Abstract
Background
Data from published articles on the relationship between MMP polymorphisms and prostate cancer risk are conflicted and inconclusive, so a meta-analysis and systematic review were performed to assess the relationship.
Methods
Relevant research articles were identified from databases using a search strategy. Studies with the same MMP polymorphisms that could be quantitatively synthesized were included in the meta-analysis. Five comparison models (homozygote, heterozygote, dominant, recessive, and additive) were applied, and a subgroup analysis by case-group sample type was performed. Studies with different polymorphisms that could not be quantitatively synthesized were included in the systematic review.
Results
Eleven articles encompassing 22 studies involving 12 MMP polymorphisms were included in this paper. Among the studies included, 13 studies involving MMP1 rs1799750, MMP2 rs243865, and MMP7 rs11568818 were quantitatively synthesized for meta-analysis, and the other nine studies involving nine polymorphisms (MMP2 rs2285053, MMP2 rs1477017, MMP2 rs17301608, MMP2 rs11639960, MMP3 11715A/6A, MMP3 1161A/G, MMP3 5356A/G, MMP9 rs17576, and MMP13 rs2252070) were included in the systematic review. Meta-analysis showed no associations between MMP1 rs1799750, MMP2 rs243865, or MMP7 rs11568818 and prostate cancer risk overall. Subgroup analysis by case-group sample type confirmed that no associations existed. The systematic review suggested that MMP3 11715A/6A and MMP9 rs17576 were associated with prostate cancer risk.
Conclusion
MMP polymorphisms are not associated with prostate cancer risk, except for MMP3 11715A/6A and MMP9 rs17576. However, it is necessary to conduct larger-scale, high-quality studies in future.
Introduction
A complex disorder resulting from the combined effects of multiple environmental and genetic factors, prostate cancer is the second-leading cause of cancer death in men.Citation1 The underlying etiology of prostate cancer is still poorly understood. Genome-wide association studies on the genetic etiology of cancer have discovered some heritability of different cancer types.Citation2 Single-nucleotide substitution, a kind of alteration in genetic sequence, can lead to cancer formation through biologically regulating a handful of molecular activities.Citation3
A family of zinc endopeptidases, MMPs can cleave nearly all components of the extracellular matrix, as well as many other soluble and cell-associated proteins.Citation4 MMPs play important roles in cancer development, invasion, and metastasis.Citation5 At the genetic level, a number of studies have been carried out to assess associations between polymorphisms of MMPs and prostate cancer risk,Citation6–Citation14 but conclusions have been conflicting and inconclusive. For example, Srivastava et al found the MMP2 rs243865 polymorphism contributed to prostate cancer susceptibility,Citation10 while Adabi et al found no association between MMP2 rs243865 polymorphism and prostate cancer risk.Citation11 Individual studies with small samples may result in incorrect conclusions. Therefore, a comprehensive meta-analysis and systematic review were necessary to assess relationships between MMP polymorphisms and prostate cancer risk precisely.
Methods
Search strategy
The entire process of this meta-analysis and systematic review followed the guidelines of the PRISMA (preferred reporting items for systematic reviews and meta-analyses) statement ().Citation15 The databases PubMed, Embase, and Web of Knowledge were searched with the following search terms: (MMP OR MMPs OR matrix metalloproteinase OR matrix metalloproteinases) AND (polymorphism OR polymorphisms OR single nucleotide polymorphism OR single nucleotide polymorphisms) AND (prostate cancer OR prostate carcinoma). The last search was on August 3, 2018. Additional published data were identified by reviewing references listed in each article. The literature search was performed by two investigators independently. Disagreement was resolved by discussion.
Inclusion and exclusion criteria
Inclusion criteria for this study were a focus on associations between MMP polymorphisms and prostate cancer risk, case–control design, available frequency of each genotype provided in both case and control groups to calculate OR and corresponding 95% CI, and written in English. Exclusion criteria were reviews, editorials, comments, and animal studies and overlap with another included article.
Data extraction
Two investigators independently extracted author names, year of publication, country of origin, case-group sample type, source of control group, genotyping method, type of MMPs, names of polymorphisms, number of cases and controls, Hardy–Weinberg equilibrium (HWE) values, and frequency of genotypes. Consensus on extracted items was reached by discussion between the two investigators.
Quality assessment
The quality of each included study was assessed according to the quality-assessment criteria in .Citation16 Quality scores of studies ranged from 0 to 15, and studies with scores ≥9 were regarded as being of high quality.
Statistical analysis
Meta-analysis was performed unless at least two studies concerning the same MMP polymorphism were included; otherwise, a systematic review was carried out. Pooled ORs and 95% CIs were calculated under five comparison models: homozygote, heterozygote, dominant, recessive, and additive. Pooled ORs assessed by Z-test were considered significant at P<0.05. HWE in the control group was checked by χ2 test, and disequilibrium was deemed present at P<0.05. Heterogeneity assumption was checked by a χ2-based Q-statistic test and quantified by I2 values. If I2<50% or Q-test P>0.10, the -effect model was used. Otherwise, a random-effect model was used. Subgroup analysis by case-group sample type was also performed. Funnel plots and Egger’s test were undertaken to examine publication bias. Publication bias was considered at P<0.05 for Egger’s test. Statistical analyses for this paper were completed with Stata (College Station, TX, USA) version 12.0.
Results
Literature search and study characteristics
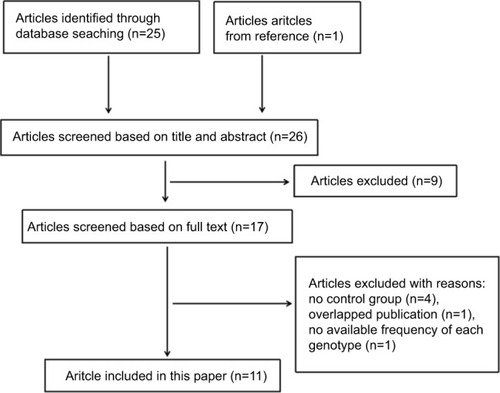
shows the selection process. A total of 26 articles were identified through the search strategy.Citation6–Citation14,Citation17–Citation33 Nine articles were removed based on the title or abstract,Citation17–Citation25 and the 17 remaining articles were screened for full text. Among these 17 articles,Citation6–Citation14,Citation26–Citation33 only eleven met inclusion criteria, because four did not have a control group,Citation26–Citation29 one overlapped with another,Citation30 and one did not provide available frequency of each genotype in either the case group or control group.Citation31 Ultimately, eleven articles encompassing 22 studiesCitation6–Citation14,Citation32,Citation33 and involving 12 polymorphisms were included in this paper. Their characteristics are listed in . Definitions of comparison models for the studies are listed in , and frequencies of genotypes from the meta-analysis and systematic review in and , respectively.
Table 1 Characteristics of included studies
Among the included studies, 13 studies with three polymorphisms (five for MMP1 rs1799750 involving 853 prostate cancer cases and 1,027 controls, six for MMP2 rs243865 involving 699 prostate cancer cases and 734 controls, and two for MMP7 rs11568818 involving 297 prostate cancer cases and 297 controls) were quantitatively synthesized for meta-analysis.Citation6,Citation8–Citation10,Citation12–Citation14,Citation32,Citation33 The remaining nine studies with nine polymorphisms (MMP2 rs2285053, MMP2 rs1477017, MMP2 rs17301608, MMP2 rs11639960, MMP3 1171-5A/6A, MMP3 1161A/G, MMP3 5356A/G, MMP9 rs17576, and MMP13 rs2252070) involving 2,054 prostate cancer cases and 2,138 controls could not be quantitatively synthesized, and so the systematic review was performed.Citation7,Citation8,Citation10,Citation11,Citation33
Meta-analysis
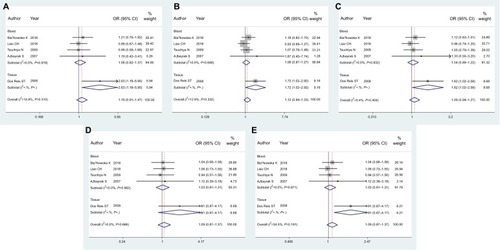
The results of meta-analysis for MMP1 rs1799750 (, ) showed that no significant associations were found in overall people (homozygote model, OR 1.16, 95% CI 0.91–1.47, P=0.237; heterozygote model, OR 1.12, 95% CI 0.94–1.33, P=0.223; dominant model, OR 1.09, 95% CI 0.94–1.27, P=0.251; recessive model, OR 1.09, 95% CI 0.87–1.37, P=0.471; additive model, OR 1.09, 95% CI 0.97–1.23, P=0.163). When the studies were stratified according to blood samples of case groups (, ), no associations existed in any comparison model. Subgroups of tissue samples could not be assessed, because there was only one study included.
Table 2 Meta-analysis of association between MMP1 rs1799750 and prostate cancer
Figure 2 Forest plots of MMP1 rs1799750 and prostate cancer risk.
Notes: (A) Homozygote model; (B) heterozygote model; (C) dominant model; (D) recessive model; (E) additive model.
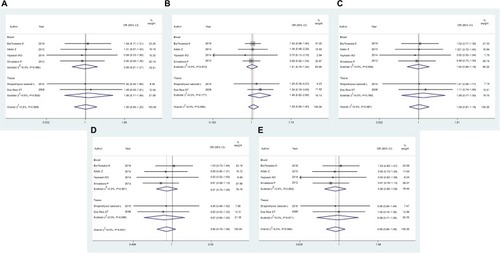
For the MMP2 rs243865 polymorphism (, ), meta-analysis showed no significant associations were found in people overall (homozygote model, OR 1.00, 95% CI 0.84–1.20, P=0.97; heterozygote model, OR 1.08, 95% CI 0.84–1.40, P=0.54; dominant model, OR 1.01, 95% CI 0.87–1.18, P=0.875; recessive model, OR 0.90, 95% CI 0.76–1.06, P=0.206; additive model, OR 0.96, 95% CI 0.86–1.08, P=0.521). Subgroup analysis by case-group sample type confirmed that no associations existed in any comparison model matter for blood or tissue samples (, ).
Table 3 Meta-analysis of association between MMP2 rs243865 and prostate cancer
Figure 3 Forest plots of MMP2 rs243865 and prostate cancer risk.
Notes: (A) Homozygote model; (B) heterozygote model; (C) dominant model; (D) recessive model; (E) additive model.
For MMP7 rs11568818 (, ), no significant associations were found in people overall (homozygote model, OR 0.95, 95% CI 0.67–1.37, P=0.796; heterozygote model, OR 0.98, 95% CI 0.72–1.33, P=0.908; dominant model, OR 0.99, 95% CI 0.77–1.26, P=0.917; recessive model, OR 0.91, 95% CI 0.66–1.27, P=0.592; additive model, OR 0.97, 95% CI 0.80–1.17, P=0.72). Subgroup analysis by case-group sample type was not performed.
Table 4 Meta-analysis of association between MMP7 rs11568818 and prostate cancer
Heterogeneity analysis
For MMP1 rs1799750, MMP2 rs243865, and MMP7 rs11568818 polymorphisms, there was no obvious heterogeneity in any comparison model for people overall or for subgroup analyses (–).
Publication-bias analysis
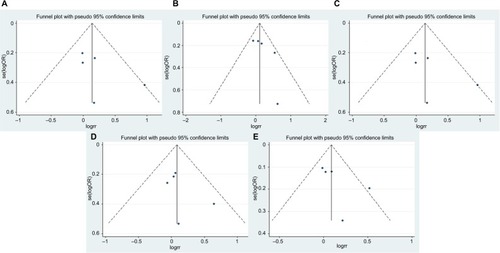
For MMP1 rs1799750, funnel plots () and Egger’s tests suggested no evidence of publication bias (homozygote model, P=0.27; heterozygote model, P=0.187; dominant model, P=0.199; recessive model, P=0.351; additive model, P=0.226).
Figure 5 Funnel plots of MMP1 rs1799750 and prostate cancer risk.
Notes: (A) Homozygote model; (B) heterozygote model; (C) dominant model; (D) recessive model; (E) additive model.
For MMP2 rs243865, funnel plots () and Egger’s tests (homozygote model, P=0.87; heterozygote model, P=0.864; dominant model, P=0.879; recessive model, P=0.826; additive model, P=0.927) suggested no evidence of publication bias in the meta-analysis either.
Figure 6 Funnel plots of MMP2 rs243865 and prostate cancer risk.
Notes: (A) Homozygote model; (B) heterozygote model; (C) dominant model; (D) recessive model; (E) additive model.
For MMP7 rs11568818, publication-bias analysis was not conducted for the two studies involved.
Systematic review
In the systematic review (), two polymorphisms (MMP3 1171-5A/6A and MMP9 rs17576) were reported to be associated with prostate cancer risk, while another seven polymorphisms (MMP2 rs2285053, MMP2 rs1477017, MMP2 rs17301608, MMP2 rs11639960, MMP3 1161A/G, MMP3 5356A/G, and MMP13 rs2252070) were not associated with prostate cancer risk.
Table 5 Systematic review of association between MMPs polymorphisms and prostate cancer
Discussion
Srivastava et al showed that MMP2 rs243865 polymorphism contributed to prostate cancer susceptibility,Citation10 while Adabi et al showed no association between MMP2 rs243865 polymorphism and prostate cancer risk.Citation13 Therefore, a comprehensive meta-analysis and systematic review were necessary. As a powerful tool for summarizing different studies, meta-analysis and systematic review refer to the use of statistical techniques to integrate results of included studies.Citation15
This meta-analysis of five studies for MMP1 rs1799750, six studies for MMP2 rs243865 and two studies for MMP7 rs11568818 demonstrated that MMP1 rs1799750, MMP2 rs243865 polymorphisms and MMP7 rs11568818 were not associated with prostate cancer. Subgroup analysis by case-group sample type confirmed that no associations existed in any comparison model. We attributed the negative conclusions of our meta-analysis to two factors: firstly, only articles in English were included, and thus other related articles failed to be included; and secondly, some lower-quality studies were included, resulting in unpersuasive conclusions.
Although this systematic review of nine studies involving nine polymorphisms revealed that MMP3 1171 5A/6A and MMP9 rs17576 were associated with prostate cancer risk, its conclusion needs more research to support it, because each polymorphism had only one study. MMP9 can produce prostate cancer indirectly via triggering TGFβ activation, because an increase in TGFβ signaling will lead to cancer development and progession.Citation34,Citation35
We noticed two previous meta-analyses had investigated the relationships of MMP1 rs1799750 or MMP2 rs243865 and prostate cancer risk.Citation17,Citation18 We read these carefully with great interest. Neither included other MMP polymorphisms, except for MMP1 rs1799750 and MMP2 rs243865.Citation17,Citation18 For MMP2 rs243865, our meta-analysis did not enroll the study by Jacobs et al, because it did not provide available frequency of genotypes.Citation7 Conversely, both the previous meta-analyses included this study and thus concluded significant association.Citation17,Citation18 For MMP1 rs1799750, our paper enrolled two additional studiesCitation32,Citation33 compared with one previous meta-analysis,Citation17 and obtained a similar result. The major strengths of our paper lie in focusing on the relationship between MMP polymorphisms and prostate cancer risk comprehensively and systematically.
Some limitations still existed in our paper. First, several included studies contained small samples, which could lead to unpersuasive conclusions. Second, departure from HWE was detected in some studies. Third, there was a lack of a unified criterion for including studies.
Conclusion
In summary, our paper shows that MMP polymorphisms are not associated with prostate cancer risk, except for MMP3 1171-5A/6A and MMP9 rs17576. However, it is necessary to conduct more large-scale and high-quality studies in future.
Supplementary materials
Table S1 PRISMA checklist
Table S2 Quality-assessment scores
Table S3 Definition of comparison models
Table S4 Frequency of genotype in studies from meta-analysis. (A) MMP1 rs1799750; (B) MMP2 rs243865; (C) MMP7 rs11568818
Table S5 Frequency of genotype in studies from systematic review
References
- AlbayrakSCangüvenOGöktaşCAydemirHKöksalVRole of MMP-1 1G/2G promoter gene polymorphism on the development of prostate cancer in the Turkish populationUrol Int200779431231518025848
- Dos ReisSTPontesJJrVillanovaFEGenetic polymorphisms of matrix metalloproteinases: susceptibility and prognostic implications for prostate cancerJ Urol200918152320232519303106
- TsuchiyaNNaritaSKumazawaTClinical significance of a single nucleotide polymorphism and allelic imbalance of matrix metalloproteinase-1 promoter region in prostate cancerOncol Rep200922349343919639194
- LiaoCHWuHCHuPSThe association of matrix metallopro-teinase-1 Promoter polymorphisms with prostate cancer in taiwanese patientsAnticancer Res20183873907391129970511
- BiałkowskaKMarciniakWMuszyńskaMAssociation of zinc level and polymorphism in MMP-7 gene with prostate cancer in Polish populationPLoS One2018137e020106530036379
- SrivastavaPLoneTAKapoorRMittalRDAssociation of promoter polymorphisms in MMP2 and TIMP2 with prostate cancer susceptibility in North IndiaArch Med Res2012432117112422374248
- YaykaşliKOKayikçiMAYamakNPolymorphisms in MMP-2 and TIMP-2 in Turkish patients with prostate cancerTurk J Med Sci201444583984325539555
- AdabiZMohsen ZiaeiSAImaniMGenetic polymorphism of MMP2 gene and susceptibility to prostate cancerArch Med Res201546754655026319608
- Shajarehpoor SalavatiLTafviziFManjiliHKThe association between MMP2 -1306 C > T (rs243865) polymorphism and risk of prostate cancerIr J Med Sci2017186110311127541146
- JacobsEJHsingAWBainEBPolymorphisms in angiogenesis-related genes and prostate cancerCancer Epidemiol Biomarkers Prev200817497297718398039
- SrivastavaPKapoorRMittalRDImpact of MMP-3 and TIMP-3 gene polymorphisms on prostate cancer susceptibility in North Indian cohortGene2013530227327723872201
Disclosure
The authors report no conflicts of interest in this work.
References
- CollakFKDemirUOzkanliSKurumEZerkPEIncreased expression of YAP1 in prostate cancer correlates with extraprostatic extensionCancer Biol Med201714440541329372107
- DaiJShenWWenWEstimation of heritability for nine common cancers using data from genome-wide association studies in Chinese populationInt J Cancer2017140232933627668986
- GengPLiJWangNPSCA rs2294008 polymorphism with increased risk of cancerPLoS One2015108e013626926308216
- RadiskyESRaeeszadeh-SarmazdehMRadiskyDCTherapeutic potential of matrix metalloproteinase inhibition in breast cancerJ Cell Biochem2017118113531354828585723
- EgebladMWerbZNew functions for the matrix metalloproteinases in cancer progressionNat Rev Cancer20022316117411990853
- AlbayrakSCangüvenOGöktaşCAydemirHKöksalVRole of MMP-1 1G/2G promoter gene polymorphism on the development of prostate cancer in the Turkish populationUrol Int200779431231518025848
- JacobsEJHsingAWBainEBPolymorphisms in angiogenesis-related genes and prostate cancerCancer Epidemiol Biomarkers Prev200817497297718398039
- dos ReisSTPontesJVillanovaFEGenetic polymorphisms of matrix metalloproteinases: susceptibility and prognostic implications for prostate cancerJ Urol200918152320232519303106
- TsuchiyaNNaritaSKumazawaTClinical significance of a single nucleotide polymorphism and allelic imbalance of matrix metalloproteinase-1 promoter region in prostate cancerOncol Rep200922349343919639194
- SrivastavaPLoneTAKapoorRMittalRDAssociation of promoter polymorphisms in MMP2 and TIMP2 with prostate cancer susceptibility in North IndiaArch Med Res201243211712422374248
- SrivastavaPKapoorRMittalRDImpact of MMP-3 and TIMP-3 gene polymorphisms on prostate cancer susceptibility in North Indian cohortGene2013530227327723872201
- YaykaşliKOKayikçiMAYamakNPolymorphisms in MMP-2 and TIMP-2 in Turkish patients with prostate cancerTurk J Med Sci201444583984325539555
- AdabiZMohsen ZiaeiSAImaniMGenetic polymorphism of MMP2 gene and susceptibility to prostate cancerArch Med Res201546754655026319608
- SalavatiLSTafviziFManjiliHKThe association between MMP2 -1306 C > T (rs243865) polymorphism and risk of prostate cancerIr J Med Sci2017186110311127541146
- MoherDLiberatiATetzlaffJAltman DGPRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementInt J Surg20108533634120171303
- ThakkinstianAMckayGJMcevoyMSystematic review and meta-analysis of the association between complement component 3 and age-related macular degeneration: a HuGE review and meta-analysisAm J Epidemiol2011173121365137921576320
- WengHZengXTWangXHLiuTZHeDLGenetic Association between Matrix Metalloproteinases Gene Polymorphisms and Risk of Prostate Cancer: A Meta-AnalysisFront Physiol2017897529249982
- LiuKGuSLiuXThe MMP2 rs243865 polymorphism increases the risk of prostate cancer: A meta-analysisOncotarget2017842729337293829069837
- HaqueSAkhterNLohaniMAliAMandalRKMatrix metal-loproteinase-2 -1306 C>T gene polymorphism is associated with reduced risk of cancer: a meta-analysisAsian Pac J Cancer Prev201516388989625735378
- LiuMZhaoYYYangFEvidence for a role of GPRC6A in prostate cancer metastasis based on case-control and in vitro analysesEur Rev Med Pharmacol Sci201620112235224827338047
- BenJJinGZhangYClass A scavenger receptor deficiency exacerbates lung tumorigenesis by cultivating a procarcinogenic microenvironment in humans and miceAm J Respir Crit Care Med2012186876377222878280
- AmankwahEKSellersTAParkJYGene variants in the angio-genesis pathway and prostate cancerCarcinogenesis20123371259126922523086
- Nangia-MakkerPWangYRazTCleavage of galectin-3 by matrix metalloproteases induces angiogenesis in breast cancerInt J Cancer2010127112530254120162566
- AhirwarDKMittalRDRe: Genetic polymorphisms of matrix metalloproteinases: susceptibility and prognostic implications for prostate cancer. S. T. dos Reis, J. Pontes, Jr., F. E. Villanova, P. M. D. Borra, A. A. Antunes, M. F. Dall’oglio, M. Srougi and K. R. M. Leite. J Urol 2009; 181: 2320-2325J Urol201018331258
- JainSChakrabortyGKunduGCThe crucial role of cyclooxygenase-2 in osteopontin-induced protein kinase C alpha/c-Src/IkappaB kinase alpha/beta-dependent prostate tumor progression and angiogenesisCancer Res200666136638664816818637
- SchveigertDValuckasKPKovalcisVUlysAChvatovicGDidziap-etrieneJSignificance of MMP-9 expression and MMP-9 polymorphism in prostate cancerTumori201399452352924326842
- JaboinJJHwangMLopaterZThe matrix metalloproteinase-7 polymorphism rs10895304 is associated with increased recurrence risk in patients with clinically localized prostate cancerInt J Radiat Oncol Biol Phys20117951330133520605361
- Gil UgarteburuRRivas del FresnoMGonzález RodríguezIMatrix metalloproteinase- 9 polymorphisms in the diagnosis of prostate cancer. A preliminary experienceArch Esp Urol201063212513220354277
- dos ReisSTVillanovaFEAndradePMMatrix metalloprotein-ase-2 polymorphism is associated with prognosis in prostate cancerUrol Oncol201028662462719117773
- dos ReisSTVillanovaFEde AndradePMPolymorphisms of the matrix metalloproteinases associated with prostate cancerMol Med Rep20081451752021479442
- Profile StudyAustralian Prostate Cancer BioResource (APCB)IMPACT StudyAssociation analyses of more than 140,000 men identify 63 new prostate cancer susceptibility lociNat Genet201850792893629892016
- LiaoCHWuHCHuPSThe association of matrix metallopro-teinase-1 Promoter polymorphisms with prostate cancer in taiwanese patientsAnticancer Res20183873907391129970511
- BiałkowskaKMarciniakWMuszyńskaMAssociation of zinc level and polymorphism in MMP-7 gene with prostate cancer in Polish populationPLoS One2018137e020106530036379
- LeeCJiaZRahmatpanahFRole of the adjacent stroma cells in prostate cancer development and progression: synergy between TGF-β and IGF signalingBiomed Res Int20142014502093825089270
- DayerCStamenkovicIRecruitment of matrix metalloproteinase-9 (MMP-9) to the fibroblast cell surface by lysyl hydroxylase 3 (LH3) triggers transforming growth factor-β (TGF-β) activation and fibroblast differentiationJ Biol Chem201529022137631377825825495