Abstract
Background
Tobacco smoking has been widely acknowledged to be the most important risk factor for bladder cancer. However, whether secondhand smoking (SHS) increases the risk of bladder cancer still remains uncertain. We conducted a meta-analysis about the risk of bladder cancer and lifetime SHS and childhood SHS.
Materials and methods
We searched PubMed, EMBASE, Web of Science, and Chinese National Knowledge Infrastructure (CNKI) up to March 12, 2018, and checked references of the retrieved articles and relevant reviews to include 14 studies. Relative risk (RR) and 95% confidence interval (CI) were used to assess this risk.
Results
The pooled RR of 14 eligible studies based on the retrieved articles and relevant reviews illustrated a significantly increased risk of bladder cancer with RR 1.22, 95% CI 1.06–1.4. No heterogeneity or publication bias was found. But we need more evidence to prove a more reliable association between childhood SHS and bladder cancer.
Conclusion
There was a statistically significant 22% increased risk of bladder cancer for lifetime SHS exposure in nonsmoking patients compared with unexposed nonsmoking population. But the association between childhood SHS exposure compared with unexposed nonsmoking population was unclear. Further research should be conducted to confirm our findings and reveal the potential biological mechanisms.
Introduction
Bladder cancer is the ninth most common cancer globally, with an estimated 430,000 new cases and 165,000 bladder cancer deaths in 2012.Citation1 A significant male predominance was observed, with male bladder cancer cases comprising three quarters of the total cases. Europe has among the highest incidence rates of bladder cancer in the world. The highest rate of incidence in men was recorded in Spain with age-standardized rate = 36.7 per 100,000 according to Cancer Registry Data. New bladder cancer diagnoses rose 1.5-fold between 1990 and 2013 with deaths rising 1.3-fold between 1990 and 2013.Citation2
Tobacco smoking has been widely acknowledged to be the most important risk factor for bladder cancer. However, whether secondhand smoking (SHS) increases the risk of bladder cancer still remains uncertain. Although a number of clinical studies were conducted to identify the association between SHS and bladder cancer,Citation3–Citation16 only one of these studies reported a significantly increased risk of SHS and bladder cancer in nonsmoking population. Six studies reported an elevated risk of bladder cancer in lifelong nonsmokers who were exposed to SHS compared with those who had no SHS exposure but without significance, while others reported a null relationship.Citation3,Citation5,Citation9,Citation10,Citation13,Citation16 This may mainly be due to the measurement error of accessing SHS and the lack of nonsmoking bladder cancer cases exposed to tobacco.
Meta-analysis could synthesize the evidence provided by studies mentioned above. Thus, we could not only reach the most extensive study population but also minimize the influence of methodological heterogeneity of each study and exclude some low-quality studies. So, we urgently need a meta-analysis that provides a higher level of evidence to draw a reliable conclusion about SHS and bladder cancer. In 2009, the only meta-analysis published reported no association between SHS and bladder cancer.Citation17 However, this meta-analysis included only eight studies. So, we did this meta-analysis including 14 studies aiming to ascertain the association between SHS and bladder cancer. Additionally, we did another meta-analysis about exposure to SHS in childhood to identify whether the risk between childhood SHS and bladder cancer is higher than lifetime exposure.
Materials and methods
Publication search
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.Citation18 We carried out a search in PubMed, EMBASE, Web of Science, and Chinese National Knowledge Infrastructure (CNKI) databases to cover all the eligible articles published up to March 12, 2018. The search strategy included terms for outcome (bladder neoplasm or bladder cancer or bladder tumor or bladder tumour or carcinoma of bladder) and exposure [Secondhand smok* or ETS*(environmental tobacco smoke) or environmental smok* or SHS or passive smok* or tobacco smoke pollution]. We carefully evaluated every potentially relevant publication by scanning their titles and abstracts. All the studies matching the inclusion criteria were retrieved. The references from retrieved articles and reviews were also thoroughly checked to identify any additional relevant study. No language restrictions were imposed. The search process was done separately by two authors independently. Disagreement was resolved by discussion; if no agreement was reached, a third independent expert acted as an arbiter.
Inclusion criteria
Studies included in this meta-analysis had to meet all the following criteria: 1) They had a cohort design or case–control design; 2) The subjects included in this study contained nonsmokers; 3) The exposure of interest included SHS; 4) The outcome of studied subjects contained incidence of bladder cancer; 5) Studies provide odds ratio (OR), hazard ratio (HR), relative risk (RR), or standardized incidence ratio (SIR) with their 95% CIs or sufficient data to calculate them. Studies concerning mortality rates of bladder cancer were not included because it could be unpredictably confounded by survival-related factors. If multiple studies researching the same population were available, the most recent and detailed study was entitled to be included in this meta-analysis.
Data extraction
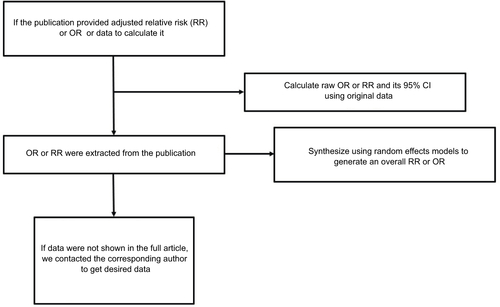
Two authors independently extracted data by using a predefined data collection form, with disagreements being resolved by consensus. For each study, the following characteristics were collected: first author’s family name, year of publication, participant characteristics (age and gender), study type, study population, range for follow-up, numbers of events and nonevent subjects, and covariates adjusted for in the analysis. From each of the studies, we optionally extracted the RR or OR estimate that was adjusted for the greatest number of potential confounders. If data were not shown in the full article, we contacted the corresponding author to get the desired data.Citation8
Quality assessment
Quality assessments of searched papers were also undertaken independently by two authors according to the Newcastle–Ottawa scale. The disagreements between authors were resolved by discussion. If no agreement was reached, a third independent expert acted as an arbiter.
Statistical methods
The OR was assumed the same as RR, and the RR was defined as the study outcome to estimate the risk of SHS and bladder cancer. These two measurements of effects can similarly estimate RR because the absolute risk of bladder cancer is low. In the study by Kabat et al,Citation11 adjusted OR or RR was not provided, so we calculated raw OR and its 95% CI using original data. If several approaches of “exposure to SHS” were reported in a publication while overall RR was not provided, the RRs with their 95% CI of each approach exposed to SHS were synthesized using random effects models to generate an RR that estimates the risk of SHS and bladder cancer in the publication (as in ).
Overall RR estimates with their corresponding 95% CIs were calculated with the random effects models, which explicitly accounts for the heterogeneity of studies through a statistical parameter representing the interstudy variation.Citation19 Heterogeneity among studies was measured by Q-test and quantified by I2, with higher value contributing to greater degree of heterogeneity. Publication bias was assessed using Begg’s test and Egger’s test. P<0.05 was considered to be representative of a significant statistical publication bias. Sensitivity analyses were also performed to assess the stability of obtained results with a single study deleted each time to manifest the influence of the individual dataset to the pooled RR. All the statistical analyses were performed with STATA 14.0 (Stata-Corp, College Station, TX, USA), using two-sided P-values.
Results
Literature search
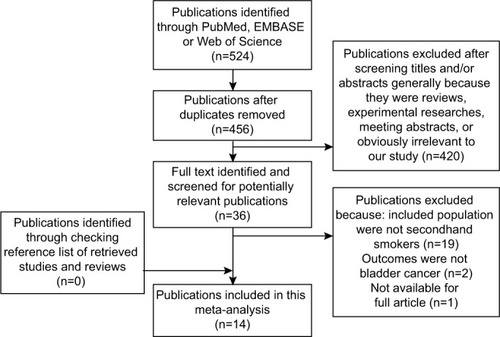
outlines our study searching and selecting process. Briefly, after removing publication duplications, our search strategy generated 524 articles. After a first scanning of the titles and abstracts of these articles, the majority were excluded generally because they were reviews, experimental researches, meeting abstracts, and obviously irrelevant to our study. Thirty- six articles seemed relevant to our study, and we tried to identify and screen the full text. We were unable to get one potentially relevant publication by Sandler et al.Citation20 After full-text reviewing of 35 papers, 21 studies were excluded for the reasons as follows: 1) Included population were not secondhand smokers (n=19); 2) Outcomes were not bladder cancer (n=2). Although we checked the reference list of retrieved studies and reviews carefully, no new articles were obtained. Thus, a total of 14 cohort studies or case–control studies,Citation3–Citation16 which met the inclusion criteria, were included in the meta-analysis. Of these studies, six were never included in former meta-analysis.Citation4,Citation8,Citation9,Citation13,Citation14,Citation16.
Study characteristics
The characteristics of the 14 studies are presented in . Of these, three were cohort studies and 11 were case–control studies. The study population in 14 studies consisted of both sexes. The included studies were published between 1986 and 2012. The sample size ranged from 126Citation7 to 220,790Citation5. The total population included in this meta-analysis reaches 325,264 with 4,002 cases and 321,262 controls. Adjustments were made for potential confounders of more than two factors in all 14 studies.
Table 1 Study characters of SHS and bladder cancer
Exposure to SHS and risk of bladder cancer
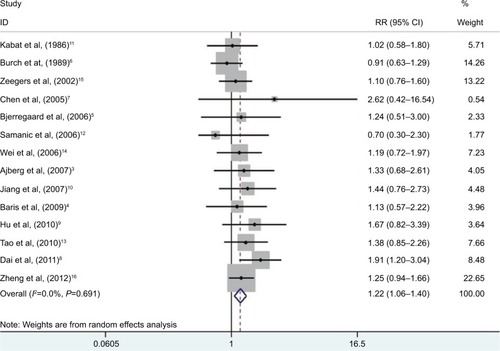
A significantly increased risk of exposure to SHS and bladder cancer was found in our review (; RR 1.22, 95% CI 1.06–1.40). There is barely any heterogeneity among these studies with Q=10.04, P=0.691, and I2=0.
Figure 2 RRs for the association between SHS and risk of bladder cancer.
Notes: Diamonds represent study-specific relative risks or summary RRs with 95% CIs; horizontal lines represent 95% CIs. Test for heterogeneity among studies: P=0.691, I2=0.0%.
Abbreviations: RR, relative risk; SHS, secondhand smoking.
When we did meta-analysis about childhood SHS and bladder cancer, an even more higher risk that is statistically significant was detected (, RR 1.43, 95% CI 1.03–1.98). But there was significant heterogeneity in these studies with Q=16.05, P=0.03, and I2=56.4%.
Publication bias
There was no evidence demonstrating significant publication bias either with Begg’s test (P=0.74) or with Egger’s test (, P=0.50). In the meta-analysis concerning childhood SHS and risk of bladder cancer, a significant publication bias was detected with Egger’s test (, P=0.09).
Sensitivity analysis
Sensitivity analysis of the pooled studies was performed to test the stability of our result. By means of the random effects model, we calculated all integrated RR or OR once again. When omitting each study in the meta-analysis, the pooled result () always remained stable. When we analyzed the sensitivity of childhood SHS and bladder cancer, the result changed several times ().
Discussion
The present meta-analysis synthesized all eligible evidence from 11 case–control studies and three cohort studies and provided a statistically significant 22% increased risk of bladder cancer for lifetime SHS exposure in nonsmoking patients compared with unexposed nonsmoking population. Our result was convincing because there was no heterogeneity detected, which indicated that the combination of these 14 studies was reasonable. Sensitivity analysis showed that when omitting each study, the result remains always consistent, which represents a good stability of this meta-analysis. There was no evidence showing obvious publication bias either.
When we conducted another meta-analysis of childhood exposure to SHS and risk of bladder cancer, we found an even higher increased risk of 43% significantly. But we found obvious heterogeneity and publication bias in this study. Meanwhile, the result of sensitivity analysis was not stable either. It means that the increasing risk between childhood SHS and bladder cancer is not reliable and should be treated with caution. In sensitivity analysis when we delete the study by Dai et al,Citation8 the heterogeneity decreased and became acceptable with I2=11.3% and P=0.343, RR=1.21 and its 95% CI 0.95–1.53. This result suggests that the study by Dai et al contributes most of the heterogeneity in this meta- analysis.Citation8 The source of heterogeneity may mainly stem from methodological diversity and measure error when conducting multicenter case–control study. More studies should be done in future to identify a relationship between childhood SHS exposure and bladder cancer.
Smoking population has a four-fold risk of bladder cancer compared with nonsmokers.Citation21 Chemically, tobacco contains over 60 species of carcinogens including polycyclic aromatic hydrocarbons and aromatic amines, which could excrete via kidney. This process could damage urethra and lead to the incidence of bladder cancer. In a basic research in vitro by Geng et al, exposure to cigarette smoke extract (CSE) induced proliferation in normal human urothelial cells via the mitogen-activated protein kinase-1/activator protein-1 pathway, which causes CSE-related carcinogenesis of bladder cancer.Citation22 In another basic research, nicotine in tobacco induces tumor growth and chemoresistance through activation of the phosphatidylinositol-3-kinase/Akt/mammalian target of rapamycin pathway in bladder cancer.Citation23 Our study suggests that secondhand smoke may contain these carcinogens and activate the pathways mentioned above. This point was proved in part by an experiment demonstrating that cigarette sidestream smoke induced mutagenic acrolein-DNA adducts, inhibited DNA repair, and enhanced anchorage-independent growth cell transformation in mice.Citation24 In the future, more basic experiments should be done to confirm that secondhand smoke contributes to the formation of bladder cancer.
Our study has several strengths. First, we take a transparent and robust approach to examine the evidence base, including adherence to PRISMA guidelines. Second, we obtained some data that were not provided in article through contacting the authorCitation8 and included two Chinese studies,Citation8,Citation9 so this meta- analysis included the broadest and most comprehensive data about SHS exposure and risk of bladder cancer. Third, our heterogeneity was inapparent with a low risk of publication bias and good result stability, which enhanced the strength and reliability of our result. Fourth, we were able to analyze the association between childhood SHS and bladder cancer and suggest that more clinical research should be done to address this issue in the future.
Of course, several limitations should be mentioned. First, most of the papers included were case–control studies; therefore, selection biases and recall biases could not be avoided. Second, only published studies were included in this meta- analysis. Even if Begg’s test and Egger’s test did not show evidence of publication bias, some inevitable publication bias may exist because studies with null results tend not to be published. Third, we could not solve the potential confounding factors like diabetes, drug use, or other unreported factors in the included studies.Citation25 In some included studies, only two confounders were adjusted.Citation8,Citation9,Citation15 Finally, we could not give an explicit evidence to prove the association between childhood SHS exposure and bladder cancer risk. Because of these above, more future cohort studies are expected to be carried out to confirm the conclusion in this meta-analysis.
In conclusion, this meta-analysis indicated that there was a statistically significant 22% increased risk of bladder cancer for lifetime SHS exposure in nonsmoking patients compared with unexposed nonsmoking population. But the association between childhood SHS exposure compared with unexposed nonsmoking population was unclear. Further research should be conducted to confirm our findings and clarify the potential biological mechanisms.
Ethics approval
All analyses were based on previous published studies; thus, no ethical approval and patient consent were required.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81472375 and No. 81702500) and Zhejiang Province Medical Technology Project (2016KYA173).
Supplementary materials
Figure S1 RRs for the association between childhood SHS and risk of bladder cancer.
Notes: Diamonds represent study-specific RRs or summary relative risks with 95% CIs; horizontal lines represent 95% CIs. Test for heterogeneity among studies: P=0.025, I2=56.4%.
Abbreviations: RR, relative risk; SHS, secondhand smoking.
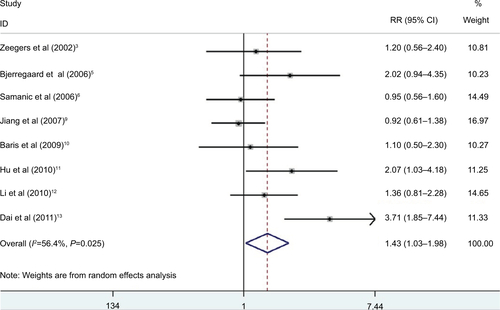
Figure S2 Funnel plot of studies evaluating the association between SHS and bladder cancer.
Notes: Egger’s regression test (P=0.50). Standardized effect was defined as the OR divided by its standard error. Precision was defined as the inverse of the standard error.
Abbreviation: SHS, secondhand smoking.
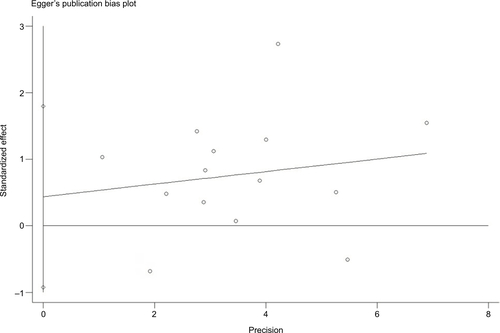
Figure S3 Funnel plot of studies evaluating the association between childhood SHS and bladder cancer.
Notes: Egger’s regression test (P=0.09). Standardized effect was defined as the OR divided by its standard error. Precision was defined as the inverse of the standard error.
Abbreviation: SHS, secondhand smoking.
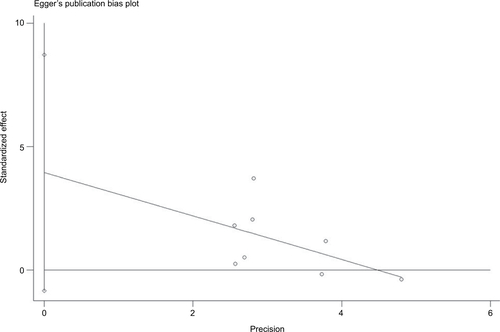
Table S1 Sensitivity analysis of SHS and bladder cancer
Table S2 Sensitivity analysis of childhood SHS and bladder cancer
References
- KabatGCDieckGSWynderELBladder cancer in nonsmokersCancer1986573623673942969
- BurchJDRohanTEHoweGRRisk of bladder cancer by source and type of tobacco exposure: A case-control studyInternational Journal of Cancer1989446226282793235
- ZeegersMPGoldbohmRAvan den BrandtPAA prospective study on active and environmental tobacco smoking and bladder cancer risk (The Netherlands)Cancer causes & control : CCC200213839011899922
- ChenYCSuHJGuoYLHousemanEAChristianiDCInteraction between environmental tobacco smoke and arsenic methylation ability on the risk of bladder cancerCancer causes & control : CCC200516758115868449
- BjerregaardBKRaaschou-NielsenOSorensenMTobacco smoke and bladder cancer–in the European Prospective Investigation into Cancer and NutritionInternational Journal of Cancer20061192412241616894557
- SamanicCKogevinasMDosemeciMSmoking and bladder cancer in Spain: effects of tobacco type, timing, environmental tobacco smoke, and genderCancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology20061513481354
- WeiZBingXYXiaSCRelationship between smoking and exposure to environmental tobacco smoking with bladder cancer..a case-control study in ShanghaiTumor2006264247
- AlbergAJKouzisAGenkingerJMA prospective cohort study of bladder cancer risk in relation to active cigarette smoking and household exposure to secondhand cigarette smokeAmerican Journal of Epidemiology200716566066617204516
- JiangXYuanJMSkipperPLTannenbaumSRYuMCEnvironmental tobacco smoke and bladder cancer risk in never smokers of Los Angeles CountyCancer Research2007677540754517671226
- BarisDKaragasMRVerrillCA Case-Control Study of Smoking and Bladder Cancer Risk: Emergent Patterns Over TimeJournal of the National Cancer Institute20091011553156119917915
- HuZSuYZengXRelationship between active smoking and environmental tobacco smoking with the risk of bladder cancer: A hospital-based case-control study in WuhanChinese Journal of Experimental Surgery20102713081310
- TaoLXiangYBWangREnvironmental tobacco smoke in relation to bladder cancer risk–the Shanghai bladder cancer study [corrected]Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology20101930873095
- DaiQSHeHCCaiCMulticenter case-control study of the relationship between smoking and bladder cancer in ChinaNational Medical Journal of China2011912407241022321786
- ZhengYLAmrSSalehDAUrinary bladder cancer risk factors in Egypt: a multicenter case-control studyCancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology201221537546
Disclosure
The authors report no conflicts of interest in this work.
References
- AntoniSFerlayJSoerjomataramIZnaorAJemalABrayFBladder cancer incidence and mortality: a global overview and recent trendsEur Urol20177119610827370177
- DyGWGoreJLForouzanfarMHNaghaviMFitzmauriceCGlobal burden of urologic cancers, 1990-2013Eur Urol201771343744628029399
- AlbergAJKouzisAGenkingerJMA prospective cohort study of bladder cancer risk in relation to active cigarette smoking and household exposure to secondhand cigarette smokeAm J Epidemiol2007165666066617204516
- BarisDKaragasMRVerrillCA case-control study of smoking and bladder cancer risk: emergent patterns over timeJ Natl Cancer Inst2009101221553156119917915
- BjerregaardBKRaaschou-NielsenOSørensenMTobacco smoke and bladder cancer–in the European Prospective Investigation into Cancer and NutritionInt J Cancer2006119102412241616894557
- BurchJDRohanTEHoweGRRisk of bladder cancer by source and type of tobacco exposure: a case-control studyInt J Cancer19894446226282793235
- ChenYCSuHJGuoYLHousemanEAChristianiDCInteraction between environmental tobacco smoke and arsenic methylation ability on the risk of bladder cancerCancer Causes Control2005162758115868449
- DaiQSHeHCCaiCMulticenter case-control study of the relationship between smoking and bladder cancer in ChinaZhonghua Yi Xue Za Zhi201191342407241022321786
- HuZSuYZengXRelationship between active smoking and environmental tobacco smoking with the risk of bladder cancer: A hospital-based case-control study in WuhanChinese J Exp Surg20102713081310
- JiangXYuanJMSkipperPLTannenbaumSRYuMCEnvironmental tobacco smoke and bladder cancer risk in never smokers of Los Angeles CountyCancer Res200767157540754517671226
- KabatGCDieckGSWynderELBladder cancer in nonsmokersCancer19865723623673942969
- SamanicCKogevinasMDosemeciMSmoking and bladder cancer in Spain: effects of tobacco type, timing, environmental tobacco smoke, and genderCancer Epidemiol Biomarkers Prev20061571348135416835335
- TaoLXiangYBWangREnvironmental tobacco smoke in relation to bladder cancer risk–the Shanghai bladder cancer study [corrected]Cancer Epidemiol Biomarkers Prev201019123087309521056942
- WeiZBingXYXiaSCRelationship between smoking and exposure to environmental tobacco smoking with bladder cancer: a case-control study in ShanghaiTumor2006264247
- ZeegersMPGoldbohmRAvan den BrandtPAA prospective study on active and environmental tobacco smoking and bladder cancer risk (The Netherlands)Cancer Causes Control2002131839011899922
- ZhengYLAmrSSalehDAUrinary bladder cancer risk factors in Egypt: a multicenter case-control studyCancer Epidemiol Biomarkers Prev201221353754622147365
- van HemelrijckMJMichaudDSConnollyGNKabirZSecondhand smoking, 4-aminobiphenyl, and bladder cancer: two meta-analysesCancer Epidemiol Biomarkers Prev20091841312132019336562
- MoherDShamseerLClarkeMPreferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statementSyst Rev20154125554246
- DersimonianRKackerRRandom-effects model for meta-analysis of clinical trials: an updateContemp Clin Trials200728210511416807131
- SandlerDPEversonRBWilcoxAJPassive smoking in adulthood and cancer riskAm J Epidemiol1985121137483964991
- FreedmanNDSilvermanDTHollenbeckARSchatzkinAAbnetCCAssociation between smoking and risk of bladder cancer among men and womenJAMA2011306773774521846855
- GengHZhaoLLiangZCigarette smoke extract-induced proliferation of normal human urothelial cells via the MAPK/AP-1 pathwayOncol Lett201713146947528123584
- YugeKKikuchiEHagiwaraMNicotine induces tumor growth and chemoresistance through activation of the PI3K/Akt/mTOR pathway in bladder cancerMol Cancer Ther20151492112212026184482
- LeeHWWangHTWengMWCigarette side-stream smoke lung and bladder carcinogenesis: inducing mutagenic acrolein-DNA adducts, inhibiting DNA repair and enhancing anchorage-independent-growth cell transformationOncotarget2015632332263323626431382
- YanHXieHYingYPioglitazone use in patients with diabetes and risk of bladder cancer: a systematic review and meta-analysisCancer Manag Res2018101627163829970962