Abstract
Background
Radical resection is the treatment of choice for hepatocellular carcinoma (HCC). However, even with this treatment, HCC prognosis and the efficacy of current predictive models for such patients remain unsatisfactory. Here, we describe an accurate and easy-to-use prognostic index for patients with HCC who have undergone curative resection.
Methods
The study population comprised of 1,041 patients with HCC who underwent curative resection at Zhongshan Hospital. This population was reduced to 768 patients who were treated in 2012 analyzed as the training cohort and 273 patients treated in 2007 who were used as a validation cohort.
Results
The lactic dehydrogenase to albumin ratio (LAR) was identified as a significant prognostic index for both overall survival and recurrence-free survival in two independent cohorts. The optimal cutoff value for LAR was determined to be 5.5. The C-index of LAR was superior to other inflammatory scores and serum parameters. This biomarker was also shown to be a stable predictive index in the validation cohort. The new nomogram combining LAR with the Barcelona Clinic Liver Cancer staging system had an improved ability to discriminate overall survival and recurrence-free survival. Nomogram predictions were consistent with observations based on calibration and decisive curve analysis in both independent cohorts.
Conclusion
LAR is a novel, convenient, reliable, and accurate prognostic predictor in patients with HCC undergoing curative resection. Our results suggest the recommendation of LAR to be used in routine clinical practice.
Background
Hepatocellular carcinoma (HCC) is the second most common cause of cancer-related death and the fifth most frequently diagnosed cancer.Citation1 Despite curative resection, metastasis and recurrence occur in 60%–70% of patients with HCC within 5 years of surgery.Citation2 However, careful selection of personalized treatment strategies has shown promising results in some patients.Citation3 Therefore, identification of a reliable prognostic index (PI) that can be applied in routine clinical practice for personalized therapy is needed.
Current staging systems used for predicting cancer prognosis include the TNM system, which depends solely on pathological characteristics,Citation4 the Barcelona Clinic Liver Cancer (BCLC) index,Citation5 the Chinese University Prognostic Index,Citation6 the Cancer of the Liver Italian Program (CLIP) score,Citation7 and the Japanese Integrated Score.Citation8 Various markers of systemic inflammatory response commonly used include: neutrophil to lymphocyte ratio (NLR),Citation9 platelet to lymphocyte ratio (PLR),Citation10 and the Glasgow Prognostic Score (GPS).Citation11 However, these scoring systems are cumbersome and their efficacy is controversial as they are not specifically formulated for postoperative prognostic prediction, greatly limiting their application in clinical practice for patients with HCC. A more reliable and easy-to-use index is desirable for HCC.
Lactate dehydrogenase (LDH), an enzyme released by necrotic cells, is a metabolic enzyme involved in anaerobic glycolysis regulated by the PI3K/Akt/mTOR pathway.Citation12 Accumulating evidence has indicated the link between LDH levels, tumor hypoxia, and tumor angiogenesis plays a role in the development of cancer.Citation13–Citation15 HIF-1, a reliable biomarker of hypoxia that is associated with LDH, is regulated by oxidative stress induced by the overproduction of reactive oxygen species.Citation16–Citation18 In order to survive in a hypoxic environment, tumor cells exploit oxidative stress ectopically, activating glycolysis to compensate for their reduced energy supply.Citation19 Additionally, elevated serum LDH levels are an independent risk factor for poor prognosis in several cancers including HCC, gastric carcinoma, lung cancer, colorectal cancer, nasopharyngeal carcinoma, and breast cancer.Citation20–Citation24 Elevated serum LDH levels have been shown to be involved in cancer pathogenesis via inflammation;Citation25–Citation27 conversely, lactate dehydrogenase inhibitors can reverse inflammation-induced changes in cancer cells.Citation28,Citation29 Increased LDH levels alone are therefore a poor prognostic factor in patients with HCC.
Serum albumin (ALB), which is produced in the liver, maintains osmotic pressure and functions as a carrier transporting various metabolic substances. Hypoalbuminemia is in indicator of malnutrition, which is associated with poor overall survival (OS) and high recurrence rates in patients with gastric, colorectal, pancreatic, lung, ovarian, breast, and liver cancers.Citation30,Citation31 Hypoalbuminemia is also closely linked to chronic inflammation. Additionally, ALB is associated with antioxidant activity, stabilization of cell growth, and DNA replication, unlike LDH.Citation32,Citation33
Elevated LDH is not only associated with hypoxia and tumor angiogenesis but also a marker of oxidative stress and inflammation, which are indicative of an elevated cancer risk and poor prognosis. Decreased ALB levels suggest impaired liver function, malnutrition, severe inflammation, and poor antioxidant capacity. Based on these findings, we sought to determine whether the ratio between LDH and ALB (LAR) could be a reasonable predictor of prognosis in postresection HCC patients.
Despite similar prognostic stratification, patients have shown different outcomes, underscoring the need to develop an individualized predictive system. A nomogram is a statistical diagram that can be used to predict prognosis and can be applied in individual evaluations. While other predictive models determine prognosis based on risk groupings, nomograms provide a more individualized prediction of outcome based on a combination of variables. Currently, different standard nomograms are used to assess various cancer types.Citation34–Citation36
The aim of this study was to assess the prognostic value of LAR in patients with HCC after curative resection. In addition, new nomograms were developed to incorporate the LAR into the BCLC staging system for survival outcome predictions for patients with HCC.
Methods
Patients and study design
A total of 1,041 patients with HCC who received curative therapy in Zhongshan Hospital, Fudan University, were included in the study. There were 768 patients in 2012 as the training cohort, and 273 patients in 2007 as the validation cohort. The inclusion criteria were as follows: 1) patients without any preoperative anticancer therapy; 2) exact pathological diagnosis of HCC; 3) radical resection, defined as removal of the tumor without residual cancer, and a cut surface free of cancer by histological examination; 4) complete clinicopathologic characteristics and follow-up data; 5) Child–Pugh score of I was selected (to eliminate fluctuations in serum ALB caused by poor liver function); and 6) no evidence of extrahepatic metastasis or primary cancer of other organs. The study protocol was approved by the Clinical Research Ethics Committee of Zhongshan Hospital, and all patients provided written informed consent.
Follow-up
The follow-up procedure was described in our previous study.Citation37 Computed tomography and magnetic resonance imaging were used for examination in cases of intrahepatic recurrence or distal metastasis. Recurrence-free survival (RFS) was defined as the time interval between the date of operation and the time when recurrence was first identified. OS was defined as the time interval from the date of surgery to the date of death. For patients without any sign of an event, the last follow-up data constituted the terminal record.
Statistical analysis
Statistical analysis was performed using SPSS version 21 (IBM Corporation, Armonk, NY, USA), and the Mann– Whitney U test was used for the comparison between two independent groups. Associations between variables were analyzed using the Pearson’s chi-squared test. The survival curves were generated using the Kaplan–Meier method, and comparisons were made using the log-rank test. Univariate and multivariate analyses of independent prognostic factors were performed using the Cox proportional hazards model. The optimal cutoff values for LAR were determined using X-tile version 3.6.1 (Yale University, New Haven, CT, USA). A nomogram was developed by R version 3.0.2 (The R Foundation, Vienna, Austria).
Results
Demographic and clinicopathological patient profiles
A total of 1,041 patients were enrolled in this study. Detailed clinicopathological characteristics of patients in the training and validation cohorts are listed in . There were significant differences between the two cohorts in the following characteristics: age, serum LDH, total bilirubin (TBIL), ALB, LAR, PLR, NLR, GPS, PI, tumor thrombus, tumor capsule, and differentiation, BCLC, and CLIP staging systems. The last follow-up data was collected on December 20, 2016. In the training cohort, the median follow-up time was 49 months (range, 2–66 months), and the 1-, 3-, and 5-year OS rates were 95.3%, 78.8%, and 67.4%, respectively. RFS rates for the same periods were 83.7, 56.6%, and 41.9%, respectively. In the validation cohort, the median follow-up time was 53 months (range, 2–72 months), and the 1-, 3-, and 5-year OS rates were 89.4%, 72.2%, and 59.2%, respectively. RFS rates were 77.1%, 62.1%, and 43.4%, respectively.
Table 1 Demographic and clinical characteristics
Relationship between LAR and clinicopathological characteristics in the training cohort
The optimal cutoff value of LAR in terms of survival prediction was 5.5 when analyzed by X-tile. Patients with a LAR level ≥5.5 (n=369) were assigned to the high-risk group, and the remaining patients were assigned to the low-risk group (n=399). A high LAR was associated with advanced BCLC stage and high CLIP score (P<0.01 for both). LAR was positively associated with AFP, GGT, ALT, tumor thrombus, tumor size, presence of microvascular invasion (MVI), and cancer cell differentiation, whereas there was no association with lymph node metastasis or tumor number. The LAR was positively related to the level of inflammatory indexes such as CRP, PLR, Prognostic Nutritional Index, NLR, and GPS ().
Table 2 The correlation between clinicopathologic characters and LAR in the training cohort
Predictive factors for prognosis and recurrence in the training cohort
Univariate analysis identified LAR as a prognostic predictor of OS and RFS (). In addition, NLR (hazard ratio [HR] =2.024, P<0.001), LAR (HR =1.905, P=0.006), and tumor-associated characteristics including multiple tumors (HR =1.620, P=0.005), tumor thrombus (HR =1.765, P=0.014), presence of MVI (HR =1.660, P=0.001), BCLC stage (HR =1.918, P<0.001), and CLIP score (HR =2.210, P<0.001) were identified as significant independent factors affecting OS (). Increased serum GGT (HR =1.302, P=0.020) was identified as a significant independent predictor of RFS. NLR (HR =1.443, P=0.001), LAR (HR =1.846, P=0.002), multiple tumors (HR =1.702, P<0.001), tumor thrombus (HR =1.665, P=0.008), MVI (HR =1.617, P<0.001), BCLC stage (HR =1.580, P<0.001), and CLIP score (HR =1.615, P<0.001) were significant factors for RFS.
Table 3 Univariate and multivariate analyses for OS and RFS in the training cohort
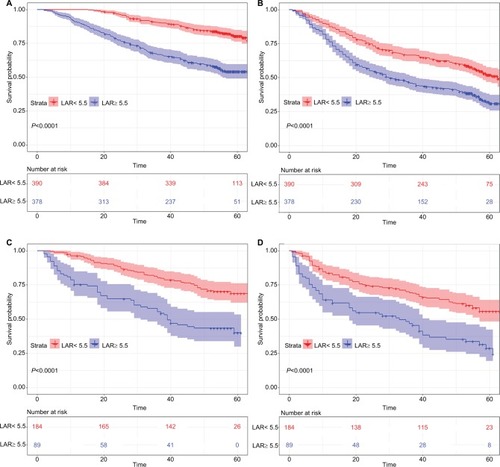
Figure 1 Kaplan–Meier survival curves for patients in the research classified by LAR.
Notes: OS curve (A, C) and RFS curve (B, D) for patients with HCC in training cohort and validation cohort respectively.
Abbreviations: HCC, hepatocellular carcinoma; LAR, lactic dehydrogenase to albumin ratio; OS, overall survival; RFS, recurrence-free survival.
Comparison between LAR and other predictive models
The C-index of nomograms for OS and RFS showed that LAR values were 0.648 and 0.586, respectively, which was superior to those of LDH (0.621 and 0.56, respectively) and ALB (0.530 and 0.504, respectively). The BCLC staging system had C-index values of 0.656 and 0.607 for OS and RFS, respectively, as well as respective CLIP scores C-index values of 0.629 and 0.591, respectively ().
Table 4 Comparison of C-index in OS and RFS in the training cohort
Validation cohort
Univariate analysis showed that the LAR was significantly associated with prognosis regarding OS and RFS (P<0.001) (). Multivariate analysis confirmed that the LAR was a significant independent predictor of OS and RFS. Patients with a high LAR were twice as likely to have a poor prognosis (P=0.005, HR =2.145) and 1.8 times more likely to experience recurrence (P=0.008, HR =1.870) (). The LAR had a C-index of 0.618 for OS and 0.594 for RFS, suggesting that it is a stable predictive index in the validation cohort ().
New nomogram for survival integrating the LAR into the BCLC staging system in two independent cohorts
New nomograms incorporating the LAR into the BCLC staging system for OS and RFS were established in . The C-index of the nomogram was 0.713, which was higher than that of BCLC (0.656) and LAR (0.648) alone for OS in the training cohort. For the prediction of RFS, the C-index of the nomogram was 0.637, which was higher than that of BCLC (0.607) and LAR (0.586). The C-index values of 0.704 and 0.683 for OS and RFS, respectively, indicated that the nomogram fit well in the validation cohort ( and S2).
Figure 2 Prognostic nomogram, calibration curve, and DCA.
Notes: Survival nomogram for patients with HCC to predict 1-, 3-, and 5-year OS and RFS (A for OS and B for RFS). The calibration curve for predicting OS of HCC patients at 3-year (C, G) and 5-year (D, H); predicting RFS at 2-years (E, I) and 3-years (F, J) in the training cohort and validation cohort respectively. Decision curve analysis described the clinical benefit in pairwise comparisons between integrated nomogram and BCLC stage. Nomogram is compared against BCLC stage in terms of 4-year OS (K, O), 5-year OS (L, P), 2-year RFS (M, Q), and 3-year RFS (N, R) in the training and validation cohorts respectively.
Abbreviations: BCLC, Barcelona Clinic Liver cancer; DCA, decision curve analysis; HCC, hepatocellular carcinoma; OS, overall survival; RFS, recurrence-free survival; LAR, lactic dehydrogenase to albumin ratio.
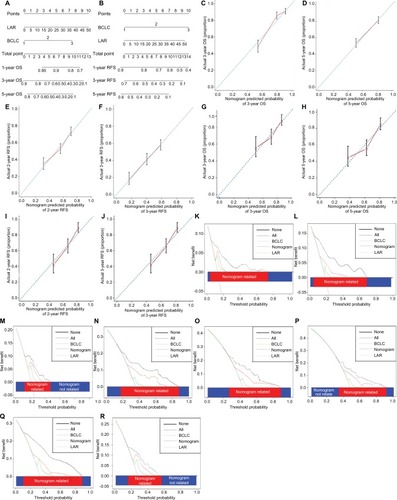
In the training cohort, the calibration curve showed good agreement between the nomogram prediction and actual observations in terms of 3-, 5-year OS (). Compared to actual observations, nomogram predictions were consistent in predicting survival at 3 and 5 years in terms of the calibration external validation curve for OS in the validation cohort (). In addition, the calibration curve confirmed the great consistency between prediction and actual observation for RFS at 2 and 3 years after curative resection in both the training cohort and validation cohort ().
The predictive ability of the nomogram in the decision curve analysis
Decision curve analysis is a novel method to evaluate the clinical net benefit of predictive models.Citation38 Our nomogram showed better net benefits with a wider range of threshold probability than the BCLC and LAR alone for OS at 4 years (), 5 years () after operation in the decision curve analysis of the two independent cohorts. And, it was also true for RFS at 2 years () and 3 years () after operation in this research.
Discussion
The present study identified and characterized LAR as an effective prognostic predictor that can be conveniently derived from preoperative serum LDH and ALB levels for use in patients with HCC who have undergone curative resection. New nomograms incorporating LAR into the BCLC staging system were generated. These nomograms were evaluated by calibration curve and decision curve analysis in two independent cohorts and showed a high discrimination ability.
Tumor inflammation and hypoxia are closely related; inflammation can be induced by hypoxia, conversely inflamed lesions can promote hypoxia.Citation39,Citation40 LDH, a metabolic enzyme, is clinically relevant to tumor hypoxia, tumor angiogenesis, and pathogenesis of inflammation.Citation13,Citation26 High levels of serum ALB are associated with antioxidant activity, whereas low levels are linked to chronic inflammation and malnutrition.Citation30,Citation33 Here, we used LAR, the ratio of LDH to ALB, as a new prognostic index for patients with HCC.
Our results indicated that a high LAR was closely related to patient clinicopathological characteristics, including advanced BCLC stage, a high CLIP score, tumor thrombus, large tumor size, MVI, and cancer cell differentiation. This suggests that the presence of a systemic inflammatory response is predictive of an aggressive clinical phenotype, which is consistent with previous studies.Citation41,Citation42 LAR was identified as a significant independent predictive factor of OS and RFS in two independent patient cohorts. These results, together with our previous findings, confirm the role of inflammation in the development and prognosis of cancer.Citation43,Citation44
The role of inflammation in the pathogenesis and progression of HCC is well defined.Citation45,Citation46 However, to the best of our knowledge, inflammation indexes are not included in routine clinical staging systems such as the BCLC staging system and CLIP scores. In addition, the heterogeneity of HCC makes predictive models for individual patients necessary. We propose that our nomogram integrating the LAR and BCLC solves both of these shortcomings. With an elevated C-index, this newly designed nomogram provides increased discriminatory ability in terms of OS and RFS. Our nomogram was tested by internal and external validation with two independent HCC patient cohorts. In the decision curve analysis, the nomogram had a wider range of threshold probability and had a better net benefit for patients.
The present study had several limitations that should be noted. First, this was a single institution, retrospective study based in People’s Republic of China. Second, the study focused only on patients with Child–Pugh I HCC who underwent curative resection. It is also necessary to point out that the majority patients involved in this study also had hepatitis B virus-related disease. At present, further evidence is required to validate our nomogram as appropriate for nonBnonC or hepatitis C virus patients. Finally, it remains unclear whether this nomogram can be applied to patients who receive treatment other than curative resection. A multicenter study including patients with advanced disease managed with different therapeutic strategies is necessary to confirm the results outlined in this report.
Conclusion
LAR is a novel, convenient, reliable, and accurate prognostic predictor of OS and RFS in patients with HCC who have undergone curative resection therapy. Nomograms integrating LAR with the BCLC system demonstrated better predictive ability and increased discriminatory capacity in terms of survival prediction.
Acknowledgments
This abstract of this paper was presented at the 2018 APPLE Conference as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Liver Cancer. This work was in part supported by National Key Sci-Tech Special Project of China (Grant No. 2012ZX10002010-001/002); the National Natural Science Foundation of China (Grant No. 81302102); Research Programs of Science and Technology Commission Foundation of Shanghai (Grant No. 13CG04, 16DZ0500301); National Natural Science Foundation of China (Grant No. 81772510); National research Programs of Science and Technology Commission Foundation (Grant No. 2017YFC0908101); Research Programs of Science and Technology Commission Foundation of Shanghai (Grant No. 15ZR1406900); and Research Programs of Science and Technology Commission Foundation of Shanghai (Grant No. 18XD1401100).
Supplementary materials
Table S1 Univariate and multivariate analyses for OS and RFS in the validation cohort
Table S2 Comparison of C-index in OS and RFS prediction in the validation cohort
Disclosure
The authors report no conflicts of interest in this work.
References
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E3598625220842
- MaluccioMCoveyARecent progress in understanding, diagnosing, and treating hepatocellular carcinomaCA Cancer J Clin201262639439923070690
- KudoMFinnRSQinSLenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trialLancet2018391101261163117329433850
- LiuCDuanLGLuWSPrognosis evaluation in patients with hepatocellular carcinoma after hepatectomy: comparison of BCLC, TNM and Hangzhou criteria staging systemsPLoS One201498e10322825133493
- PinatoDJSharmaRAllaraEThe ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinomaJ Hepatol201766233834627677714
- ChanSLJohnsonPJMoFInternational validation of the Chinese university prognostic index for staging of hepatocellular carcinoma: a joint United Kingdom and Hong Kong studyChin J Cancer2014331048149125223914
- LiuPHHsuCYHsiaCYPrognosis of hepatocellular carcinoma: Assessment of eleven staging systemsJ Hepatol201664360160826551516
- TannusRKAlmeida-CarvalhoSRLoureiro-MatosCAEvaluation of survival of patients with hepatocellular carcinoma: A comparative analysis of prognostic systemsPLoS One2018134e019492229617435
- TempletonAJPezaroCOmlinASimple prognostic score for metastatic castration-resistant prostate cancer with incorporation of neutrophil-to-lymphocyte ratioCancer2014120213346335224995769
- ZhaoQTYuanZZhangHPrognostic role of platelet to lymphocyte ratio in non-small cell lung cancers: A meta-analysis including 3,720 patientsInt J Cancer2016139116417026915723
- PinatoDJStebbingJIshizukaMA novel and validated prognostic index in hepatocellular carcinoma: the inflammation based index (IBI)J Hepatol20125751013102022732513
- DemeDTelekesAA laktátdehidrogenáz (LDH) prognosztikai jelentősége az onkológiában. [Prognostic importance of lactate dehydrogenase (LDH) in oncology]Orv Hetil20171585019771988 Hungarian29226713
- BakLKSchousboeAMisconceptions regarding basic thermodynamics and enzyme kinetics have led to erroneous conclusions regarding the metabolic importance of lactate dehydrogenase isoenzyme expressionJ Neurosci Res201795112098210228150419
- SundstrømTEspedalHHarterPNMelanoma brain metastasis is independent of lactate dehydrogenase A expressionNeuro Oncol201517101374138525791837
- KoukourakisMIGiatromanolakiAPanteliadouMLactate dehydrogenase 5 isoenzyme overexpression defines resistance of prostate cancer to radiotherapyBr J Cancer201411092217222324714743
- BelaidiEMorandJGrasEPépinJLGodin-RibuotDTargeting the ROS-HIF-1-endothelin axis as a therapeutic approach for the treatment of obstructive sleep apnea-related cardiovascular complicationsPharmacol Ther201616811127492897
- LuHLiXLuoZCetuximab reverses the Warburg effect by inhibiting HIF-1-regulated LDH-AMol Cancer Ther201312102187219923920275
- HorakPCrawfordARVadysirisackDDNegative feedback control of HIF-1 through REDD1-regulated ROS suppresses tumorigenesisProc Natl Acad Sci USA2010107104675468020176937
- SimJCowburnASPalazonAThe Factor Inhibiting HIF Asparaginyl Hydroxylase Regulates Oxidative Metabolism and Accelerates Metabolic Adaptation to HypoxiaCell Metab2018274898913.e729617647
- MuchtarEDispenzieriALacyMQElevation of serum lactate dehydrogenase in AL amyloidosis reflects tissue damage and is an adverse prognostic marker in patients not eligible for stem cell transplantationBr J Haematol2017178688889528699650
- XieHHanaiJRenJGTargeting lactate dehydrogenase–a inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cellsCell Metab201419579580924726384
- GiampieriRPuzzoniMDanieleBFirst-line FOLFIRI and bevacizumab in patients with advanced colorectal cancer prospectively stratified according to serum LDH: final results of the GISCAD (Italian Group for the Study of Digestive Tract Cancers) CENTRAL (ColorEctalavastiNTRiAlLdh) trialBr J Cancer201711781099110428926529
- ZhaoDZouSWLiuYLysine-5 acetylation negatively regulates lactate dehydrogenase A and is decreased in pancreatic cancerCancer Cell201323446447623523103
- LiuXMengQHYeYHildebrandtMAGuJWuXPrognostic significance of pretreatment serum levels of albumin, LDH and total bilirubin in patients with non-metastatic breast cancerCarcinogenesis201536224324825524924
- YuSLXuLTQiQSerum lactate dehydrogenase predicts prognosis and correlates with systemic inflammatory response in patients with advanced pancreatic cancer after gemcitabine-based chemotherapySci Rep201774519428345594
- RhoJMInhibition of lactate dehydrogenase to treat epilepsyN Engl J Med2015373218718926154793
- BarbosaCVSilvaASde OliveiraCVEffects of Sesame (Sesamum indicumL.) Supplementation on Creatine Kinase, Lactate Dehydrogenase, Oxidative Stress Markers, and Aerobic Capacity in Semi-Professional Soccer PlayersFront Physiol2017819628408889
- AugoffKHryniewicz-JankowskaATabolaRLactate dehydrogenase 5: an old friend and a new hope in the war on cancerCancer Lett201535811725528630
- ManerbaMDiILGovoniMRobertiMRecanatiniMDiSGLactate dehydrogenase inhibitors can reverse inflammation induced changes in colon cancer cellsEur J Pharm Sci201796374427622920
- WuNChenGHuHPangLChenZLow pretherapeutic serum albumin as a risk factor for poor outcome in esophageal squamous cell carcinomasNutr Cancer201567348148525706773
- YamashitaKUshikuHKatadaNReduced preoperative serum albumin and absence of peritoneal dissemination may be predictive factors for long-term survival with advanced gastric cancer with positive cytology testEur J Surg Oncol2015411013243226251341
- Garcia-MartinezRAndreolaFMehtaGImmunomodulatory and antioxidant function of albumin stabilises the endothelium and improves survival in a rodent model of chronic liver failureJ Hepatol201562479980625450713
- DasSMarasJSHussainMSHyperoxidized albumin modulates neutrophils to induce oxidative stress and inflammation in severe alcoholic hepatitisHepatology201765263164627775820
- CustodioACarmona-BayonasAJiménez-FonsecaPNomogram-based prediction of survival in patients with advanced oesophagogastric adenocarcinoma receiving first-line chemotherapy: a multicenter prospective study in the era of trastuzumabBr J Cancer2017116121526153528463962
- NecchiASonpavdeGLo VulloSNomogram-based Prediction of Overall Survival in Patients with Metastatic Urothelial Carcinoma Receiving First-line Platinum-based Chemotherapy: Retrospective International Study of Invasive/Advanced Cancer of the Urothelium (RISC)Eur Urol201771228128927726966
- BattersbyNJBouliotisGEmmertsenKJDevelopment and external validation of a nomogram and online tool to predict bowel dysfunction following restorative rectal cancer resection: the POLARS scoreGut201867468869628115491
- YiYHeHWWangJXThe functional impairment of HCC-infiltrating γδ T cells, partially mediated by regulatory T cells in a TGFβ- and IL-10-dependent mannerJ Hepatol201358597798323262246
- FitzgeraldMSavilleBRLewisRJDecision curve analysisJAMA201531344091025626037
- LiXFChenCXiangDMChronic inflammation-elicited liver progenitor cell conversion to liver cancer stem cell with clinical significanceHepatology20176661934195128714104
- ZhangJZhangQLouYHypoxia-inducible factor-1α/interleukin-1β signaling enhances hepatoma epithelial-mesenchymal transition through macrophages in a hypoxic-inflammatory microen-vironmentHepatology20186751872188929171040
- LaiQNicoliniDInostrozaNMA Novel Prognostic Index in Patients With Hepatocellular Cancer Waiting for Liver Transplantation: Time-Radiological-response-Alpha-fetoprotein-INflammation (TRAIN) ScoreAnn Surg2016264578779627429025
- AzizMHSiderasKAzizNAThe Systemic-Immune-Inflammation Index Independently Predicts Survival and Recurrence in Resectable Pancreatic Cancer and its Prognostic Value Depends on Bilirubin Levels: A Retrospective Multicenter Cohort StudyAnn Surg Epub2018112
- MeiZLiuYLiuCTumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysisBr J Cancer201411061595160524504370
- CabillicFCorluARegulation of Transdifferentiation and Retrodifferentiation by Inflammatory Cytokines in Hepatocellular CarcinomaGastroenterology2016151460761527443822
- SaxenaAIzmirlyPMHanSWSerum Biomarkers of Inflammation, Fibrosis, and Cardiac Function in Facilitating Diagnosis, Prognosis, and Treatment of Anti-SSA/Ro-Associated Cardiac Neonatal LupusJ Am Coll Cardiol201566893093926293764
- BrennanCAGarrettWSGut microbiota, inflammation, and colorectal cancerAnnu Rev Microbiol20167039541127607555
