocyte repertoire generation, development, selection and evolutionImmunol Lett20121481112222902400
- WangJYangJIdentification of CD4+CD25+CD127− regulatory T cells and CD14+HLA−DR−/low myeloid-derived suppressor cells and their roles in the prognosis of breast cancerBiomed Rep20165220821227446543
- DemirLYigitSEllidokuzHPredictive and prognostic factors in locally advanced breast cancer: effect of intratumoral FOXP3+ TregsClin Exp Metastasis20133081047106223836289
- SageEKSchmidTESedelmayrMComparative analysis of the effects of radiotherapy versus radiotherapy after adjuvant chemotherapy on the composition of lymphocyte subpopulations in breast cancer patientsRadiother Oncol2016118117618026683801
- SongQRenJZhouXCirculating CD8 + CD28 − suppressor T cells tied to poorer prognosis among metastatic breast cancer patients receiving adoptive T-cell therapy: A cohort studyCytotherapy201820112613328988693
- SongGWangXJiaJElevated level of peripheral CD8+CD28− T lymphocytes are an independent predictor of progression-free survival in patients with metastatic breast cancer during the course of chemotherapyCancer Immunology, Immunotherapy20136261123113023604172
- DattaJFracolMMcmillanMTAssociation of Depressed Anti-HER2 T-Helper Type 1 Response With Recurrence in Patients With Completely Treated HER2-Positive Breast CancerJAMA Oncol20162224224626719971
- DattaJBerkEXuSAnti-HER2 CD4+ T-helper type 1 response is a novel immune correlate to pathologic response following neoadjuvant therapy in HER2-positive breast cancerBreast Cancer Research20151717125997452
- O’ConnorJPAboagyeEOAdamsJEImaging biomarker roadmap for cancer studiesNat Rev Clin Oncol201714316918627725679
- ChagparABMiddletonLPSahinAAAccuracy of Physical Examination, Ultrasonography, and Mammography in Predicting Residual Pathologic Tumor Size in Patients Treated With Neoadjuvant ChemotherapyAnn Surg2006243225726416432360
- FowlerAMMankoffDAJoeBNImaging Neoadjuvant Therapy Response in Breast CancerRadiology2017285235837529045232
- CroshawRShapiro-WrightHSvenssonEErbKJulianTAccuracy of Clinical Examination, Digital Mammogram, Ultrasound, and MRI in Determining Postneoadjuvant Pathologic Tumor Response in Operable Breast Cancer PatientsAnn Surg Oncol201118113160316321947594
- KeuneJDJeffeDBSchootmanMHoffmanAGillandersWEAftRLAccuracy of ultrasonography and mammography in predicting pathologic response after neoadjuvant chemotherapy for breast cancerAm J Surg2010199447748420359567
- HeineJJMalhotraPTissueMbreast cancer risk, serial image analysis, and digital mammography. Part 1. Tissue and related risk factorsAcad Radiol20029329831611887946
- JafariSHSaadatpourZSalmaninejadABreast cancer diagnosis: Imaging techniques and biochemical markersJ Cell Physiol201823375200521329219189
- SchellingMAvrilNNährigJPositron emission tomography using [18F]Fluorodeoxyglucose for monitoring primary chemotherapy in breast cancerJ Clin Oncol20001881689169510764429
- LeeHWLeeHMChoiSEThe Prognostic Impact of Early Change in 18F-FDG PET SUV After Neoadjuvant Chemotherapy in Patients with Locally Advanced Breast CancerJ Nucl Med20165781183118827033896
- Dose SchwarzJBaderMJenickeLHemmingerGJänickeFAvrilNEarly prediction of response to chemotherapy in metastatic breast cancer using sequential 18F-FDG PETJ Nucl Med20054671144115016000283
- KostakogluLDuanFIdowuMOA Phase II Study of 3’-Deoxy-3’-18F-Fluorothymidine PET in the Assessment of Early Response of Breast Cancer to Neoadjuvant Chemotherapy: Results from ACRIN 6688J Nucl Med201556111681168926359256
- LindholmPLapelaMNågrenKLehikoinenPMinnHJyrkkiöSPreliminary study of carbon-11 methionine PET in the evaluation of early response to therapy in advanced breast cancerNucl Med Commun2009301303619306512
- KennyLMContractorKBHinzRReproducibility of [11C] choline-positron emission tomography and effect of trastuzumabClin Cancer Res201016164236424520682702
- UlanerGAGoldmanDACorbenAProspective Clinical Trial of 18F-Fluciclovine PET/CT for Determining the Response to Neoadjuvant Therapy in Invasive Ductal and Invasive Lobular Breast CancersJ Nucl Med20175871037104227856630
- PinkerKHelbichTHMorrisEAThe potential of multiparametric MRI of the breastBr J Radiol20179010692016071527805423
- MariscottiGHoussamiNDurandoMAccuracy of mammography, digital breast tomosynthesis, ultrasound and MR imaging in preoperative assessment of breast cancerAnticancer Res20143431219122524596363
- LobbesMBIPrevosRSmidtMThe role of magnetic resonance imaging in assessing residual disease and pathologic complete response in breast cancer patients receiving neoadjuvant chemotherapy: a systematic reviewInsights Imaging20134216317523359240
- YuanYChenXSLiuSYShenKWAccuracy of MRI in prediction of pathologic complete remission in breast cancer after preoperative therapy: a meta-analysisAJR Am J Roentgenol2010195126026820566826
- MarinovichMLHoussamiNMacaskillPMeta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapyJ Natl Cancer Inst2013105532133323297042
- YlGPanSMRenJYangZXJiangGQRole of Magnetic Resonance Imaging in Detection of Pathologic Complete Remission in Breast Cancer Patients Treated With Neoadjuvant Chemotherapy: A Meta-analysisClin Breast Cancer201717424525528209330
- MarinoMAHelbichTBaltzerPPinker-DomenigKMultiparametric MRI of the breast: A reviewJ Magn Reson Imaging201847230131528639300
- MarinovichMLSardanelliFCiattoSEarly prediction of pathologic response to neoadjuvant therapy in breast cancer: Systematic review of the accuracy of MRIBreast201221566967722863284
- PrevosRSmidtMLTjan-HeijnenVCGPre-treatment differences and early response monitoring of neoadjuvant chemotherapy in breast cancer patients using magnetic resonance imaging: a systematic reviewEur Radiol201222122607261622983282
- WoolfDKPadhaniARTaylorNJAssessing response in breast cancer with dynamic contrast-enhanced magnetic resonance imaging: Are signal intensity–time curves adequate?Breast Cancer Res Treat2014147233534325129341
- LiSPMakrisABeresfordMJUse of dynamic contrast-enhanced MR imaging to predict survival in patients with primary breast cancer undergoing neoadjuvant chemotherapyRadiology20112601687821502383
- RaunigDLMcshaneLMPennelloGQuantitative imaging biomarkers: A review of statistical methods for technical performance assessmentStat Methods Med Res2015241276724919831
- PartridgeSCMcdonaldESDiffusion weighted magnetic resonance imaging of the breast: protocol optimization, interpretation, and clinical applicationsMagn Reson Imaging Clin N Am201321360162423928248
- ParkSHMoonWKChoNDiffusion-weighted MR Imaging: Pretreatment Prediction of Response to Neoadjuvant Chemotherapy in Patients with Breast CancerRadiology20102571566320851939
- BelliPCostantiniMIerardiCDiffusion-weighted Imaging in Evaluating the Response to Neoadjuvant Breast Cancer TreatmentBreast J201117661061921929557
- WuLMHuJNGuHYHuaJChenJXuJRCan diffusion-weighted MR imaging and contrast-enhanced MR imaging precisely evaluate and predict pathological response to neoadjuvant chemotherapy in patients with breast cancer?Breast Cancer Res Treat20121351172822476850
- SardanelliFCarbonaroLAMontemezziSCavedonCTrimboliRMClinical Breast MR Using MRS or DWI: Who Is the Winner?Front Oncol20166Suppl 121727840809
- JagannathanNRKumarMSeenuVEvaluation of total choline from in-vivo volume localized proton MR spectroscopy and its response to neoadjuvant chemotherapy in locally advanced breast cancerBr J Cancer20018481016102211308247
- MeisamySBolanPJBakerEHNeoadjuvant Chemotherapy of Locally Advanced Breast Cancer: Predicting Response with in Vivo 1H MR Spectroscopy—A Pilot Study at 4 TRadiology2004233242443115516615
- BaekHMChenJHNalciogluOSuMYMySProton MR spectroscopy for monitoring early treatment response of breast cancer to neo-adjuvant chemotherapyAnn Oncol20081951022102418372283
- YamaguchiKNakazonoTEgashiraRDiagnostic Performance of Diffusion Tensor Imaging with Readout-segmented Echo-planar Imaging for Invasive Breast Cancer: Correlation of ADC and FA with Pathological Prognostic MarkersMagn Reson Med Sci201716324525227853053
- Furman-HaranENissanNRicart-SelmaVMartinez-RubioCDeganiHCamps-HerreroJQuantitative evaluation of breast cancer response to neoadjuvant chemotherapy by diffusion tensor imaging: Initial resultsJ Magn Reson Imaging2017
- FanMWuGChengHZhangJShaoGLiLRadiomic analysis of DCE-MRI for prediction of response to neoadjuvant chemotherapy in breast cancer patientsEur J Radiol20179414014728712700
- LambinPLeijenaarRTHDeistTMRadiomics: the bridge between medical imaging and personalized medicineNat Rev Clin Oncol2017141274976228975929
- WangJKatoFOyama-ManabeNIdentifying Triple-Negative Breast Cancer Using Background Parenchymal Enhancement Heterogeneity on Dynamic Contrast-Enhanced MRI: A Pilot Radiomics StudyPLoS One20151011e014330826600392
- WuJGongGCuiYLiRIntratumor partitioning and texture analysis of dynamic contrast-enhanced (DCE)-MRI identifies relevant tumor subregions to predict pathological response of breast cancer to neoadjuvant chemotherapyJ Magn Reson Imaging20164451107111527080586
- FanMChengHZhangPDCE-MRI texture analysis with tumor subregion partitioning for predicting Ki-67 status of estrogen receptor-positive breast cancersJ Magn Reson Imaging201848123724729219225
- HaSParkSBangJ-IKimE-KLeeH-YMetabolic Radiomics for Pretreatment 18F-FDG PET/CT to Characterize Locally Advanced Breast Cancer: Histopathologic Characteristics, Response to Neoadjuvant Chemotherapy, and PrognosisSci Rep201771155628484211
- ParkHLimYKoESEsKRadiomics Signature on Magnetic Resonance Imaging: Association with Disease-Free Survival in Patients with Invasive Breast CancerClinical Cancer Research Epub2018618
- EisenhauerEATherassePBogaertsJNew response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1)Eur J Cancer200945222824719097774
- SeymourLBogaertsJPerroneAiRECIST: guidelines for response criteria for use in trials testing immunotherapeuticsLancet Oncol2017183e143e15228271869
- WahlRLJaceneHKasamonYLodgeMAFrom RECIST to PER-CIST: Evolving Considerations for PET response criteria in solid tumorsJ Nucl Med200950Suppl 1122S15019403881
- WeaverOLeungJWTBiomarkers and Imaging of Breast CancerAJR Am J Roentgenol2018210227127829166151
- LiHZhuYBurnsideESMR Imaging Radiomics Signatures for Predicting the Risk of Breast Cancer Recurrence as Given by Research Versions of MammaPrint, Oncotype DX, and PAM50 Gene AssaysRadiology2016281238239127144536
- PinkerKShitanoFSalaEBackground, current role, and potential applications of radiogenomicsJ Magn Reson Imaging201847360462029095543
- CxMGaoFLuoJNeoPalAna: Neoadjuvant Palbociclib, a Cyclin-Dependent Kinase 4/6 Inhibitor, and Anastrozole for Clinical Stage 2 or 3 Estrogen Receptor-Positive Breast CancerClinical cancer research: an official journal of the American Association for Cancer Research201723154055406528270497
- JovanovićBMayerIAMayerELA Randomized Phase II Neoadjuvant Study of Cisplatin, Paclitaxel With or Without Everolimus in Patients with Stage II/III Triple-Negative Breast Cancer (TNBC): Responses and Long-term Outcome Correlated with Increased Frequency of DNA Damage Response Gene Mutations, TNBC Subtype, AR Status, and Ki67Clinical Cancer Research201723154035404528270498
- CuriglianoGRomieuGCamponeMA phase I/II trial of the safety and clinical activity of a HER2-protein based immunotherapeutic for treating women with HER2-positive metastatic breast cancerBreast Cancer Res Treat2016156230131026975189
- SturgeonCHillRHortinGLThompsonDTaking a new biomarker into routine use - A perspective from the routine clinical biochemistry laboratoryProteomics Clin Appl201041289290321137030
