Abstract
Purpose
Improvement in the control of delayed chemotherapy-induced nausea and vomiting (CINV) is needed. There is limited information on antiemetic prophylaxis for patients undergoing low-emetic-risk chemotherapy (LEC), and the optimal antiemetic treatment is not well understood. Therefore, we analyzed the risk factors for delayed CINV to aid in the development of individualized treatments.
Patients and methods
This prospective multicenter study was conducted in 13 hospitals and included patients with solid cancers undergoing LEC. A total of 222 patients were enrolled between September 2013 and November 2014. The participants completed a daily diary for 5 days after the commencement of the first cycle of LEC to describe the daily incidence of CINV (yes/no). Furthermore, the participants described the severity of nausea and the amount of food intake with the help of VAS.
Results
Two hundred and ten patients provided their data that were analyzed using multivariate logistic regression to examine the risk factors for delayed CINV. History of CINV, Eastern Cooperative Oncology Group performance status score ≥1, acute CINV, and single-day antiemetic prophylaxis were identified as independent risk factors for delayed CINV.
Conclusion
The current use of antiemetic prophylaxis according to the recommended guideline appears to effectively control delayed CINV in patients undergoing LEC. Therefore, patients with the abovementioned risk factors should be carefully observed, and their treatment should be adjusted according to their symptoms. The use of multiple-day dexamethasone may be beneficial for those patients who develop acute CINV, especially when it is accompanied by anorexia.
Introduction
CINV is a well-known potential adverse effect of cancer chemotherapy that impairs the patients’ quality of life, including that of patients undergoing LEC.Citation1,Citation2 The control of delayed CINV, a particularly important issue, remains unresolved. In fact, in our previous study, delayed CINV was observed more frequently than that of predicted CINV, and the severity of nausea gradually increased from day 1, peaking on days 4 and 5.Citation3 Both treatment- and patient-related risk factors need to be considered to ensure the optimal control of CINV.Citation4–Citation12 However, until recently, the lack of clinical trials performed in patients treated with LEC has made it difficult to identify patients at risk of CINV. Moreover, antiemetic guidelines recommend the use of a single agent, such as low-dose dexamethasone on the first day of chemotherapy, stating that it is not necessary to administer antiemetics to prevent delayed CINV in patients undergoing LEC; however, this recommendation is not based on the results of clinical trials, rather it reflects a consensus among experts in the field.Citation13–Citation16 Identifying patients at a high risk of delayed CINV while undergoing LEC may enhance the clinical management by health care providers to reduce the incidence and severity of delayed CINV. Therefore, in this study, we aimed to assess both risk factors and a candidate treatment strategy for delayed CINV in patients with solid cancers undergoing LEC.
Patients and methods
Study design
The study design, including patient enrollment, data collection, and treatment, has been described previously.Citation3 Briefly, this prospective, observational, multicenter study was conducted from September 2013 to November 2014 in 13 hospitals affiliated with the National Hospital Organization in Kyushu, Japan. Adult patients beginning LEC were consecutively recruited at the study sites.
This study is registered in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (protocol ID: UMIN000020800).
Patients
Men and women ≥20 years of age who were LEC-naïve and scheduled to undergo at least 1 cycle of a single-day cytotoxic LEC were eligible for inclusion in this study. The intended cancer treatment comprised at least one of the following injectable agents: docetaxel, paclitaxel, gemcitabine, pemetrexed, liposomal doxorubicin, eribulin, and 5-fluorouracil. Patients were excluded from the study if they had undergone treatment with chronic systemic corticosteroid therapy, concurrent abdominal or pelvic radiation therapy, or had undergone LEC within 120 hours (5 days) of initiating chemotherapy. Patients were also excluded if they had brain metastases or had vomited in the 24-hour period preceding chemotherapy initiation.
Patients were provided with a diary before the commencement of chemotherapy and were asked to record their digestion-related symptoms (development and severity of nausea, frequency of vomiting, food intake, and the number of salvage treatments received) each day during a 5-day period after commencing LEC. The incidence of nausea was identified by patients. A 100 mm linear VAS was used to quantify food intake (100 mm, no oral food intake; 0 mm, eating as usual) and severity of nausea (100 mm, worst nausea; 0 mm, no nausea). Before or at the time of the initial chemotherapy treatment, we recorded the following patient information on a case report form: initials, sex, hospital number, date of birth, treatment history, alcohol consumption, history of motion sickness, ECOG performance status, cancer chemotherapy regimen, and antiemetic as well as salvage antiemetic treatments. The patients were asked to send their completed diaries to the Central Office using the preaddressed return envelopes provided. Likewise, the investigators sent their case reports to the Central Office in such return envelopes.
Statistical analyses
Patient demographics, clinical characteristics, and antiemetic treatments prescribed for the acute and delayed phases were summarized using contingency tables. Independent risk factors for delayed CINV incidence (dependent variable) were evaluated using logistic regression analysis with backward elimination method. The following independent factors were included in the model: sex, CINV history, development of acute CINV, opioid use, motion sickness, morning sickness, alcohol consumption, ECOG performance status, antiemetic prophylaxis, LEC other than taxane, and age. The severity patterns of nausea and food intake in relation to the occurrence of acute CINV were evaluated by the transition of the VAS score (without conducting further statistical analysis). All reported P-values corresponded to two-sided tests; and P-values <0.05 were considered statistically significant. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Ethics approval and informed consent
All procedures involving human participants were performed in accordance with the ethical standards of the institutional research committees of each participating institution, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from individual participants, prior to their inclusion in the study.
Results
Participants
In this study, 222 patients were enrolled who were undergoing LEC for the first time. After excluding patients who withdrew within 5 days of undergoing LEC or who did not submit a diary, the data of 210 patients (94.6% of all patients enrolled) were finally analyzed (). summarizes the demographic and clinical characteristics of the 210 patients.
Table 1 Patient demographics and clinical characteristics (n=210)
Figure 1 Enrollment of patients.
Notes: A total of 222 patients were registered, and 211 patients’ diaries were paired with the case report forms and staff reports. One patient who had an incomplete diary was excluded from the analysis; hence, the data of 210 patients (94.6% of all patients registered) were finally analyzed.

Antiemetic treatment
The CINV guidelines recommend the use of single-day anti-emetic agents (e.g., dexamethasone) for patients undergoing LEC.Citation13–Citation16 Such patients should not undergo routine antiemetic prophylaxis during the delayed phase. However, 78 patients (37.1%) received antiemetic agents on multiple days. summarizes the antiemetic treatments prescribed for the acute and delayed phases in the first cycle of LEC.
Table 2 Incidence of delayed CINV
Incidence of delayed CINV
Delayed CINV (of any grade) was reported by 27.3% of the patients who underwent the single-day antiemetic prophylaxis and by 11.5% of the patients who underwent the multiple-day antiemetic prophylaxis. Among patients who developed acute CINV, 68.8% and 33.3% who underwent the single- and multiple-day prophylaxis, respectively, developed delayed CINV (). The antiemetic most commonly used on day 2 or later, for multiple-day prophylaxis was dexamethasone.
Analysis of risk factors
Univariate and multivariate logistic regression analyses were performed to determine the degree of delayed CINV risk associated with various CINV-related factors. The multivariate analysis identified the history of nausea and/or vomiting, ECOG performance status score ≥1, acute CINV, and single-day antiemetic prophylaxis as independent risk factors for delayed CINV ().
Table 3 Risk factors for delayed CINV
Severity of nausea and amount of food intake
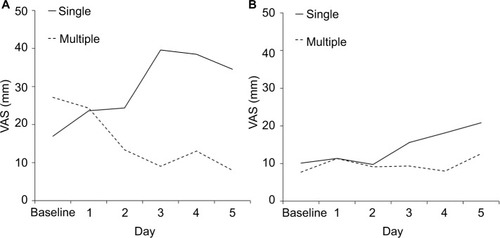
and shows the daily mean VAS scores for severity of nausea and the amount of food intake, respectively, on days 1–5 postchemotherapy. Because the low incidence of vomiting precluded the observation of a visible difference in vomiting, the incidence of nausea alone was assessed. In patients who developed acute CINV, those who underwent multiple-day antiemetic prophylaxis had a lesser reduction in food intake than those who underwent the single-day antiemetic prophylaxis.
Figure 2 Severity of nausea.
Notes: Daily mean visual analog scale (VAS) scores for severity of nausea on days 1–5 after the initiation of low-emetic-risk chemotherapy in patients (A) with and (B) without acute chemotherapy-induced nausea and vomiting. The difference in the severity of nausea is shown between the single- and multiple-day antiemetic prophylaxis groups. VAS (100 mm, worst nausea; 0 mm, no nausea).
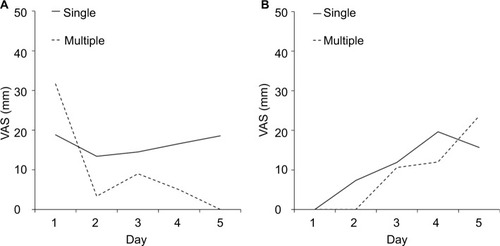
Figure 3 Food intake.
Notes: Daily mean visual analog scale (VAS) scores for food intake during the first 5 days after the initiation of low-emetic-risk chemotherapy in patients (A) with and (B) without acute chemotherapy-induced nausea and vomiting. The difference in VAS-rated food intakes between the single- and multiple-day antiemetic prophylaxis groups is shown. VAS (100 mm, no oral food intake; 0 mm, eating as usual).
Discussion
This study demonstrated both risk factors and a candidate treatment strategy for delayed CINV in patients with solid cancers undergoing LEC. History of CINV, ECOG performance status score ≥1, CINV in the acute phase, and undergoing single-day prophylactic antiemetics were found to be the independent risk factors for delayed CINV in patients undergoing LEC.
We found that younger age was not a risk factor for CINV; this finding is consistent with previous reports.Citation17,Citation18 However, it differs from the widely accepted clinical view that younger patients are more prone to CINV than that of older patients. It is possible that age was not identified as a risk factor in this study because of the age strata bias—the median age of the participants in this study was found to be 64 years.
Single-day antiemetic prophylaxis was identified as a risk factor for delayed CINV. Patients undergoing multiple-day antiemetic prophylaxis experienced delayed CINV less frequently than those undergoing single-day antiemetic prophylaxis. However, the VAS-based evaluation of the severity of nausea revealed that patients experienced only mild nausea; hence, the change of antiemetic treatment, from the single-day to the routine multiple-day prophylaxis, was unnecessary for all patients undergoing LEC. “No significant nausea” has historically been defined as a VAS score <25.Citation18,Citation19 However, a more robust and modern approach would be to use “no nausea” as the primary endpoint, especially in the context of LEC. This is the most patient-centered clinical outcome; this parameter was also used in a recent study.Citation20 In this study, we relied on the information provided by the patients when assessing the incidence of nausea. Therefore, we defined a VAS score of 0 mm as “no nausea.”
Of the patients who developed acute CINV, the incidence of delayed CINV was found to be higher in those undergoing single-day antiemetic prophylaxis than those undergoing multiple-day antiemetic prophylaxis. Therefore, acute CINV is a possible predictor of delayed CINV. Patients who underwent multiple-day antiemetic prophylaxis had less severe nausea and lesser reduction in food intake than patients who underwent single-day antiemetic prophylaxis. Molassiotis et al reported the symptom cluster related to nausea as loss of appetite, dry mouth, feeling drowsy and bloated, and vomiting; nausea, rather than vomiting, was associated with the loss of appetite and dry mouth.Citation21 Olver et al reported that fatigue often accompany chemotherapy-induced nausea.Citation22 In this study, dexamethasone was the most common antiemetic used to prevent delayed CINV. It improves anorexia and fatigue; thus, it may be useful in patients experiencing these adverse effects. Ito et al suggested that administration of dexamethasone on days 1–3, compared to only day 1, reduces the incidences of nausea, anorexia, depression, and fatigue during the delayed phase.Citation23 Thus, in patients who develop acute CINV, dexamethasone may be useful in preventing nausea and anorexia in the delayed phase. However, because dexamethasone has several side effects, including insomnia, indigestion/epigastric discomfort, agitation, and increased appetite,Citation24,Citation25 we need to be mindful of these side effects when using dexamethasone for delayed antiemetic prophylaxis.
In a randomized controlled trial comparing the efficacy of palonosetron plus dexamethasone on day 1 with or without dexamethasone on days 2 and 3 for the prevention of CINV, patients receiving dexamethasone on multiple days experienced a significantly higher incidence of insomnia than that of patients receiving single-day dexamethasone. In another study, the incidence of adverse events potentially attributable to dexamethasone, such as abdominal pain and hiccups, tended to be lower in the single-day than in the multiple-day dexamethasone group, but this difference was not statistically significant.Citation26 Therefore, multiple-day dexamethasone could be a candidate alternative prophylactic antiemetic regimen for patients undergoing LEC. However, randomized comparative studies with large patient populations are needed to confirm the efficacy of the salvage treatment. Hesketh et al demonstrated that palonosetron was well tolerated and effectively prevented CINV in both acute and delayed phases in patients with the history of CINV.Citation27 Although palonosetron is a useful antiemetic treatment option, it is expensive and does not increase the appetite or improve fatigue.
Breakthrough CINV is also an important issue. In this study, rescue antiemetics were administered to 28.9% of the patients with breakthrough CINV during the delayed phase, while metoclopramide was prescribed most frequently in this study (data not shown). Guidelines on breakthrough CINV recommend the use of antiemetics with a different mechanism of action from those used as initial prophylaxis (e.g., dopamine receptor antagonists, glucocorticoids, and antipsychotic or antianxiety agents) or a first generation 5-hydroxytryptamine 3 receptor antagonist different from that used as initial prophylaxis.Citation13–Citation16 However, the recommended treatment is unable to effectively control the breakthrough CINV.Citation28 Navari et al reported that olanzapine was significantly better than metoclopramide in controlling breakthrough CINV in patients undergoing HEC.Citation29 As reported, the repeated use of rescue palonosetron is useful in controlling breakthrough CINV in HEC or moderate-emetic-risk chemotherapy, even when it was already used as prophylaxis.Citation30 However, the repeated use of palonosetron does not appear to be reasonable in LEC from an economic point of view. The antiemetic treatment for breakthrough CINV is not well established, and optimal antiemetic regimen for breakthrough CINV in LEC is still unclear. Further studies are needed to establish the strategy to prevent and suppress breakthrough CINV. However, the novel strategy, multiple-day dexamethasone, as shown in this study, can be expected to reduce the incidence of breakthrough CINV in the delayed phase.
This study has some limitations. First, its design was neither randomized nor blinded, and the sample size was not very large. Second, more than half of the patients in this study had undergone prior chemotherapy treatment. Compared with chemotherapy-naïve patients, previous chemotherapy might have affected the incidence of CINV among patients in this study. Finally, patients who underwent taxane therapy (e.g., docetaxel or paclitaxel) accounted for ~70% of the study population. Despite these limitations, we believe that the results show the risk factors for delayed CINV in routine clinical practice, as opposed to a controlled trial design, and may therefore, be more realistic.
Conclusion
The current use of antiemetic prophylaxis, according to the recommended guideline, controls the delayed CINV in patients undergoing LEC. However, patients with the identified risk factors (i.e., the history of nausea and/or vomiting, ECOG performance status score ≥1, acute CINV, and single-day antiemetic prophylaxis) should be carefully observed, and treatment should be adjusted according to their symptoms. The use of multiple-day dexamethasone may be beneficial in patients who develop acute CINV, especially when it is accompanied by anorexia.
Abbreviations
| CINV | = | chemotherapy-induced nausea and vomiting |
| ECOG | = | Eastern Cooperative Oncology Group |
| HEC | = | high-emetic-risk chemotherapy |
| LEC | = | low-emetic-risk chemotherapy |
| VAS | = | visual analog scale |
Acknowledgments
We thank the study participants and their families as well as the support personnel. This study included the following 13 medical institutions: the National Kyushu Medical Center (Fukuoka, Fukuoka), National Kyushu Cancer Center (Fukuoka, Fukuoka), National Hospital Organization (NHO) Fukuoka National Hospital (Fukuoka, Fukuoka), NHO Kokura Medical Center (Kitakyushu, Fukuoka), NHO Saga National Hospital (Saga, Saga), NHO Ureshino Medical Center (Ureshino, Saga), NHO Nagasaki Medical Center (Omura, Nagasaki), NHO Kumamoto Medical Center (Kumamoto, Kumamoto), NHO Kumamoto Saishunso National Hospital (Koushi, Kumamoto), NHO Kumamoto Minami National Hospital (Uki, Kumamoto), NHO Beppu Medical Center (Beppu, Oita), NHO Miyakonojo Medical Center (Miyakonojo, Miyazaki), and NHO Kagoshima Medical Center (Kagoshima, Kagoshima). Funding for this study was provided by the Policy-Based Medical Service Foundation. The Foundation was not involved in the research design or manuscript writing.
Data availability
All datasets supporting the results reported in this article are kept by the corresponding author and can be provided upon reasonable request.
Disclosure
The authors report no conflicts of interest in this work.
References
- GrunbergSMOsobaDHeskethPJEvaluation of new antiemetic agents and definition of antineoplastic agent emetogenicity--an updateSupport Care Cancer2005132808415599601
- Bloechl-DaumBDeusonRRMavrosPHansenMHerrstedtJDelayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatmentJ Clin Oncol200624274472447816983116
- HayashiTShimokawaMMiyoshiTA prospective, observational, multicenter study on risk factors and prophylaxis for low emetic risk chemotherapy-induced nausea and vomitingSupport Care Cancer20172592707271428341971
- du BoisAMeerpohlHGVachWKommossFGFenzlEPfleidererACourse, patterns, and risk-factors for chemotherapy-induced emesis in cisplatin-pretreated patients: a study with ondansetronEur J Cancer1992282–34504571534250
- HeskethPNavariRGroteTDouble-blind, randomized comparison of the antiemetic efficacy of intravenous dolasetron mesylate and intravenous ondansetron in the prevention of acute cisplatin-induced emesis in patients with cancer. Dolasetron Comparative Chemotherapy-induced Emesis Prevention GroupJ Clin Oncol1996148224222498708713
- OsobaDZeeBPaterJWarrDLatreilleJKaizerLDeterminants of postchemotherapy nausea and vomiting in patients with cancer. Quality of Life and Symptom Control Committees of the National Cancer Institute of Canada Clinical Trials GroupJ Clin Oncol19971511161238996132
- PaterJSlametLZeeBOsobaDWarrDRusthovenJInconsistency of prognostic factors for post-chemotherapy nausea and vomitingSupport Care Cancer1994231611668032701
- PolleraCFGiannarelliDPrognostic factors influencing cisplatin-induced emesis. Definition and validation of a predictive logistic modelCancer1989645111711222667749
- Persistence of efficacy of three antiemetic regimens and prognostic factors in patients undergoing moderately emetogenic chemotherapyItalian Group for Antiemetic ResearchJ Clin Oncol1995139241724267666102
- RoilaFBoschettiETonatoMPredictive factors of delayed emesis in cisplatin-treated patients and antiemetic activity and tolerability of metoclopramide or dexamethasone. A randomized single-blind studyAm J Clin Oncol19911432382422031511
- RoilaFTonatoMBasurtoCAntiemetic activity of high doses of metoclopramide combined with methylprednisolone versus metoclopramide alone in cisplatin-treated cancer patients: a randomized double-blind trial of the Italian Oncology Group for Clinical ResearchJ Clin Oncol1987511411493543234
- TamuraKAibaKSaekiCINV Study Group of JapanTesting the effectiveness of antiemetic guidelines: results of a prospective registry by the CINV Study Group of JapanInt J Clin Oncol201520585586525681876
- OlverIRuhlmannCHJahnF2016 Updated MASCC/ESMO Consensus Recommendations: Controlling nausea and vomiting with chemotherapy of low or minimal emetic potentialSupport Care Cancer201725129730127572335
- HeskethPJKrisMGBaschEAntiemetics: American Society of Clinical Oncology Clinical Practice Guideline UpdateJ Clin Oncol201735283240326128759346
- National Comprehensive Cancer NetworkClinical Practice Guidelines in Oncology (2017) Antiemetics. Version 22017 Available from: https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdfAccessed April 30, 2018
- Japan Society of Clinical OncologyGuidelines for Antiemetics in Oncology 2015TokyoKanehara & Co, Ltd2015 Available from: https://www.kanehara-shuppan.co.jp/books/detail.html?isbn=9784307101745Accessed September 26, 2018 Japanese
- PirriCKatrisPTrotterJBaylissEBennettRDrummondPRisk factors at pretreatment predicting treatment-induced nausea and vomiting in Australian cancer patients: a prospective, longitudinal, observational studySupport Care Cancer201119101549156320811914
- MolassiotisAAaproMDicatoMEvaluation of risk factors predicting chemotherapy-related nausea and vomiting: results from a European prospective observational studyJ Pain Symptom Manage2014475839848.e424075401
- HeskethPJGrunbergSMGrallaRJAprepitant Protocol 052 Study GroupThe oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin--the Aprepitant Protocol 052 Study GroupJ Clin Oncol200321224112411914559886
- NavariRMQinRRuddyKJOlanzapine for the prevention of chemotherapy-induced nausea and vomitingN Engl J Med2016375213414227410922
- MolassiotisAFarrellCBourneKBrearleySGPillingMAn exploratory study to clarify the cluster of symptoms predictive of chemotherapy-related nausea using random forest modelingJ Pain Symptom Manage201244569270322672920
- OlverINEliottJAKoczwaraBA qualitative study investigating chemotherapy-induced nausea as a symptom clusterSupport Care Cancer201422102749275624805911
- ItoYTsudaTMinatogawaHPlacebo-controlled, double-blinded phase III study comparing dexamethasone on day 1 with dexamethasone on days 1 to 3 with combined neurokinin-1 receptor antagonist and palonosetron in high-emetogenic chemotherapyJ Clin Oncol201836101000100629443652
- VardyJChiewKSGalicaJPondGRTannockIFSide effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapyBr J Cancer20069471011101516552437
- NakamuraMIshiguroAMuranakaTA prospective observational study on effect of short-term periodic steroid premedication on bone metabolism in gastrointestinal cancer (ESPRESSO-01)Oncologist201722559260028341762
- KomatsuYOkitaKYukiSOpen-label, randomized, comparative, phase III study on effects of reducing steroid use in combination with PalonosetronCancer Sci2015106789189525872578
- HeskethPJMorrowGKomorowskiAWAhmedRCoxDEfficacy and safety of palonosetron as salvage treatment in the prevention of chemotherapy-induced nausea and vomiting in patients receiving low emetogenic chemotherapy (LEC)Support Care Cancer201220102633263722733373
- TamuraKAibaKSaekiTCINV Study Group of JapanBreakthrough chemotherapy-induced nausea and vomiting: report of a nationwide survey by the CINV Study Group of JapanInt J Clin Oncol201722240541227909835
- NavariRMNagyCKGraySEThe use of olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapySupport Care Cancer20132161655166323314603
- MussoMScaloneRBonannoVPalonosetron (Aloxi) and dexamethasone for the prevention of acute and delayed nausea and vomiting in patients receiving multiple-day chemotherapySupport Care Cancer200917220520918839220
