Abstract
Background
Glucose-6-phosphate isomerase (GPI) is a glycolytic-related enzyme that inter-converts glucose-6-phosphate and fructose-6-phosphate in the cytoplasm. This protein is also secreted into the extracellular matrix by cancer cells and is, therefore, also called autocrine motility factor (AMF).
Methods
To clarify the roles of AMF/GPI in gastric cancer (GC), we collected 335 GC tissues and the corresponding adjacent noncancerous tissues, performed immunohistochemical studies, and analyzed the relationship between AMF/GPI expression and the patients’ clinicopathologic features.
Results
AMF/GPI expression was found to be significantly higher in the GC group than in the corresponding noncancerous tissue group (P<0.001). Additionally, AMF/GPI expression positively associated with a higher TNM stage and poorer prognosis in patients. Through Kaplan–Meier analysis and according to the Oncomine database, we found that AMF/GPI was overexpressed in GC tissues compared to normal mucosa, and the patients with higher AMF/GPI expression had poorer outcomes. We used AMF/GPI-silenced GC cell lines to observe how changes in AMP/GPI affect cellular phenotypes. AMF/GPI knockdown suppressed proliferation, migration, invasion, and glycolysis, and induced apoptosis in GC cells.
Conclusion
These findings suggest that AMF/GPI overexpression is involved in carcinogenesis and promotes the aggressive phenotypes of GC cells.
Introduction
Gastric cancer (GC) is the fifth common cancer and a major cause of cancer-related deaths in worldwide.Citation1 Adenocarcinoma is the major type of GC. According to Lauren’s classification, it can be further subdivided into intestinal and diffuse types.Citation2 GC is often either asymptomatic or it may cause only nonspecific symptoms in its early stages. By the time of diagnosis, the cancer often reached an advanced stage with poor prognosis. When the tumor is invading the serosa or distant metastases, 5-year overall survival (OS) rate is only <5%.Citation3 Therefore, there is a critical challenge to identify new diagnostic and treatment targets to improve GC patients’ outcomes.
Glucose-6-phosphate isomerase (GPI) is encoded by a GPI gene and is a member of the glucose phosphate isomerase protein family. In the cytoplasm, GPI functions as a glycolytic enzyme that interconverts glucose-6-phosphate and fructose-6-phosphate.Citation4 GPI is also named as phosphoglucose isomerase or phosphohexose isomerase, involved in gluconeogenesis and the pentose phosphate pathway.Citation5 Additionally, the same protein is secreted into the extracellular matrix by cancer cells, acts as a tumor-secreted cytokine and angiogenic factor, and is, therefore, called autocrine motility factor (AMF).Citation6 Finally, GPI is also studied as a neurotrophic factor that promotes survival of skeletal motor neurons and sensory neurons and induces immunoglobulin secretion.Citation7
AMF/GPI was widely studied in rheumatoid arthritis as a prognosis and therapy marker.Citation8–Citation10 In cancer research, AMF/GPI is a known biomarker of cancer progression that is found in the serum and urine of patientsCitation11,Citation12 and explored for early diagnosis purposes.Citation13 AMF/GPI is also known as a tumor-secreted cytokine that plays a role in induction of cell migration,Citation14 tumor angiogenesis,Citation15 tumor metastasis,Citation16 cell proliferation,Citation17 and resistance to apoptosis.Citation18 Remarkably, higher AMF/GPI expression was found to correlate with poor prognosis of human GC.Citation19 Additionally, as the second glycolytic enzyme, AMF/GPI is possibly involved in the “Warburg effect”, which proposes that for energy production, tumor cells rely more on glycolysis than on mitochondrial respiration.Citation20 AMF/GPI targeting might offer therapeutic benefits for drug-resistant cancers and allow effective treatment of GC. Therefore, we aimed to clarify the clinicopathologic and prognostic significance of AMF/GPI expression in GC and its role in the regulation of the phenotype of GC cells.
Materials and methods
Study population and immunohistochemistry
Tissue microarrays (TMA) included 335 cases of GC tissues and paired adjacent nonneoplastic mucosa from GC patients who underwent radical gastrectomy at Peking University Beijing Cancer Hospital from January 2004 to December 2012. All patients signed a general informed consent to agree to the use of GC tissues for clinical research. The study was approved by Ethics Committee of Beijing Cancer Hospital. The original data of patients were reviewed in the context of clinicopathologic and follow-up information. Stage of GC was classified according to 2010 TNM classification recommended by the American Joint Committee on Cancer (AJCC, seventh edition). T and N classification were assessed based on the final pathologic result and M classification was determined by surgical findings. The OS was calculated starting from the date of the initial surgery to the time of death, counting death from tumor cause as the end point or the last date of follow-up as the end point, if no event was documented. All patients were followed up until 2012. None of the patients received chemotherapy or radiation therapy prior to surgery. Formalin-fixed and paraffin-embedded tissue blocks were cut into 4-μm-thick sections, which were deparaffinized in xylene and rehydrated. Antigen retrieval was done upon incubation in an EDTA solution (pH 8.0; Santa Cruz Biochemistry, Dallas, TX, USA) for 10 minutes in a pressure cooker. Endogenous peroxidase activity was blocked with a 3% H2O2 solution at 25°C for 15 minutes. After blocking with 5% BSA, the sections were incubated with anti-AMF/GPI antibody (1:800, Bethyl Laboratories, Inc.) at 4°C overnight, followed by washing with PBS and incubation with goat anti-rabbit IgG (1:1000, Santa Cruz Biotechnology, Inc.) at 25°C for 1 hour. All TMA slide sections were quantified by an automated image analysis software. The percentage of cells with positive AMF/GPI immunostaining was estimated from 0 to 100. The staining intensity was scored as “1” (no staining or weakly stained), ‘‘2” (moderate staining), or ‘‘3” (strong staining). The value indicating the percentage of stained cells was multiplied by the corresponding intensity value (intensity×proportion) to obtain a score ranging from 0 to 300: a final value <50 indicated negative expression and ≥50 positive expression.
Cell culture
SGC7901 and BGC823 cells were obtained from the Cell Research Institute (Shanghai, China) and routinely cultured as previously reported.Citation21 All the cells were cultured in DMEM (Sigma-Aldrich Co., St Louis, MO, USA) with 10% FBS (Sigma-Aldrich) and 1% antibiotics at 37°C and 5% CO2 incubator.
shRNA-mediated RNA interference
shRNAs against AMP/GPI were designed with MIT’s siRNA designer (http://sirna.wi.mit.edu/home.php). At least four quadruplexes were designed, and the most effective shRNAs were used for subsequent studies. The sequences of the effective shRNA were provided as follows: 5′-gttgccctgtctactaaca-3′. shRNAs against AMP/GPI and control hairpins were cloned into pSICO R vector. Production of lentiviral particles and transduction of GC cell lines were performed according to protocols from the RNAi consortium (http://www.broadinstitute.org/rnai/trc). Cells were infected with lentiviral constructs expressing shAMP/GPI or shControl as described earlier for 24 hours. Stable cells were selected with puromycin. Then, cells were collected for protein and RNA analysis.
Proliferation assays
Cell proliferation was measured with the MTT assay. Cells were seeded into 96-well plates and incubated for 0, 24, 48, and 72 hours. At each time point, the supernatant was removed, and 10 μL of MTT solution was added to each well for a 4-hour incubation. Then, the supernatant was discarded, and 100 μL dimethyl-sulfoxide was added to each well. Absorbance was measured with a reference wavelength of 450 nm in a microplate reader (Bio-Rad, Hercules, CA, USA). Cell viability was calculated as (absorbance of test sample/absorbance of control)×100%.
Transwell assay
Cell migration/invasion was assayed using transwell chambers (Cell Biolabs, San Diego, CA, USA). Cells (5×104) were harvested and added to the upper chambers with serum-free medium; culture medium containing 10% FBS was added into the lower chambers. For cell invasion assays, the transwell chambers were coated with 100 μL Matrigel before the cells were added. Finally, the cells on the upper surface of the filters were removed. The membranes were fixed with methanol for 10 minutes and stained with 0.5% crystal violet for 10 minutes. The cells on the underside of the filter were photographed and counted in five randomly selected microscopic views.
Flow cytometry
Cells were synchronized in the G0/G1 phase by incubating them in a serum-free medium overnight and then with medium containing 10% FBS for 24 hours. The cells were then trypsinized, washed three times with PBS, and fixed with 70% ethanol for 16 hours at −20°C. The samples were washed with PBS and stained with staining buffer for 15 minutes before flow cytometry (BD Biosciences, San Jose, CA, USA). For apoptotic analysis, the cells were stained using an Annexin V/propidium iodide (PI) double staining kit (DOJINDO, Kumamoto, Japan).
Seahorse XF24 analysis
Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were measured with the Seahorse XF24 analyzer (Seahorse Bioscience, North Billerica, MA, USA). Cells (2×104 cells/well) were cultured in DMEM in Seahorse XF24 plates at 37°C. Each well was sequentially injected with three mitochondrial inhibitors, oligomycin, carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone, and rotenone. OCR and ECAR were calculated with the Seahorse XF-24 software.
Western blotting
Cells were lysed in RIPA lysis buffer (Pierce Biotechnology, Rockford, IL, USA) containing a protease inhibitor cocktail (Roche, Basel, Switzerland), and centrifuged at 15,000×g for 20 minutes. Proteins (100 μg) were separated by 10% SDS-PAGE and transferred onto a 0.45 μm polyvinylidene difluo-ride membrane (Whatman, Germany). The membrane was blocked with blocking buffer (5% skim milk in 0.1% tween tris-buffered saline solution) for 1 hour at 25°C, and then incubated with diluted primary antibodies (1:1000, Bethyl Laboratories, Inc., Montgomery, TX, USA) in the blocking buffer at 4°C overnight. The membrane was then washed with PBS and incubated in horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:1000, Santa Cruz) for 1 hour. Finally, the membrane was developed using a chemiluminescence detection system (Pierce Biotechnology).
Animal studies
Animal studies were performed with the approval of the Ethics Committee of Peking University Beijing Cancer Hospital and conducted according to the institutional and national guidelines. The shControl and shAMF/GPI transfectants of SGC7901 and BGC823 cells (~2×106 cells in 200 μL volume) were injected into both forelegs of BALB/c-nude mice (20 mice total, five mice per group). Tumors were monitored every 3 days and measured using a caliper. The tumor volume was calculated with the formula, V=0.5×L×WCitation2 (with L, length and W, width).
Statistical analysis
The demographic and clinical information of the patients and samples were summarized by descriptive analyses. The chi-squared test was used to evaluate the correlation between AMF/GPI expression and the clinicopathologic characteristics of the patients with GC. Survival curves were obtained with the Kaplan–Meier (KM) method and compared with the log-rank test. The Cox proportional hazard regression model was used to estimate the effect of AMF/GPI expression on mortality risk, ultimately controlling for confounders. The 95% CI for the median time to event was computed. Differences were considered significant at P<0.05. All the statistical analyses were performed using STATA 15.0.
Results
AMF/GPI expression in GC tissues
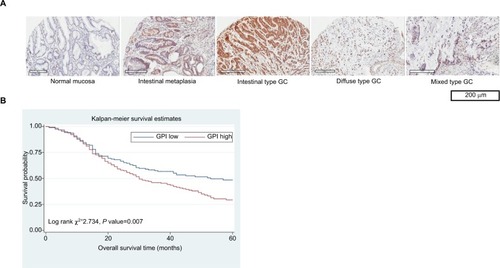
Using the Oncomine database, we found that AMF/GPI expression was significantly higher in GC tissues than in normal tissues (). To confirm this observation, we collected four pairs of fresh GC and adjacent noncancerous tissues and, by Western blot, found a higher AMF/GPI expression in GC tissues than in the paired mucosa (). As shown in , AMF/GPI expression in the GC group was significantly higher than that in adjacent nonneoplastic mucosa (53.73%vs 36.72%, P<0.001).
Table 1 AMF/GPI expression in matched adjacent noncancerous and GC tissues
Figure 1 AMF/GPI expression in primary GC tissues and the survival in patients with GC.
Notes: (A) Expression of AMF/GPI detected by immunohistochemical staining. (B) Kaplan–Meier survival curves of overall survival in all 335 patients of AMF/GPI negative vs AMF/GPI positive.
Abbreviations: AMF, autocrine motility factor; GC, gastric cancer; GPI, glucose-6-phosphate isomerase.
Association between AMF/GPI expression and clinicopathologic features of GC
As shown in , higher AMF/GPI expression was positively associated with lymph node metastasis (P=0.021) and pathologic TNM staging (P=0.022). Additionally, the diffuse-type GC displayed a lower AMF/GPI expression than intestinal-type and mixed-type ones (P=0.033), in agreement with the results from the Oncomine database.
Table 2 Relationship between AMF/GPI expression and clinicopathologic features of gastric cancer patients
Higher AMF/GPI expression predicts worse outcomes in patients with GC
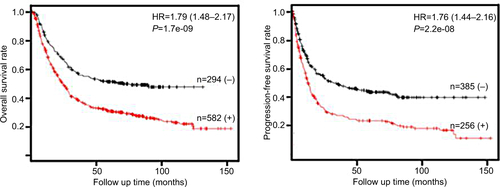
KM analysis showed that among all 335 cases, patients with higher AMF/GPI expression have a lower 5-year OS rate than those with lower AMF/GPI expression (P=0.007 with the log-rank test; ). Also, patients with lower AMF/GPI expression showed a better OS rate in 231 cases with intestinal type (P=0.003 with the log-rank test; ). Additionally, by analyzing data from KM plotter data set, we found that the OS rate and progression-free (PF) survival rate of patients with GC in the AMF/GPI-positive group are poorer than those of patients in the AMF/GPI-negative group (, P<0.05).Citation22 Multivariate Cox regression revealed that there was some evidence to suggest the expression of AMF/GPI to be an independent prognostic factor in patients with GC (HR=1.30, 95% CI=0.96–1.76; ).
Table 3 Predictors of mortality using the univariate and multivariate Cox proportional hazard model among participants (N=335)
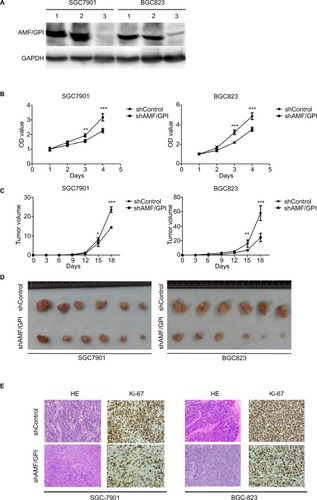
Knockdown of AMF/GPI restrains proliferation and tumorigenesis of GC cells
AMF/GPI knockdown with a lentivirus expressing anti-AMF/GPI shRNA was confirmed by Western blotting (). As shown in , shRNA knockdown groups had a lower cell viability than the shControl ones in both SGC7902 and BGC823 cell lines (P<0.05). In the mouse xenograft models injected with shAMF/GPI- or shControl-infected cells, tumors were smaller and grew slower in the shAMF/GPI groups than the shControl groups (). The xenografts were formalin-fixed paraffin-embedded and sectioned for H&E and Ki-67 staining. As shown in , the xenografts injected with shAMF/GPI-infected cells exhibited lower cellular proliferation levels compared to their respective control ones.
Figure 2 Effects of AMF/GPI knockdown in GC cell growth and proliferation in vitro and in vivo.
Notes: (A) AMF/GPI expression confirmed by Western blotting. Lane 1, wild type; lane 2, scrambled shControl; lane 3, shAMF/GPI. (B) Cell proliferation measured by MTT assay. (C) Tumor growth curve of shControl and shAMF/GPI cells. (D) Pictures show tumor formation in nude mice injected with shControl and shAMF/GPI cells. (E) H&E and Ki-67 stainings of xenograft tumor tissues. Magnification: ×100. The data are shown as mean±SD. *p<0.05, **p<0.01, ***p<0.001.
Abbreviations: AMF, autocrine motility factor; GC, gastric cancer; GPI, glucose-6-phosphate isomerase.
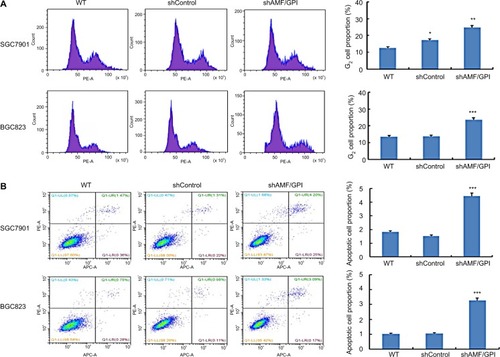
Knockdown of AMF/GPI induced G2/M arrest and apoptosis in GC cells
To clear whether AMF/GPI commands cell growth by influencing DNA synthesis or apoptosis, we monitored cell cycle progression using fluorescence-activated cell sorting. A higher proportion of shAMF/GPI-infected cells both in SGC7901 and BGS 823 were arrested in the G2/M phase of the cell cycle compared to the shControl-infected ones (). In addition, apoptosis was assessed by annexin V and PI staining in in SGC7901 and BGS 823 cells. shAMF/GPI-infected cell groups showed increased apoptosis rate compared to their corresponding control ones ().
Figure 3 AMF/GPI expression is related to G2/M arrest and apoptosis of GC cells.
Notes: (A) GC cell cycle analysis by flow cytometry. (B) Effects of AMF/GPI knockdown in GC cell apoptosis. Bars, mean±SD of three independent experiments.*p<0.05, **p<0.01, ***p<0.001.
Abbreviations: AMF, autocrine motility factor; GC, gastric cancer; GPI, glucose-6-phosphate isomerase; WT, wild type.
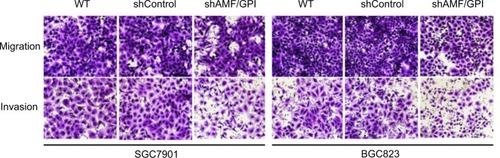
Knockdown of AMF/GPI attenuates GC cell migration and invasion
We had found that AMF/GPI expression was associated with the TNM stage in patients with primary GC. We hypothesized that AMF/GPI augmented the migration and invasion capabilities of GC cells. Therefore, we performed Transwell assays to investigate whether changes in AMF/GPI levels affected the migration and invasion of the cells. We found that migration was inhibited and found a significant reduction of invasion in AMF/GPI-knockdown cells ().
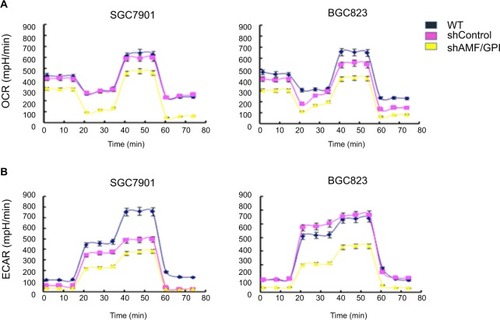
Knockdown of AMF/GPI disrupts the metabolism in GC cells
To determine the metabolic effect of AMF/GPI on GC cells, we performed Seahorse XF24 assays and analyzed OCR and ECAR in each group. We found blockage of these two metabolic indicators in AMF/GPI-silenced cells (). The results confirmed that AMF/GPI knockdown causes a metabolic disturbance in GC cells.
Figure 5 Effects of AMF/GPI knockdown on metabolism in GC cells.
Notes: (A) OCR of shControl and shAMF/GPI cells. (B) ECAR of shControl and shAMF/GPI cells.
Abbreviations: AMF, autocrine motility factor; ECAR, extracellular acidification rates; GC, gastric cancer; GPI, glucose-6-phosphate isomerase; OCR, oxygen consumption rates; WT, wild type.
Discussion
AMF/GPI is a housekeeping enzyme that catalyzes the interconversion between glucose-6-phosphate and fructose-6-phosphate in glycolysis and gluconeogenesis and also acts as a multifunctional cytokine associated with invasion and metastasis in cancer cells.Citation5,Citation6 According to the theory of the “Warburg effect”, cancer cells favor glycolysis for ATP production even in an aerobic environment.Citation20 In previous studies, de Padua et alCitation23 found that GPI knockdown can suppress glucose consumption and lactic acid secretion in human colon cancer and mouse melanoma. Niizeki et alCitation24 proved that hypoxia enhances the expression of AMF/GPI and the motility of human pancreatic cancer cells, and inhibitors of AMF/GPI could counteract these effects. A different study has shown that overexpression of hypoxia-inducible factor 1-alpha (HIF-1α) or a dominant-negative HIF-1α enhances or suppresses the expression of AMF/GPI and cell motility, respectively.Citation25 These findings suggest that AMF/GPI overexpression is involved in the molecular mechanism of carcinogenesis caused by the adaption of cancer cells to hypoxia. In this study, we found that AMF/GPI overexpression was related to the metabolism disruption in GC cells.
A previous study has shown that AMF/GPI binds to HER2, and triggers HER2 phosphorylation and metalloprotease-mediated ectodomain shedding, and activation of the phosphoinositide 3-kinase and mitogen-activated protein kinase (MAPK) signaling, finally interfering with the ability of trastuzumab to inhibit breast carcinoma cell growth.Citation26 Another study has found that an anti-AMF/GPI monoclonal antibody (9A-4H) significantly suppresses the growth of tumors in Balb/c nude mice transplanted with the human GC cell line NCI-N87.Citation27 Therefore, AMF/GPI overexpression may cause drug resistance in GC and its suppression may increase the sensitivity to chemotherapy.
AMF/GPI expression was positively linked to lymph node metastasis and TNM staging of GC, indicating that AMF overexpression may play a positive role in the progression and differentiation of cancer. Gong et alCitation19 have found AMF/GPI overexpression in GC with lymph node metastasis, an observation that supports our data. Our functional experiments showed the inhibitory effect of AMF/GPI knockdown on proliferation, metabolism, and metastasis of GC cells, in accordance with the previous findings. In breast cancer, AMF/GPI overexpression increases the DNA-binding activity of NF-κB to upregulate the expression of ZEB1/ZEB2, which induces epithelial–mesenchymal transition (EMT).Citation28 In hepatocellular carcinoma, AMF/GPI enhances cell invasion by stimulating the adhesion, motility, and matrix metalloproteinase 2 secretion.Citation29 In melanoma, migration induced by AMF/GPI is mediated by autocrine production of IL-8.Citation30 AMF/GPI knockdown inhibits cell invasion, migration, and proliferation, and tumor metastasis in endometrial carcinoma via the suppression of the MAPK-ERK1/2 pathway.Citation31 Moreover, Tsutsumi et alCitation32 indicated that AMF/GPI overexpression stimulates invasion in vitro, tumor growth in vivo, and promotes liver metastasis of pancreatic cancer cells through downregulation of E-cadherin and upregulation of SNAIL. These data support the hypothesis that AMF/GPI may be positively linked to the progression of GC by inducing proliferation and EMT.
Previously, AMF/GPI overexpression has been found to be an independent prognostic factor of poor survival.Citation11,Citation19 Furthermore, high levels of AMF/GPI and its receptor have been reported in patients with breast cancer and high AMF expression has been related to local recurrence.Citation33 In our study, the results of immunohistochemistry and bioinformatics analysis showed that AMF/GPI overexpression is positively correlated with lower OS and PF survival rates in patients with GC. This result might be explained by the positive correlation between AMF/GPI expression and aggressive cell behavior. Hence, we propose that AMF/GPI might be a novel marker for adverse prognosis in patients with GC. Future studies are necessary to confirm the biologic effects of AMF/GPI on drug resistance and/or angiogenesis and to explore the relationship between tumor metabolism and other aggressive features.
Collectively, we found that AMF/GPI overexpression was associated with carcinogenesis and tumor progression and with a poorer prognosis in patients with GC. AMF/GFI knockdown reverses the aggressive phenotypes of GC and might, therefore, be a novel target for the treatment of GC.
Acknowledgments
The authors would like to thank Dr Han-Chen Huang and Dr Jian-Jun Lou at Institute of Biophysics, Chinese Academy of Sciences to make a suggestion and kindly provide shAMF/GPI expression vector. We also thank Dr Zheng HC at China Medical University for providing excellent guidance for seahorse XF24 analyses. This work was supported by Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No. ZYLX201701), Beijing Cancer Hospital (support code A001538), Research Fund for the Doctoral program of Higher Education (No. 2012001120135), and 985 cooperation program between clinical and basic medicine of Peking University (Grant No. A001047).
Supplementary materials
Figure S1 AMF/GPI mRNA in normal gastric tissues and primary gastric cancer tissues (Oncomine).
Abbreviations: AMF, autocrine motility factor; GPI, glucose-6-phosphate isomerase.
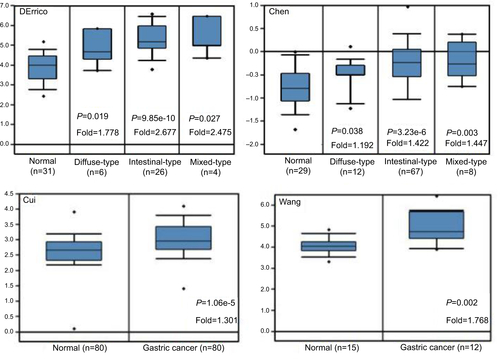
Figure S2 Kaplan–Meier survival curves of overall survival in 56 patients of diffuse type and 231 patients of intestinal type (AMF/GPI negative vs AMF/GPI positive).
Abbreviations: AMF, autocrine motility factor; GPI, glucose-6-phosphate isomerase.
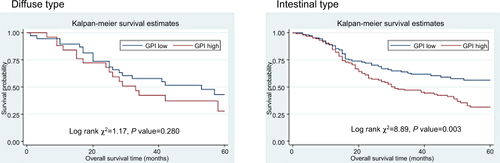
Figure S3 Kaplan–Meier survival curves of overall and progression-free survival for patients with AMF/GPI-negative and AMF/GPI-positive, from Kaplan–Meier Plotter (Szasz, Lanczky et al. 2016).
Abbreviations: AMF, autocrine motility factor; GPI, glucose-6-phosphate isomerase.
Reference
- SzaszAMLanczkyANagyACross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patientsOncotarget2016731493224933327384994
Disclosure
The authors report no conflicts of interest in this work.
References
- FitzmauriceCAkinyemijuTFAl LamiFHGlobal, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease studyJAMA Oncol20173452454827918777
- LaurenPThe two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histoclinicalActa Pathol Microbiol Scand196564314914320675
- van CutsemESagaertXTopalBHaustermansKPrenenHGastric cancerLancet2016388100602654266427156933
- HuBEl HajjNSittlerSLammertNBarnesRMeloni-EhrigAGastric cancer: classification, histology and application of molecular pathologyJ Gastrointest Oncol20123325126122943016
- AchariAMarshallSEMuirheadHPalmieriRHNoltmannEAGlucose-6-phosphate isomerasePhilos Trans R Soc Lond B Biol Sci198129310631451576115414
- LiottaLAMandlerRMuranoGTumor cell autocrine motility factorProc Natl Acad Sci U S A19868310330233063085086
- WatanabeHTakehanaKDateMShinozakiTRazATumor cell autocrine motility factor is the neuroleukin/phosphohexose isomerase polypeptideCancer Res19965613296029638674049
- XuJLiuJZhuLZhangXWLiZGZgLSignificance of glucose-6-phosphate isomerase assay in early diagnosis of rheumatoid arthritisBeijing Da Xue Xue Bao Yi Xue Ban201648694294627987494
- FanLYZongMWangQDiagnostic value of glucose-6-phosphate isomerase in rheumatoid arthritisClin Chim Acta201041123–242049205320826128
- DaiLZhuLJZhengDHElevated serum glucose-6-phosphate isomerase correlates with histological disease activity and clinical improvement after initiation of therapy in patients with rheumatoid arthritisJ Rheumatol201037122452246120810510
- BaumannMKapplALangTBrandKSiegfriedWPaterokEThe diagnostic validity of the serum tumor marker phosphohexose isomerase (PHI) in patients with gastrointestinal, kidney, and breast cancerCancer Invest199083–43513562207761
- FilellaXMolinaRJoJMasEBallestaAMSerum phosphohexose isomerase activities in patients with colorectal cancerTumour Biol19911263603671798910
- DevillersMAhmadLKorri-YoussoufiHSalmonLCarbohydrate-based electrochemical biosensor for detection of a cancer biomarker in human plasmaBiosens Bioelectron20179617818528500945
- FunasakaTHagaARazANagaseHTumor autocrine motility factor is an angiogenic factor that stimulates endothelial cell motilityBiochem Biophys Res Commun2001285111812811437381
- MurataJLeeHYClairTcDNA cloning of the human tumor motility-stimulating protein, autotaxin, reveals a homology with phosphodiesterasesJ Biol Chem19942694830479304847982964
- LiYCheQBianYAutocrine motility factor promotes epithelial-mesenchymal transition in endometrial cancer via MAPK signaling pathwayInt J Oncol20154731017102426201353
- TsutsumiSYanagawaTShimuraTRegulation of cell proliferation by autocrine motility factor/phosphoglucose isomerase signalingJ Biol Chem200327834321653217212783864
- HagaAFunasakaTNiinakaYRazANagaseHAutocrine motility factor signaling induces tumor apoptotic resistance by regulations Apaf-1 and Caspase-9 apoptosome expressionInt J Cancer2003107570771414566819
- GongWJiangYWangLExpression of autocrine motility factor correlates with the angiogenic phenotype of and poor prognosis for human gastric cancerClin Cancer Res200511165778578316115916
- WarburgOThe metabolism of carcinoma cellsJ Cancer Res192591148163
- LiSLiZGuoTMaternal embryonic leucine zipper kinase serves as a poor prognosis marker and therapeutic target in gastric cancerOncotarget2016756266628026701722
- SzászAMLánczkyANagyÁCross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patientsOncotarget2016731493224933327384994
- de PaduaMCDelodiGVučetićMDisrupting glucose-6-phosphate isomerase fully suppresses the “Warburg effect” and activates OXPHOS with minimal impact on tumor growth except in hypoxiaOncotarget2017850876238763729152106
- NiizekiHKobayashiMHoriuchiIHypoxia enhances the expression of autocrine motility factor and the motility of human pancreatic cancer cellsBr J Cancer200286121914191912085186
- FunasakaTYanagawaTHoganVRazARegulation of phosphoglucose isomerase/autocrine motility factor expression by hypoxiaFaseb J200519111422143016126909
- KhoDHNangia-MakkerPBalanVAutocrine motility factor promotes HER2 cleavage and signaling in breast cancer cellsCancer Res20137341411141923248119
- JungHSLeeSIKangSHMonoclonal antibodies against autocrine motility factor suppress gastric cancerOncol Lett20171364925493228599497
- AhmadAAboukameelAKongDPhosphoglucose isomerase/autocrine motility factor mediates epithelial-mesenchymal transition regulated by miR-200 in breast cancer cellsCancer Res20117193400340921389093
- BayoJFioreEAquinoJBIncreased migration of human mesenchymal stromal cells by autocrine motility factor (AMF) resulted in enhanced recruitment towards hepatocellular carcinomaPLoS One201494e9517124736611
- ArakiKShimuraTYajimaTPhosphoglucose isomerase/autocrine motility factor promotes melanoma cell migration through ERK activation dependent on autocrine production of interleukin-8J Biol Chem200928447323053231119801670
- LiYJiaYCheQZhouQWangKWanXPAMF/PGI-mediated tumorigenesis through MAPK-ERK signaling in endometrial carcinomaOncotarget2015628263732638726308071
- TsutsumiSYanagawaTShimuraTKuwanoHRazAAutocrine motility factor signaling enhances pancreatic cancer metastasisClin Cancer Res200410227775778415570012
- JiangWGRazADouglas-JonesAManselREExpression of autocrine motility factor (AMF) and its receptor, AMFR, in human breast cancerJ Histochem Cytochem200654223124116204225