Abstract
Purpose
Sarcopenia is distinguished by decreased skeletal muscle plus low muscle strength and/or physical performance. This study was designed to demonstrate the relationship between sarcopenia and systemic inflammatory response (neutrophil/lymphocyte ratio [NLR], platelet/lymphocyte ratio [PLR], and large platelet/lymphocyte ratio [LPLR]) prior to radical gastrectomy for gastric cancer.
Patients and methods
We conducted a prospective study of gastric cancer patients who underwent radical gastrectomy. The clinical utility of the NLR, PLR, and LPLR was evaluated by receiver operating characteristic curves. Sarcopenia components including skeletal muscle index, handgrip strength, and 6 m usual gait speed were measured. Logistic analysis was used to identify the independent indices associated with sarcopenia.
Results
A total of 670 patients were included, representing 504 men and 166 women. Of these, 104 patients (15.5%) were diagnosed with sarcopenia and 567 (84.5%) were non-sarcopenia. PLR has a diagnostic sensitivity of 91.3% for sarcopenia. In addition to the indicators of preoperative age, nutritional risk screening, body mass index, preoperative albumin, and diabetes, the NLR and PLR were independent predictors for sarcopenia (P<0.05).
Conclusion
The present study showed that at-diagnosis sarcopenia was associated with inflammation in patients with operable gastric cancer. Due to the complex assessment of muscle condition, PLR may be used as a primary screening test for sarcopenia. How systemic inflammatory response influences changes in sarcopenia may provide new therapeutic perception toward improving outcomes.
Introduction
Gastric cancer is the fifth most frequently diagnosed cancer all over the world with one million new cases occurring every year.Citation1 However, despite progress in tumor diagnosis, surgery, and drug therapy, the prognosis of gastric cancer remains poor.Citation2,Citation3 Malnutrition is common in gastric cancer patients consequent to inadequate digestion and nutrient absorption.Citation4 In comparison, sarcopenia, an age-related syndrome associated with functional disturbance and disability, is distinguished by reduced skeletal muscle and low muscle strength plus physical performance.Citation5 This condition can result due to many causes, such as malnutrition, aging, inactivity, inflammatory disease, and cancer.Citation6 This syndrome has come up as a measure of the physiological reserve for the body. Besides age, body mass index (BMI), albumin, and comorbidities,Citation7–Citation10 previous studies confirmed that sarcopenia influenced total postoperative complications and overall survival of gastric cancer patients.Citation11,Citation12
From the beginning of production of cancer cells to the occurrence of cancer cachexia, inflammation plays crucial role in each step of cancer progression.Citation13–Citation15 Currently, the platelet/lymphocyte ratio (PLR)Citation16 and neutrophil/lymphocyte ratio (NLR)Citation17 are gradually gaining more attention as indicators of systemic inflammatory response (SIR). Several studies have revealed that PLR and NLR could be identified as indicators predictive of advanced stage, lymphatic metastasis, treatment response, and prognosis in some types of carcinoma.Citation18–Citation21 A pilot study has suggested that the accuracy of large platelet/lymphocyte ratio (LPLR) was superior to that of PLR in patients with cancer.Citation22 We assumed that SIR may be relevant in identifying patients with sarcopenia.
To our knowledge, however, no relevant research has elucidated the association between SIR and sarcopenia for gastric carcinoma, especially for operable gastric cancer. Furthermore, the majority of studies regarding sarcopenia have been retrospective and all have used skeletal muscle mass as the single diagnostic parameter of sarcopenia, neglecting the importance of muscle strength and physical ability, which constitute indispensable parts of sarcopenia as well.Citation23 Therefore, this prospective study was designed to analyze whether SIR, including PLR, NLR, and LPLR, is associated with sarcopenia in gastric cancer patients who undergo radical gastrectomy.
Patients and methods
Study population
We identified consecutive patients of the First Affiliated Hospital of Wenzhou Medical University, Zhejiang, China, who were diagnosed with operable gastric cancer between February 1, 2015 and December 31, 2016. The inclusion criteria were: 1) need to undergo radical gastrectomy; 2) performed preoperative CT examinations less than 2 weeks prior to the operation; and 3) able to walk and grip within 7 days before the operation. Considering the stable blood parameters, the exclusion criteria were the following: 1) occurrence of another malignancy during the 3 years prior to surgery; 2) an emergency operation; 3) preoperative chemotherapy or radiotherapy; 4) severe bleeding or immune system disease; 5) severe inflammation, such as secondary peritonitis. Based on the abovementioned criteria, 73 patients were excluded. Finally, 670 patients were included in this study.
Preoperative factors
All the data about patients were collected prior to surgery: 1) personal information, including age, sex, BMI, ASA grade (according to the standard proposed by the American Society of Anesthesiologists), and surgery history; 2) tumor characteristics, including tumor location, histological type, and TNM stage of tumor; and 3) blood routine index, including neutrophil, platelet, lymphocyte, and large platelet count. The histopathological types were defined as “differentiated (well/moderate)” or “undifferentiated (poor)”. We performed nutritional risk screening (NRS-2002) within 24 hours of admission. Total score of 3 or greater than 3 were regarded as nutritional risk.Citation24 The treatment for gastric cancer was based on the Japanese Gastric Cancer Treatment Guidelines 2010.Citation25 Surgery history was defined as surgery that has been performed in the past including abdominal surgery.
Definition of sarcopenia
Based on the European Working Group on SarcopeniaCitation23 and the Asian Working Group for Sarcopenia,Citation26 low skeletal muscle mass plus low muscle strength were considered as sarcopenia.
A cross-sectional CT image of the third lumbar vertebra (L3) was selected for valuing muscle mass.Citation27 Skeletal muscles were separated from other tissues by a Hounsfield units threshold range from 30 to 150.Citation28 The muscles of L3 region include psoas, external obliques, internal obliques, erector spinae, quadratus lumborum, transversus abdominis, and rectus abdominis. Tissue borderlines were manually drawn out. To decrease the bias, one investigator who was blinded for the patient was well-trained to identify and measure the muscle area by a professional imaging software (INFINITT PACS software version 3.0.11.3 BN17 32 bit; INFINITT Healthcare Co., Ltd, Seoul, Korea). was well-trained to identify and measure the muscle area. L3 muscle cross-sectional areas computed from each image were normalized for height (m2) to acquire the L3 skeletal muscle index (L3 SMI, cm2/m2).Citation29
Being indispensable indices, preoperative grip strength and 6 meter usual gait speed were also determined, which, respectively, reflect muscle strength and physical performance.Citation12 Grip strength was calculated by electronic hand dynamometer (EH101; Camry, Guangdong Province, China). Patients were asked to squeeze the device with all strength by using the dominant hand. Six-meter usual gait speeds were calculated by asking patients to walk through 6 m, clocking from the first foot to the last foot over the finish line. The two tests were performed within 7 days before the operation. The maximal value of three consecutive tests was recorded.
In consideration of the ethnic differences between Asians and Europeans,Citation23,Citation26 sarcopenia was diagnosed based on the following parameters: 1) muscle mass (L3 SMI ≤40.8 cm2/m2 for men and 34.9 cm2/m2 for women);Citation12 2) muscle strength (hand grip strength <26 kg for men and <18 kg for women); and 3) muscle performance (6 m usual gait speed <0.8 m/s).Citation26
SIR evaluation
Blood specimens were prospectively gathered within 7 days before the operation and translocated to sterile centrifuge tubes, which were carefully delivered to the clinical laboratory department. The neutrophil, lymphocyte, platelet, and large platelet counts were calculated by a hemocounter (XE2100; Sysmex Co., Kobe, Japan). The maximal Youden index values were selected as the cutoff points for NLR, PLR, and LPLR in the resultant receiver operating characteristic (ROC) curves.Citation30 The patients were divided into two groups, NLR and PLR, according to the cutoff points.
Validation data for PLR
According to the same criteria as described in the study population section, 162 patients were selected in our prospective database from January 2017 to June 2017 as validation group. The data about preoperative factors, definition of sarcopenia, and SIR evaluation were collected as mentioned earlier.
Statistical analysis
We carried out the Kolmogorov–Smirnov test to confirm the normality of continuous parameters, for example, the neutrophil, platelet, and lymphocyte counts, along with the NLR and PLR. The Mann–Whitney U test was used to compare the non-normal distributed variables between the sarcopenia and non-sarcopenia groups. The performance of SIR in diagnosing sarcopenia was examined using the area under the ROC (AUROC) curves. The AUROC was expressed as plots of the test sensitivity vs 1-specificity. The sensitivity and specificity were also assessed. The chi-squared test was used to analyze the relationship between clinicopathological characteristics and the variables as well as in validation cohort. A multivariate logistic regression analysis was used to calculate the OR and 95% CI of the confirmed independent variables, based on the univariate analysis results. A value of P<0.05 was regarded as statistically significant. SPSS software version 22.0 (SPSS Inc., Chicago, IL, USA) was used to perform statistical analyses.
Results
Patient characteristics
Of the 670 patients selected, 504 were men and 166 were women. The median age was 65.0 years (interquartile range 58.0–73.0). Hypoalbuminemia was found in 172 (25.7%) patients. One hundred and four patients (15.5%) exhibited sarcopenia and 567 (84.5%) showed non-sarcopenia. reports the detailed demographics and clinical characteristics of the selected patients.
Table 1 Systemic inflammatory response markers according to sarcopenia (IQR)
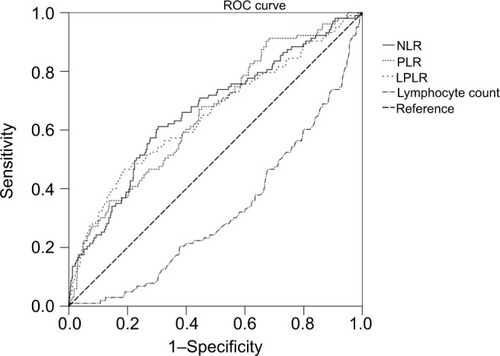
Among the features of preoperative SIR markers in patients with sarcopenia, NLR (P<0.001), PLR (P<0.001), and LPLR (P<0.001) were significantly higher in patients with gastric cancer and sarcopenia than in those with non-sarcopenia. Lymphocyte count (P<0.001) was significantly lower in patients with gastric cancer and sarcopenia than in those with non-sarcopenia (). White blood cell, neutrophil, and platelet counts were not relevant with sarcopenia. The ROC curves were used to further evaluate the variables that differed significantly. shows that the AUCs of preoperative NLR (0.663, 95% CI 0.603–0.723), PLR (0.655, 95% CI 0.598–0.712), and LPLR (0.649, 95% CI 0.587–0.712) were wider than those of the lymphocyte count (0.321, 95% CI 0.266–0.375).
Clinicopathological characteristics associated with SIR
Based on the ROC curve shown in , the cutoff values of the NLR, PLR, and LPLR for sarcopenia were set as 2.67, 116.85, and 59.41, respectively. According to the cutoff value, NLR sensitivity and specificity for sarcopenia diagnosis were 62.0% and 70.1%, whereas those for PLR were 91.3% and 31.8%, respectively. Groups of “high NLR (>2.67)” and “low NLR (≤2.67)” or “high PLR (>116.85)” and “low PLR (≤116.85)” were dichotomized. The data of groups of LPLR were not shown below. The number of patients with high NLR and high PLR was 236 (35.2%) and 480 (71.6%), respectively. The clinicopathological characteristics associated with PLR and NLR were analyzed further. The number of sarcopenia cases was statistically higher in the high PLR and NLR groups (both P<0.001) (). Concerning the other clinicopathological characteristics, high NLR was significantly associated with old age (>65 years, P=0.001), low BMI (<18 kg/m2, P=0.043), high NRS (>1, P<0.001), hypoalbuminemia (<35 g/L, P<0.001), hypohemoglobinemia (<100 g/L, P<0.001), poor pathological type (undifferentiated, P=0.045), high node status (P<0.001), and depth of invasion (P<0.001). PLR was significantly increased with older age (P=0.005), high NRS (P<0.001), hypoalbuminemia and hypohemoglobinemia (P<0.001), poor pathological type (P=0.05), depth of invasion (P<0.001), and high node status (P=0.008). No significant difference was observed with regard to the remainder of the clinicopathological characteristics.
Table 2 Clinical characteristics according to NLR and PLR (%)
Univariate and multivariate analysis of clinicopathological characteristics
Univariate analysis of clinicopathological characteristics indicated that age (P<0.001), BMI (P<0.001), ASA (P=0.001), NRS (P<0.001), surgery history (P=0.036), histologic type (P=0.042), hemoglobin (P<0.001), diabetes (P=0.006), albumin (P<0.001), NLR (P<0.001), PLR (P<0.001), LPLR (P<0.001), node status (P=0.017), and T stage (P<0.001) showed significant differences according to sarcopenia status (). Other variables such as sex and hypertension were found to have no significant association with sarcopenia.
Table 3 Univariate analysis and multivariate analysis of the risk of sarcopenia
Fourteen variables from univariate analysis (P<0.05) were chosen for multivariate analysis as potential independent risk factors. demonstrates that seven of the 14 variables differed significantly (P<0.05). Finally, we confirmed that NLR (HR 2.065; 95% CI 1.234–3.455; P=0.006), PLR (HR 2.194; 95% CI 1.015–4.741; P=0.046), age (HR 4.386; 95% CI 2.297–8.376; P<0.001), NRS ([2 vs 1; HR 3.588; 95% CI 1.171–10.996; P=0.025]; [3 vs 1; HR 7.246; 95% CI 2.381–22.050; P<0.001]; [>3 vs 1; HR 7.563; 95% CI 2.460–23.248; P<0.001]), albumin (HR 0.488; 95% CI 0.292–0.815; P=0.006), BMI (HR 0.384; 95% CI 0.162–0.90; P=0.003), and diabetes (HR 2.514; 95% CI 1.269–4.984; P=0.008) were independent indicators predictive of sarcopenia, while ASA grade, surgical history, hemoglobin, histologic type, LPLR, tumor stage, and node status were not independently associated with sarcopenia.
Validation cohort
Considering sensitivity of PLR as an important outcome measure in this study, we externally validated PLR. Detailed patient characteristics are listed in . The cutoff value of PLR was defined as 116.85. The incidence in the group of sarcopenia was 16.7%. PLR (P=0.035) was significantly higher in patients with sarcopenia. PLR sensitivity of sarco-penia diagnosis was 88.9%.
Table 4 Clinical characteristics of validation cohort (%)
Discussion
This represents the first study to examine the relationship of SIR with sarcopenia in gastric cancer. In our cohort of 670 patients, we observed that greater NLR and PLR were associated with sarcopenia at diagnosis. The findings support the hypothesis that host-related SIR increases the incidence of sarcopenia. Furthermore, there were direct relationships between age, NRS, albumin, BMI, and diabetes with sarcopenia. Inflammation may cause malnutrition status; nevertheless, we suggested that inflammation and malnutrition jointly lead to sarcopenia in this study.Citation31 Sarcopenia may be a good indicator of malnutrition. Malnutrition, particularly protein deficiency, has been reported to cause immune impairment and reduce body strength. Conversely, although a pilot study had shown that the accuracy of LPLR was superior to that of PLR in patients with cancer,Citation22 justifying the inclusion of LPLR as one of our preoperative factors, this measure was found to be irrelevant to sarcopenia (P=0.137).
Age-related factors, inactivity, inappetence, and insufficient nutritional intake are defined as mechanisms that alter skeletal muscle strength.Citation32 Moreover, several lines of evidence show that the host-related inflammatory response reflects the body composition. Another report has suggested that the inflammatory response, previously acknowledged as a marker of prognosis in gastrointestinal cancer patients, is related with the basic characteristics of muscle wastage, for instance, decreased quality of life or increased risk of morbidity and mortality.Citation33 Our study shows that a similar relationship exists in gastric cancer: preoperative elevated NLR and PLR were associated with sarcopenia. In this study, elevated NLR and PLR were observed in half of the sarcopenia patients (57.7%). The finding that inflammation is associated with sarcopenia is consistent with a well-established prior literature on non-metastatic colon carcinoma.Citation21
The close correlation between sarcopenia and short-term and long-term prognosis of gastric cancer highlights the need for early nutritional status assessment.Citation11,Citation12 Given the complex diagnosis of sarcopenia, the presence of motor dysfunction or the lack of clinically acquired computed tomography images will misjudge the nutrition status of gastric cancer patients. Using PLR as primary screening test (a sensitivity of 91.3%) aids with the early, simple, and convenient identification of sarcopenia, which may facilitate the use of therapeutic intervention to ensure a successful perioperative management and postoperative rehabilitation, a high quality of life, and a longer likelihood of survival. Such treatments might include taking anti-inflammatory drugs,Citation34 following a Mediterranean-style diet,Citation35 and/or partaking in regular resistance exercise, which are evidenced to be valid and reliable in keeping and enhancing muscle mass and function in cancer patients, improve life quality, and effectively prolong survival.Citation36–Citation38
This study was strengthened by its large sample size and prospective data collection. However, our study has several limitations. Firstly, we were unable to disentangle the network of two-way relationships among inflammation, sarcopenia, and cancer. Further research is needed to address this issue. Secondly, this was a single institution study. A multicenter prospective study including other regions is essential to overcome this limitation and broaden the generalizability of the study results. Thirdly, we defined 18 as the cutoff point of BMI, by which a group of people with sarcopenic obesity may have been ignored. However, in Asia, the incidence of sarcopenic obesity is low, with only rare individuals being ascertained in this study.
Conclusion
The present study showed that PLR and NLR could be related with sarcopenia in gastric cancer patients. The understanding of their mechanisms should be helpful as potential therapeutic targets. Furthermore, PLR, NLR, and other indicators may aid in distinguishing gastric cancer patients with sarcopenia, which would likely be helpful for further planning of timely nutritional intervention prior to surgery. Together with our findings, additional studies on NLR and PLR measures in combination with other known indicators should be explored to predict the risk of malnutrition in patients with gastric cancer in the future.
Acknowledgments
The authors thank all the participants in this study and the members of our research team. This work was supported by the Science and Technology Incubation Program of Wenzhou Medical University, Zhejiang Province, China (FHY2014039) and Project of Wenzhou Municipal Science and Technology Bureau, Zhejiang Province, China (Y20170104).
Ethics approval and consent to participate
All participants provided their written informed consent, and the protocol for this study was approved by the ethics committee of The First Affiliated Hospital of Wenzhou Medical University (2014 NO.063).
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- KimYEjazASpolveratoGConditional survival after surgical resection of gastric cancer: a multi-institutional analysis of the us gastric cancer collaborativeAnn Surg Oncol201522255756425287440
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E359E38625220842
- EsfahaniASomiMHAsghari JafarabadiMA new score for screening of malnutrition in patients with inoperable gastric adenocar-cinomaJpn J Clin Oncol201747647547928334893
- MorleyJEBaumgartnerRNRoubenoffRMayerJNairKSSarco-peniaJ Lab Clin Med2001137423124311283518
- FuggleNShawSDennisonECooperCSarcopeniaBest Pract Res Clin Rheumatol201731221824229224698
- TakamaTOkanoKKondoAPredictors of postoperative complications in elderly and oldest old patients with gastric cancerGastric Cancer201518365366124874161
- TsujinakaTSasakoMYamamotoSInfluence of overweight on surgical complications for gastric cancer: results from a randomized control trial comparing D2 and extended para-aortic D3 lymphadenec-tomy (JCOG9501Ann Surg Oncol200714235536117146738
- ParkDJLeeHJKimHHYangHKLeeKUChoeKJPredictors of operative morbidity and mortality in gastric cancer surgeryBr J Surg20059291099110215931657
- KimMCKimWKimHHRisk factors associated with complication following laparoscopy-assisted gastrectomy for gastric cancer: a large-scale Korean multicenter studyAnn Surg Oncol200815102692270018663532
- WangSLZhuangCLHuangDDSarcopenia Adversely Impacts Postoperative Clinical Outcomes Following Gastrectomy in Patients with Gastric Cancer: A Prospective StudyAnn Surg Oncol201623255656426668085
- ZhuangCLHuangDDPangWYSarcopenia is an Independent Predictor of Severe Postoperative Complications and Long-Term Survival After Radical Gastrectomy for Gastric Cancer: Analysis from a Large-Scale CohortMedicine (Baltimore)20169513e316427043677
- GrivennikovSIGretenFRKarinMImmunity, inflammation, and cancerCell2010140688389920303878
- BalkwillFMantovaniAInflammation and cancer: back to Virchow?Lancet2001357925553954511229684
- CoussensLMWerbZInflammation and cancerNature2002420691786086712490959
- TempletonAJAceOMcnamaraMGPrognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysisCancer Epidemiol Biomarkers Prev20142371204121224793958
- TempletonAJMcnamaraMGŠerugaBPrognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysisJ Natl Cancer Inst20141066dju12424875653
- ZhouXDuYHuangZPrognostic value of PLR in various cancers: a meta-analysisPLoS One201496e10111924968121
- GuthrieGJCharlesKARoxburghCSHorganPGMcmillanDCClarkeSJThe systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancerCrit Rev Oncol Hematol201388121823023602134
- PangWLouNJinCCombination of preoperative platelet/lymphocyte and neutrophil/lymphocyte rates and tumor-related factors to predict lymph node metastasis in patients with gastric cancerEur J Gastroenterol Hepatol201628549350226854795
- FelicianoEMCKroenkeCHMeyerhardtJAAssociation of Systemic Inflammation and Sarcopenia With Survival in Nonmetastatic Colorectal Cancer: Results From the C SCANS StudyJAMA Oncol2017312e17231928796857
- SeretisCSeretisFLagoudianakisEPolitouMGemenetzisGSalemisNSEnhancing the accuracy of platelet to lymphocyte ratio after adjustment for large platelet count: a pilot study in breast cancer patientsInt J Surg Oncol201220127
- Cruz-JentoftAJBaeyensJPBauerJMSarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older PeopleAge Ageing201039441242320392703
- KondrupJRasmussenHHHambergOStangaZAd Hoc ESPEN Working Group, Ad Hoc ESPEN Working GroupNutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trialsClin Nutr200322332133612765673
- SanoTAikoTNew Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised pointsGastric Cancer20111429710021573921
- ChenLKLiuLKWooJSarcopenia in Asia: consensus report of the Asian Working Group for SarcopeniaJ Am Med Dir Assoc20141529510124461239
- MourtzakisMPradoCMLieffersJRReimanTMccargarLJBaracosVEA practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine careAppl Physiol Nutr Metab2008335997100618923576
- LieffersJRBatheOFFassbenderKWingetMBaracosVESarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgeryBr J Cancer2012107693193622871883
- PradoCMLieffersJRMccargarLJPrevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based studyLancet Oncol20089762963518539529
- FlussRFaraggiDReiserBEstimation of the Youden Index and its associated cutoff pointBiom J200547445847216161804
- GondaKShibataMSatoYElevated neutrophil-to-lymphocyte ratio is associated with nutritional impairment, immune suppression, resistance to S-1 plus cisplatin, and poor prognosis in patients with stage IV gastric cancerMol Clin Oncol2017761073107829285377
- FearonKArendsJBaracosVUnderstanding the mechanisms and treatment options in cancer cachexiaNat Rev Clin Oncol2013102909923207794
- RichardsCHRoxburghCSMacmillanMTThe relationships between body composition and the systemic inflammatory response in patients with primary operable colorectal cancerPLoS One201278e4188322870258
- EwaschukJBAlmasudAMazurakVCRole of n-3 fatty acids in muscle loss and myosteatosisAppl Physiol Nutr Metab201439665466224869970
- YarlaNSPolitoAPelusoIEffects of Olive Oil on TNF-α and IL-6 in Humans: Implication in Obesity and FrailtyEndocr Metab Immune Disord Drug Targets2018181637429165098
- FochtBCClintonSKDevorSTResistance exercise interventions during and following cancer treatment: a systematic reviewJ Support Oncol2013112456023967493
- HardeeJPPorterRRSuiXThe effect of resistance exercise on all-cause mortality in cancer survivorsMayo Clin Proc20148981108111524958698
- StrasserBSteindorfKWiskemannJUlrichCMImpact of resistance training in cancer survivors: a meta-analysisMed Sci Sports Exerc201345112080209023669878